Recent advances in the management of gastrointestinal stromal tumor
Monjur Ahmed
Monjur Ahmed, Division of Gastroenterology and Hepatology, Department of Internal Medicine,Thomas Jefferson University, Philadelphia, PA 19107, United States
Abstract
Key words:Gastrointestinal stromal tumor;Mesenchymal tumor of gastrointestinal tract;Gastrointestinal subepithelial tumors;Management of gastrointestinal stromal tumor;Familial gastrointestinal stromal tumor;Risk stratification
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are rare gastrointestinal (GI) neoplasms accounting for 0.1 to 3% of all GI malignancies[1].But they are the most common (80%)mesenchymal tumors of the GI tract[2].They arise from the spindle shaped mesenchymal cells called interstitial cells of Cajal (ICCs) or stem cell precursors to these cells.ICCs are the GI pacemaker cells (specialized nerve cells) present in the muscularis propria and around the myenteric plexus.Most (50%-70%) of the GISTs are located in the stomach (70% in gastric body, 15% in antrum and 15% in cardia) or small intestine (30% in jejunum or ileum, 5% in duodenum) but they can arise in any part of the GI tract (colon, rectum, appendix together–5% and esophagus 2%-3%), and rarely in the extra-GI site (mesentery, omentum, retroperitoneum and pancreas) where they arise from stem cell precursors to ICC[3].The incidence of GISTs has been increasing over the last few decades.They generally occur in the middle aged and older population (median age 60-65 years) with slight male predominance (3:1)[4].Patients may remain asymptomatic for long period of time until complication or metastasis occurs.Most of the time, GISTs are diagnosed incidentally in imaging studies, endoscopic evaluations, during surgical procedures or autopsy.Instead of classifying as benign or malignant, they are stratified as low risk and high risk GISTs depending on mitotic index, tumor size and tumor locations.They can have slow growing indolent course or fast growing, metastasizing and life threatening course.The prognosis can be excellent if they can be diagnosed and treated early.In the past,GISTs were thought to be originating from smooth muscles as they have smooth muscle features on light microscopy.In 1980s, they were named as stromal tumors as they were found to have no immunophenotypic features of smooth muscles on immunohistochemistry.In 1998, Kindblom and associates first reported that GISTs originated from ICCs[5].In this article, the epidemiology, pathophysiology, histology,clinical aspects, diagnosis, the updated treatment strategy and prognosis of GISTs will be reviewed.
EPIDEMIOLOGY
In the United States (US), about 5000–6000 new cases of GISTs are diagnosed per year[6].As per Surveillance, Epidemiology, and End Results database, the incidence of GIST increased from 0.55/100000 population in 2001 to 0.78/100000 population in 2011[7].Another study published in 2006 showed that there was 25-fold increase in incidence of GISTs in the US in 10 years since 1992[8].In Europe, the incidence of GIST varies from 6.5 to 14.5 per million per year[9-11].The exact of prevalence of GIST is not known.But it assumed that as many cases remain silent throughout their life, the prevalence of GISTs is possibly high.One German study on consecutive autopsies found the presence of subcentimeter GISTs in 22.5% of individuals older than 50 years[12].The real incidence of GIST is not known because a lot of tumors have not been tested for theKITor the Platelet derived growth factor receptor alpha (PDGFRA) gene mutations.
PATHOPHYSIOLOGY
Uncontrolled proliferation of ICCs leads to the development of GISTs.Thec-kitprotooncogene located on chromosome 4q 11-12 encodes the transmembrane tyrosine kinase KIT[13].The great discovery of gain-of-function mutation of c-KIT in human GISTs was published by Hirotaet al[14]in 1998.Exon 11 (transmembrane domain) is involved in 90% ofKITgene mutation[15].KIT-activating mutations lead to ICCs hyperplasia and GISTs.KITgene mutation with constitutive activation of tyrosine kinase is found in 75% of GISTs.PDGFRAgene at chromosome 4q12 controls production ofPDGFRAwhich is a part of a family of proteins called receptor tyrosine kinase (RTKs).The most commonPDGFRAmutation is Asp842Val substitution in exon18.Intragenic activation mutation ofPDGFRAgene with production of RTKs was found in about 35% of GISTs lackingKITgene mutations[16].Thus the growth of GISTs is propelled by mutation of eitherKITgene (75% of cases) orPDGFRAgene (10% of cases).The oncogenic mechanisms appear to be alternative and mutually exclusive.GISTs associated with eitherKITorPDGFRAgene mutations are indistinguishable with respect to cytogenetic changes associated with tumor progression.But GISTs associated withPDGFRAmutation due to Asp842Val substitution in exon18 is resistant to tyrosine kinase inhibitor (TKI) imatinib mesylate.About 15% of GISTs do not have detectableKITorPDGFRAgene mutation.These are so-called ‘wild-type’GISTs or pediatric GISTs.Wild-type GISTs should be tested for germ-line mutation in theSDHgene (SDHdeficient by immunohistochemistry).Clinically, they cannot be distinguished fromKITorPDGFRA-mutant GISTs and have the same morphology,express high levels ofKIT, and occur in any part of the GI tract[17].Few other genetic mutations have been detected in wild-type GISTs.These includeBRAF,HRAS,NRAS,PIK3CAgene mutations.Germline mutation ofKITorPDGFRAoccurs in familial GIST which is extremely rare (<0.1%) and autosomal dominant.Patients generally present in middle age of life with multiple GISTs.Familial GISTs have benign course and do not decrease life span[18].GISTs can also be associated with certain syndromes.These include:(1) Neurofibromatosis type 1- multifocal small GISTs with low risk feature occur in small intestine.GISTs do not haveKITorPDGFRAmutation;(2) Carney-Stratakis syndrome–SDH-deficient GIST and paraganglioma due to germline mutation ofSDHA,SDHB, orSDHC[19];and (3) Carney triad–gastric GISTs,paraganglioma and pulmonary chondromas.GISTs are multifocal with high local recurrence rate.The above syndromes lack KIT or PDGFRA gene mutation.
