Mutagenesis reveals that the rice OsMPT3 gene is an important osmotic regulatory factor
Shengi Hung,Shiho Xin, Guoqing Xie, Jio Hn,1,Zhongli Liu, Bing Wng,Shuqing Zhng, Qingyu Wu, Xinguo Cheng,*
aLaboratory of Plant Nutrition and Biology, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences,Beijing 100081,China
bKey Laboratory of Rubber Biology and Genetic Resources of the Ministry of Agriculture, Rubber Research Institute, Chinese Academy of Tropical Agricultural Sciences,Haikou 571011,Hainan,China
cJiujiang Academy of Agricultural Sciences,Jiujiang 332101,Jiangxi,China
ABSTRACT Plant mitochondrial phosphate transporters regulate phosphate transport and ATP synthesis. Determining whether they function in abiotic stress response process would shed light on their response to salt stress.We used the CRISPR/Cas9 gene-editing system to mutagenize two mitochondrial phosphate transporters, OsMPT3;1 and OsMPT3;2, to investigate their regulatory roles under salt stress. Two cas9 (CRISPR-associated protein 9)-free homozygous mutants,mpt33 and mpt30,were confirmed to be stable.Both OsMPT3;1 and OsMPT3;2 were markedly induced by salt stress, and their mutagenesis strongly inhibited growth and development, especially under salt stress. Mutagenesis sharply reduced the accumulation of ATP, phosphate, calcium, soluble sugar, and proline and increased osmotic potential, malondialdehyde, and Na+/K+ ratio under salt stress. Both mutants demonstrate normal growth and development in the presence of ATP, revealing high sensitivity to exogenous ATP under salt stress. The mutants showed lowered rates of Na+efflux but also of K+and Ca2+influx under salt stress.Mutagenesis of OsMPT3;2 altered the enrichment profiles of differentially expressed genes involved mainly in synthesis of secondary metabolites, metabolism of glycolysis, pyruvate, tricarboxylic acid cycle, in response to salt stress. The mutant displayed significant accumulation differences in 14 metabolites involved in 17 metabolic pathways, and strongly up-regulated the accumulation of glutamine,a precursor in proline synthesis,under salt stress.These findings suggest that the OsMPT3 gene modulates phosphate transport and energy supply for ATP synthesis and triggers changes in accumulation of ions and metabolites participating in osmotic regulation in rice under salt stress, thus increasing rice salt tolerance. This study demonstrates the effective application of CRISPR/Cas9 gene-editing to the investigation of plant functional genes.
1. Introduction
High salinity in soils limits crop growth and development by triggering ion toxicity, osmotic, and oxidative stresses in plants [1]. Plants have evolved a series of physiological mechanisms based on responding to external signals to adapt to environmental changes [2]. Both energy transfer and energy acquisition act as important signaling processes in plant metabolism. The mitochondrion is an organelle that hosts respiration and releases free energy for adenosine triphosphate (ATP) production by oxidative phosphorylation[3]. ATP, the most important energy source in living organisms, directly participates in the regulation of numerous metabolic pathways by controlling the Na+/K+balance, redox balance, and signaling pathways in response to salt stress[4,5]. Unlike ATP, as important Na+/H+exchangers, Na+/H+exchangers (NHXs) transport Na+from the cytoplasm to the vacuole, in a process driven by the establishment of an H+gradient potential regulated by vacuolar H+-pyrophosphatase and H+-ATPase [6,7]. Zhou et al. [8] reported that STRK1 increases salt tolerance by phosphorylating and activating the catalase domain containing protein (CatC) and thereby regulating H2O2homeostasis.Two Arabidopsis mutants,MKK4 and MKKK20, have been confirmed to be sensitive to salt stress [9], indicating that mitogen-activated protein kinases(MAPKs) play important regulatory roles in plant signal transduction under osmotic stress. Like MAPK, SnRK2 is also an ABA-dependent protein family, and participates in signal transduction in response to osmotic stress [10]. Activation of SnRK2 facilitates the phosphorylation of downstream genes to decompose starch into soluble sugar,thus maintaining the balance of osmotic potentials in plant cells[10].
Mitochondrial phosphate transporters (MPTs) are composed of six transmembrane helices localized to the inner mitochondrial membrane.They are essential for the transport of orthophosphate (Pi) from the cytoplasmic matrix to the mitochondria,thereby providing substrate for the synthesis of ATP in the matrix [11]. Although the first MPT gene was identified in betulaceous tree,most studies of MPT genes have been conducted in Arabidopsis. Three typical AtMPT genes:AtMPT1,AtMPT2,and AtMPT3,have highly conserved domains and have been characterized in Arabidopsis [12,13]. Both AtMPT2 and AtMPT3 genes are induced specifically by salt stress, but transgenic lines overexpressing the three AtMPTs showed increased sensitivity to salt stress [4]. In addition to this sensitivity, Arabidopsis overexpressing the MPT3 gene showed developmental defects including dwarfing, dark green leaf curling, and infertility because the overexpression of the MPT3 gene accelerates electron transfer and causes excessive accumulation of reactive oxygen species (ROS) in plants [14]. Interestingly, transgenic Arabidopsis overexpressing either the AtMPT1 or AtMPT2 gene showed a normal phenotype, indicating that the AtMPT3 gene indeed plays a specific regulatory role in energy transport or accumulation of ROS in the plant [14]. MPT genes are also induced by low phosphorus. Transgenic rice overexpressing McMPT3 or SlMPT3;1 