Immunohistochemistry is important to identify GISTs from other spindle cell or epitheloid tumors.GISTs are generally negative for desmin (smooth muscle marker)and S100 protein (Schwann cell marker) but can be variably positive for smooth muscle actin and muscle-specific actin[20].70% of GISTs stain positive for CD34 which was the first immunohistochemical marker identified in1984 to differentiate GIST from leiomyoma and leiomyosarcoma.CD117 (KIT) is a sensitive and more specific marker for GIST discovered in 1998[21].DOG1 (a calcium-dependent chloride channel protein anoctamin) is a novel marker ubiquitously expressed in GISTs irrespective ofKITorPDGFRAmutation status[22].It was first discovered in 2004.CD117 and DOG1 each stain positive in more than 95% of GISTs.KITreceptor tyrosine kinase is expressed by almost all GISTs[23].It is not sufficient to establish the diagnosis of GIST just on the basis of immunohistochemical staining (IHS).Some extra-intestinal malignancies which can stain positive forKITinclude small cell lung cancer, metastatic melanoma,angiosarcoma and Ewing’s sarcoma[24].
HISTOLOGY
GISTs are well-circumscribed tumors ranging in size from <1 cm to >40 cm in diameter, the median size varies from 2.7 cm (when diagnosed incidentally) to 8.9 cm(when a patient is symptomatic)[10,25].Histologically, they are of 3 types:spindle cell type (70%), epitheloid type (20%) and mixed type (10%).Spindle cell types have compact and highly cellular spindle shaped cells which have fibrillary eosinophilic cytoplasm, ovoid nuclei with frequent paranuclear vacuolization, syncytial cell borders and are arranged in a storiform, fascicular pattern with minimal stroma.Dense deposits of collagen are also seen in the extracellular space.In epitheloid type,the cells have abundant pale eosinophilic to clear cytoplasms, round nuclei, distinct cellular borders and are arranged in sheets, nests or cords.Mixed types have combination of spindle cells and epitheloid cells.Rarely, GISTs contain acellular PAS stain positive collagen bundles known as skenoid fibers.Irrespective of the type, a variable inflammatory infiltrate (lymphocytes and plasma cells), hemorrhage, necrosis and sclerosis can be present.Degeneration and necrosis can be seen in GIST histology.There can be complete absence or high mitotic activity[26,27].Mitotic index calculated by counting the number of mitoses per 50 high-power fields is an important indicator of proliferative activity and prognosis of GIST.
Extragastrointestinal stromal tumors
Less than 5% of all GISTs are located outside the GI tract[28].Most of the extragastrointestinal stromal tumors (EGISTs) are found in the mesentery or omentum but other sites of involvement include retroperitoneum, pancreas, spleen, falciform ligament, hepatogastric ligament, mediastinum and pelvis[29].EGISTs are generally discovered during imaging studies and surgery.Tissue diagnosis is made by computerized tomography (CT)-guided or ultrasound-guided biopsy with IHS.They carry an aggressive course and poor prognosis if the GIST size is >5 cm with mitotic rate of >5/50 HPF.
Composite tumors with a GIST component
Coexistence of two different tumorsi.e.collision tumors are extremely rare and generally detected in the surgically resected specimen.Collision tumors with coexistence of adenocarcinoma and GIST in the stomach and small intestine have been reported[30].
CLINICAL ASPECTS
As mentioned before, GISTs are now-a-days detected more and more by various imaging studies like CT, magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS).Small GISTs do not produce any symptom or sign.But when they become larger in size, patients generally present with GI bleeding due to mucosal ulceration, abdominal pain or obstruction due to mass effect.In about 50% of cases,mucosal ulceration is seen on the luminal surface of the GIST[31].Of course, symptoms and signs depend on the location of the tumor.Gastric GISTs may present with hematemesis, melena, dyspepsia, nausea, vomiting or early satiety.Duodenal GISTs are well-defined round or oval subepithelial masses, generally located in the second through the fourth part of duodenum.They do not cause duodenal obstruction or lymphadenopathy but they may cause bleeding, ampullary obstruction and jaundice.They may also metastasize frequently to the liver and peritoneum[32].About 10% of gastric GISTs and 15% of duodenal GISTs may present emergently with upper GI bleeding.Small intestinal (jejunal–40%, ileal - 60%) GISTs may present with small bowel bleeding, fatigue (due to anemia), abdominal pain and intestinal obstruction.
They cause acute abdominal symptoms more frequently than duodenal GISTs[33].Esophageal GISTs may present with dysphagia.Mesenteric or omental GISTs may present as abdominal masses.8% of GISTs may present with acute abdominal emergency due to bowel wall rupture and peritonitis[34].Occasionaally tumor rupture into the peritoneal cavity may also cause intraabdominal bleeding and peritoneal seeding of tumor cells[35].GISTs generally metastasize to the liver and intra-abdominal cavity but rarely they metastasize to the lungs and bones.Anorectal GISTs are welldefined subepithelial tumors with an average size of 6.9 cm.They represent 5% of all GISTS.Most of the anorectal GISTs are asymptomatic, and are detected during endoscopic or imaging studies.Symptomatic patients may present with proctalgia(34%) or recal bleeding (25%)[36].
DIAGNOSIS
There is no supportive or confirmatory Laboratory test for GISTs.Imaging studies or endoscopic evaluations are done according to patient’s clinical presentation.As most of the GISTs occur in the stomach or small bowel, contrast-enhanced CT may show an intramural endophytic or exophytic (as GISTs generally involve the outer longitudinal muscular layer) hypervascular mass in the gastric or small bowel wall.Small (<5 cm)GISTs are homogenous, smooth-walled and sharply margined masses whereas large(>5 cm) GISTs are heterogenous (due to hemorrhage, necrosis or cystic degeneration)masses with well-defined or ill-defined margins and rare calcifications.CT is the most commonly used modality in the diagnosis of primary and metastatic GISTs.It is helpful not only in the initial diagnosis but also in following the natural progression of GISTs and the response of GISTs to treatment[37].On CT, GISTs are mainly iso to minimally hypodense to the muscle layer.In response to effective treatment, GISTs decreases in size and becomes more homogenous with disappearance of nodules.On the other hand, appearance of nodule in the GIST following treatment indicates recurrence of the disease.The main drawback of CT is inability to differentiate between inflammatory adhesions and involvement of contiguous organs.In case of gastric GISTs it is difficult to decide if the tumor arises from the stomach, pancreas,liver or colon.MRI is adjunctive to CT in the evaluation of GISTs.On MRI, small GISTs are generally round and homogenous with strong arterial enhancement while large GISTs are usually lobulated and mildly heterogenous with gradual enhancement due to hemorrhage, necrosis and cystic changes[38].MRI is more useful in the evaluation of anorectal GISTs and metastatic hepatic GISTs.On MRI, GISTs are iso to minimally hypointense to the muscle layer on T1-weighted images and hyperintense on T2-weighted images.More than 90% of GISTs are fluorodeoxyglucose (FDG) avid with a mean baseline standard uptake value of 5.8 on FDG-positron emission tomography(FDG-PET)[39].A baseline FDG-PET should be done prior to initiation of therapy.It is an ideal test not only for staging of GISTs but also for assessing therapeutic response,evaluating primary and secondary resistance to treatment, and resolving discrepant findings between clinical status and CT results.Primary resistance to TKI therapy can be identified by seeing lack of metabolic response on FDG-PET shortly after initiation of therapy.Secondary resistance to TKI will show re-emergence of metabolic activity(hot spot) on a quiescent area within the GIST following a period of therapeutic response.Combined hybrid PET/CT scanning can be the optimal test for the evaluation of patients treated with TKI[40].Endoscopically, GISTs appear as spherical or hemispherical smooth, subepithelial polypoid lesions which are further evaluated by EUS.Most commonly GISTs originate from the muscularis propriai.e., echo-poor 4thEUS layer of the GI wall but rarely they can originate from the echo-poor 2ndlayeri.e.,muscularis mucosae[41].Endosonographically, they appear as inhomogeneous,hypoechoic lesions, their differential diagnoses include leiomyoma, lymphoma and schwannoma[42].Tissue diagnosis should be done by EUS guided fine needle aspiration(FNA) for >1 cm lesion or true cut biopsy (TCB) for 2 to 5 cm lesion, particularly if non-GIST histology like leiomyoma is suspected[43].EUS-guided FNA/TCB should also be considered if it is a very large GIST needing neoadjuvant therapy with TKI.Tissue acquisition may not be necessary if it is a very large, symptomatic GIST with malignant features on EUS and has to be resected by surgery irrespective of FNA/TCB result.High risk malignant features on EUS include large size of the GIST (≥ 2 cm),irregular borders, presence of heterogenous echogenicity, anechoic (cystic) spaces,ulceration, echogenic foci, a marginal halo and increase in size during follow up[44,45].Endoscopically, focal mucosal ulceration of the GIST is common and is not related to malignancy[46].Endoscopic, endosonographic or imaging features are suggestive but not diagnostic of GIST.Histopatholgy with hematoxylin and eosin staining alone is not sufficient to establish the diagnosis of GIST.Definitive diagnosis of GIST is established when there is concordance between histology and IHS with KIT, CD34 or DOG1.
RISK STRATIFICATION OF GISTs
The aggressiveness of GISTs depends on the size and mitotic index of GISTs, and also anatomic site of involvement and patient’s age.As per the National Institute Health(NIH) GIST consensus criteria, GISTs are categorized into very low risk, low risk,intermediate risk and high risk groups as shown in Table 1[47].
As per NIH classification, 44% of GISTs belong to high-risk category, 24%intermediate risk and 32% low risk/very low risk category.The significance of mitotic index also depends on location of the GISTs.Small bowel, esophageal and rectal GISTs are more aggressive and are associated with worse prognosis and more recurrence than gastric GISTs of the same size, mitotic index and tumor rupture[48].Size ≥ 10 cm,mitotic rate ≥ 5 per 50 HPF and location of the GIST (non-gastric) and tumor rupture can independently predict recurrence of the GIST after complete surgical resection[49].A modified version of NIH risk assessment has been proposed by Joensuu as shown in Table 2.Currently, the Joensuu classification is widely used.Tumor size, mitotic index,primary tumor site and tumor rupture are included in this version.Patients belonging to high-risk category have 15%-20% risk of having recurrent disease[50].Besides the above factors, certain mutations at the genetic level can be associated with poor outcome.These includeKITexon 9 mutations and deletions affecting codons 557-558(exon 11 deletions) of thec-KITgene[51].KITexon 9 mutations are almost exclusively found in the small bowel.As mentioned beforePDGFRAmutation due to Asp842Val substitution in exon18 leads to resistance to tyrosine kinase inhibitor imatinib mesylate.PDGFRAmutations are usually seen in gastric GISTs.A simplified way of stratifying the risk of GISTs is to use “rule of fives” for low risk and high risk groups[52].Both high risk and intermediate risk gastric GISTs are >5 cm in size and have >5 mitosis per 50 HPF whereas non-gastric GISTs belong to high risk category if they are either >5 cm in size or have >5 mitosis per 50 HPF.Gastric GISTs <10 cm and <5 mitosis per HPF can metastasize in about 2 to 3% of cases whereas gastric GISTs >10 cm and >5 mitosis per 50 HPF can metastasize in about 86% of cases.11%of gastric GISTs >10 cm with <5 mitosis per HPF and 15% of gastric GISTs <5 cm with >5 mitosis per 50 HPF can metastasize.Gastric antral GISTs carry a better prognosis than gastric fundic or gastroesophageal junction GISTs[53].Small intestinal GISTs >10 cm with mitosis ≤ 5 per 50 HPF or ≤ 5 cm with mitosis >5 per 50 HPF have metastatic potential of >50% whereas small intestinal GISTs >5 to 10 cm with mitosis<5 per 50 HPF have metastatic potential of 24%[54].
The latest staging system of GIST is the 2018 American Joint Committee of Cancer tumor node metastasis system as shown in Table 3 and Table 4[55].
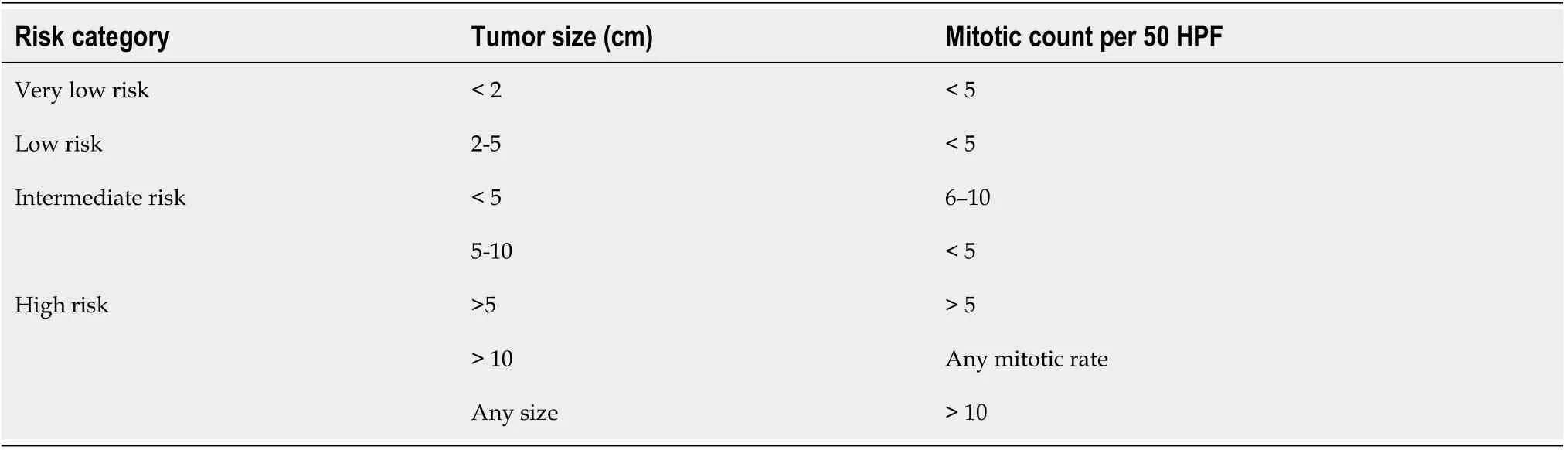
Table 1 National Institute Health gastrointestinal stromal tumor consensus criteria
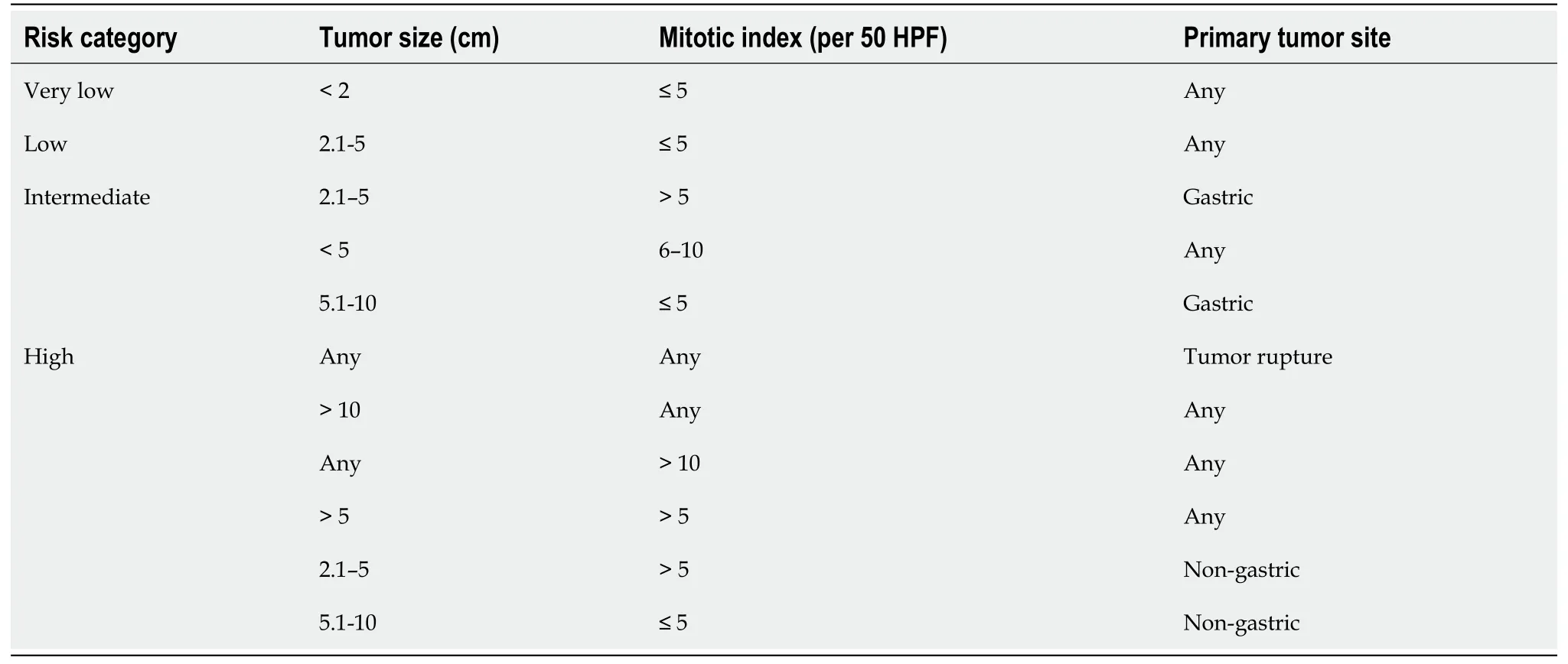
Table 2 Joensuu criteria for gastrointestinal stromal tumor risk assessment
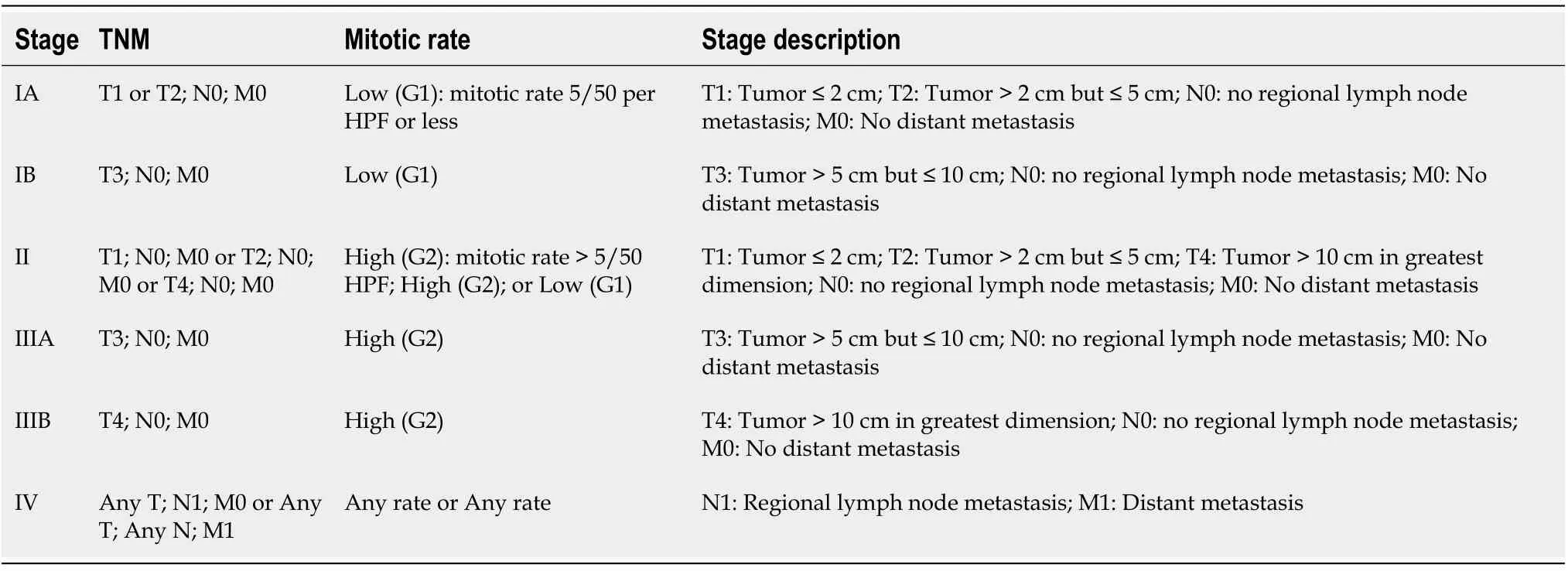
Table 3 American Joint Committee on Cancer tumor node metastasis system for gastric and omental gastrointestinal stromal tumor
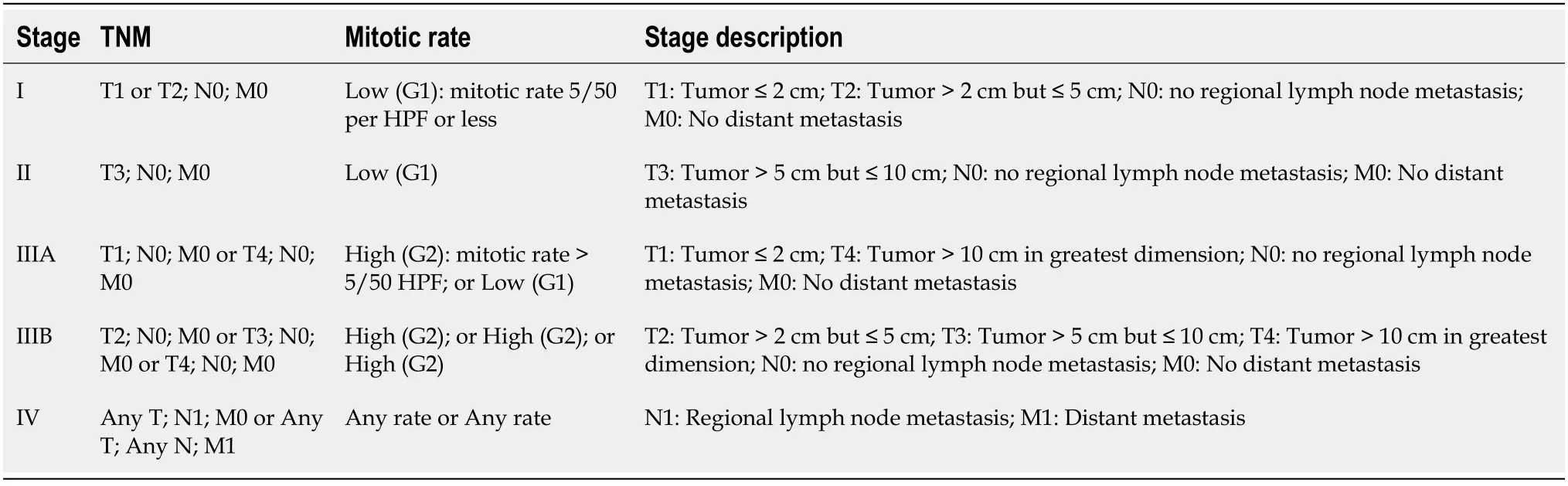
Table 4 American Joint Committee on Cancer tumor node metastasis system for small intestine, esophagus, colon, rectum, or peritoneum
TREATMENT
The treatment of GISTs depends on risk stratification.The different therapeutic modalities of GISTs include surgery, endoscopic resection, medical therapy,radiotherapy, chemotherapy, hepatic artery embolization, chemoembolization and radiofrequency ablation.
SURGERY
Surgery is the treatment of choice for localized symptomatic GISTs, ≥ 2 cm GISTs and GISTs with suspicious EUS features of malignancy as mentioned before[56].According to National Comprehensive Cancer Network (NCCN), asymptomatic patients with GISTs <2 cm with benign EUS features can be followed conservatively with annual EUS and/or esophagogastroduodenoscopy[57].But surgery should be considered if the GIST increases in size during follow-up.R0 resection (negative margin) of the GIST is the main aim of surgery.As GISTs almost never metastasize to the lymph nodes,routine local lymph node dissection is not necessary unless suspected on imaging or endosonography.The surgical approach depends on the location of the GIST, its adherence or invasion into adjacent structures and the patient’s general medical status.Laparoscopic surgery (wedge resection) is indicated if the GIST is less than 5 cm in size[58].As GISTs are usually surrounded by a pseudocapsule, enucleation technique is tried for small posterior wall GIST of the stomach at the gastroesophageal junction or small esophageal GIST to preserve the organ’s function.Esophagectomy is done for large esophageal GIST.Depending on the size, number and location of gastric GISTs,surgical options include wedge partial resection of the stomach, Biliroth I partial gastrectomy or gastroduodenostomy, Biliroth II partial gastrectomy or gastrojejunostomy and total gastrectomy with Roux-en-Y reconstruction.If the duodenal GIST is small and far enough away from the ampulla of Vater, segmental duodenal resection with end to end anastomosis can be done.But if the GIST is close to the ampulla of Vater or adherent to adjacent organs, pancreaduodenectomy or Whipple resection is required.In case of jejunal, ileal or colonic GISTs, segmental resection with anastomosis of the divided ends are done.Surgery for rectal GISTs depends on the location:if the GIST is in the upper rectum or not too close to the anal sphincter, rectal resection with anastomosis of the divided colonic end to the remaining rectum can be done with or without temporary colostomy.If the GIST is close to the anal sphincter, abdominoperineal resection of the rectum (resection of rectum and anus with permanent sigmoid colostomy) is done[59].Novel techniques to remove rectal GISTs have been described.These include transanal endoscopic operation[60]and transanal minimal invasive surgery[61].Appendiceal GISTs are extremely rare and treated by simple laparoscopic appendectomy.
Total 80% of the GISTs are single and surgery is considered as the first line therapy.But if the GIST is large in size and surgical resection can be difficult, shrinkage of the GIST can be done by giving TKI therapy (neoadjuvant therapy) prior to surgery.Sometimes, debulking surgery is also considered in case of potentially resectable metastatic GISTs.Surgery is also required emergently in case of bleeding gastric or duodenal GISTs.
ENDOSCOPIC RESECTION
Endoscopic treatment is mainly used for the treatment of GISTs of the upper GI tract for patients with no recurrence or metastasis[62].The available endoscopic resection methods include endoscopic enucleation, endoscopic band ligation (EBL), endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), submucosal tunneling endoscopic resection (STER), endoscopic full-thickness resection (EFTR),and laparoscopic and endoscopic cooperative surgery (LECS)[63-71].Endosonographically, gastric GIST can be classified into 4 types according to their location in the mucularis propria (MP).Type I GIST protrudes into the lumen like a polyp and is attached to the MP by a narrow connection.Type II GIST also protrudes into the lumen but is attached to the MP by a wider connection.Type III GIST is centrally located in the gastric wall.Type IV protrudes outwards towards the serosa of the gastric wall[46].Endoscopic enucleation can be done for type I and possibly for type II GISTs.Type III and type IV GISTs can be treated by other endoscopic methods which include EBL, ESD, ESE, STER, EFTR and LECs.Small (≤ 4 cm) gastric GISTs can be resected safely by endoscopic methods although intermediate or high risk GISTs may require adjuvant TKI therapy and/or additional surgery to reduce the chance of metastasis and recurrence[72].
MEDICAL THERAPY
Medical therapy includes TKIs which has revolutionized the treatment of metastatic and unresectable GISTs.TKI is given in patients with metastatic and unresectable GISTs as a definitive therapy.Metastatic lesion should be biopsied to confirm the diagnosis and mutational analysis should be assessed.Adjuvant therapy is given after surgery and continued for 3 years to decrease the chance of recurrence of GISTs with high (>50%) risk of recurrence[73].A prognostic nomogram was developed at the Memorial Sloan-Kettering Cancer Center for recurrence-free survival (RFS) after complete surgical resection of localized GISTs using tumor size (in cm), location(stomach, small intestine, colon/rectum, or other), and mitotic index (<5 or ≥ 5 mitoses per 50 high power fields).The nomogram is able to predict RFS for patients with completely resected GIST and can be used to select patients for adjuvant therapy[74].Neoadjuvant therapy is given preoperatively for 6 to 12 mo (although the optimal duration is not known) in patients with large GISTs to decrease the size of the tumor to enable resectability.Neoadjuvant therapy should also be considered for organ and function-preserving surgeries like esophagectomy for esophageal GIST,pancreatoduodenectomy for duodenal GIST and abdominoperineal resection for rectal GIST[75].PET/CT should be done one month after beginning neoadjuvant therapy to evaluate response to the treatment.TKI is also recommended in case of tumor rupture during surgery and should be continued for at least 3 years to lifelong.Some authors also recommend neoadjuvant therapy when GISTs present emergently with acute upper GI bleeding[76].There is no guideline on the post-operative management of patients who received neoadjuvant therapy.Patients with high-risk GISTs, TKI should be continued for several years.Imatinib is the first-line therapy Sunitinib, the secondline and Regorafenib, the third-line TKI used as adjuvant and neoadjuvant therapy[77].The effectiveness of TKI depends onKITandPDGFRAmutation.90% of GISTs containingKITexon 11 mutation and 50% of GISTs containingKITexon 9 mutation respond to imatinib.Dose escalation of TKI may be needed to improve response.GISTs containingKITexon 13 and 14 mutation are sensitive to sunitinib.Regorafenib improves progression free survival for locally advanced and unresectable GISTs which do not respond to imatinib or sunitinib[78].Recently, a new TKI known as Avapritinib has been added for unresectable/metastatic GISTs withPDGFRAexon 18 mutation,includingPDGFRAD842V[79].Avapritinib is also indicated in patients with GISTS which keep growing despite imatinib or other TKI therapy.So testing forKITandPDGFRAgene mutation is suggested, particularly when medical therapy is going to be administered.Multi-disciplinary team with experience and expertise in GISTs should be involved prior to initiation of therapy.According to NCCN guideline, resectable GISTs with minimum morbidity should be resected and risk assessment should be evaluated on the pathology of the GISTs as shown in Table 5.Patients with significant risks of recurrence (intermediate or high risk depending on size, mitotic rate and location) should be given TKI therapy.Resectable GISTs with significant morbidity should be considered for preoperative TKI therapy to decrease surgical morbidity.Imaging should be done to evaluate treatment response.If the patient responds to TKI therapy, TKI should be continued and surgery should be done.If the GIST progresses on TKI therapy, surgery should be done if feasible, but if surgery is not feasible:(1) For limited progression–(a) continue TKI (imatinib or avapritinib) and consider other options like resection, if feasible or radiofrequency ablation (RFA), embolization,chemoembolization and palliative radiation therapy for bone metastasis;and (b)increase the dose of imatinib as tolerated or change to sunitinib.Imaging should be done to evaluate therapeutic response and patient adherence;and (2) For widespread progression–(a) increase the dose of imatinib as tolerated or change imatinib to sunitinib;(b) If GISTs progresses on sunitinib, then change to regorafenib;and (c) If GISTs progresses on regorafinib, change to avapritinib.
Imaging should be done to evaluate therapeutic response and patient adherence.
If GISTs keep progressing despite giving imatinib, sunitinib, regorafinib and avapritinib, other options include enrolling the patients into clinical trials, use systemic therapy and agents against GIST (sorafenib, nilotinib, pazopanib, everolimus plus TKI and dasatinib for patients with D842V mutation) based on limited data or to give best supportive care.
Small gastric GIST (<2 cm) should be evaluated by EUS-guided FNA and imaging.If the GIST has high-risk features, complete resection should be done.But if there is no high-risk feature, periodic endoscopic and imaging surveillance should be done.Micro-GISTs (<1 cm) are generally benign irrespective of their mitotic rate.
TREATMENT OF UNRESECTABLE, METASTATIC OR RECURRENT GISTs
Baseline imaging and percutaneous image guided biopsy should be appropriate to establish metastatic disease.Patient should be started on imatinib or avapritinib.Follow-up imaging should be done to evaluate for treatment response.Wanget al[80]did a single center analysis in which patients with advanced and metastatic/recurrent GISTs were given imatinib mesylate for a median period of 8.2 mo preoperatively and tumor shrinkage was observed in almost 30% of cases.If the response is good and the disease remains stable, continue imatinib or avapritinib, and surgery consult should be done to consider resection.If resection is not possible, continue imatinib or avapritinib.If the disease is progressive, dose escalation and change of TKI should be considered.If GISTs keep progressing despite giving imatinib, sunitinib, regorafinib and avapritinib, other options include to enroll the patients into clinical trials, use systemic therapy and agents against GIST (sorafenib, nilotinib, pazopanib, everolimus plus TKI and dasatinib for patients with D842V mutation) based on limited data or to give best supportive care.

Table 5 Treatment options of resectable gastrointestinal stromal tumors
FOLLOW UP AND SURVIVAL
There is no current guideline for follow up of patients who have undergone GIST resection to evaluate for recurrence or metastasis.Current practice is based on clinician’s opinion taking into account the tumor site, size and mitotic index[81].The imaging modality and follow up interval varies from institution to institution.Very low risk category patients may not need routine follow up although the risk of recurrence is not zero.Low risk category patients can be followed up by doing CT scan every 6 mo for 5 years.Intermediate to high risk category patients should be more closely followed-up with CT scan every 3 to 4 mo for 3 years, then every 6 months for 5 years, and then yearly[82].PET/CT is more sensitive than CT in detecting response,resistance and recurrence following TKI therapy when it is used for neoadjuvant,adjuvant or definitive therapy[83].
The survival of patients with GIST depends on several factors which include risk category or stage of the GIST, the type of treatment patient received and recurrence of GIST after treatment.If the GIST is localized, the 5-year survival rate is 94%.It becomes 82% if there is local spread of the GIST.If the GIST has distant metastasis at the time of diagnosis, the 5-year survival rate becomes 52%[84].
SUMMARY
GISTs are well-circumscribed mesenchymal tumors (median size 2.7 cm to 8.9 cm)originating from the muscular layer of the gastrointestinal tract mostly seen in the stomach and small bowel but they can be found in any part of the gastrointestinal tract.The incidence of GISTs has increased over the last few decades possibly due to increased detection due to increased availability of imaging studies, endoscopic and endosonographic procedures.C-KITgene mutation with constitutive activation of tyrosine kinase and ICC hyperplasia is the pathogenic mechanism seen in most GISTs.Mutation inPDGFRAgene can also lead to the formation of GISTs.The importance ofPDGFRAmutation (due to Asp842Val substitution in exon18) is that they are resistant to imatinib therapy.KITandPDGFRAmutations are not detectable in 15% of GISTs when they are called wild-type GISTs.Immunohistochemistry is important to differentiate GISTs from other mesenchymal tumors like leiomyoma.GISTs stain positive forKIT,CD34,CD117andDOG1.Histologic features of GISTs include spindle cell type, epitheloid type or mixed type.GISTs remain silent when they are small in size but when they increase in size, they can produce various manifestations which include gastrointestinal bleeding, abdominal pain, small bowel obstruction, dysphagia and proctalgia depending on their location.CT, MRI, PET/CT, endoscopic procedures and EUS are done in the diagnostic and metastatic evaluation as well as in following response to treatment.Tissue acquisition is done by EUS FNA/TCB, or postoperatively or sometimes by percutaneous biopsy in case of metastatic GISTs.Surgery is the treatment of choice for any potentially resectable GIST greater than 2 cm in size or if the GIST is localized and symptomatic or if the GIST has suspicious malignant features on EUS.Type of surgery depends on the location of GISTs.Now there is an increased interest in endoscopic resection of gastric GISTs <4 cm in size, and various endoscopic resection methods are available.TKI has multiple roles in the treatment of GISTs:(1) As a neoadjuvant therapy preoperatively for 6 to 12 mo for tumor cytoreduction of a large GIST to make it operable;(2) As an adjuvant therapy postoperatively to prevent recurrence of GISTs for a minimum of 3 years;and (3) As a neoadjuvant therapy (to be considered) for organ and function-preserving surgeries like esophagectomy, pancreatoduodenectomy and abdominoperineal resection for esophageal, duodenal and rectal GISTs.
CONCLUSION
GIST is an important gastrointestinal tumor.Although they can be asymptomatic when small, they can have a variety of clinical presentations when they are large or rapidly growing.They are diagnosed by imaging, endoscopic and endosonographic studies or sometimes post-operatively.They have distinct genetic mutations,immunohistochemical features and risk stratification criteria.Surgical or endoscopic resection and TKI are the main modalities of treatment.Clinical trials are ongoing for refractory cases.Future treatment options will change as we discover more and more drugs against GISTs.
 World Journal of Clinical Cases2020年15期
World Journal of Clinical Cases2020年15期
- World Journal of Clinical Cases的其它文章
- Facial and bilateral lower extremity edema due to drug-drug interactions in a patient with hepatitis C virus infection and benign prostate hypertrophy:A case report
- Total laparoscopic segmental gastrectomy for gastrointestinal stromal tumors:A case report
- COVID-19 with asthma:A case report
- Computed tomography, magnetic resonance imaging, and Fdeoxyglucose positron emission computed tomography/computed tomography findings of alveolar soft part sarcoma with calcification in the thigh:A case report
- Acute suppurative oesophagitis with fever and cough:A case report
- Coexistence of ovarian serous papillary cystadenofibroma and type A insulin resistance syndrome in a 14-year-old girl:A case report
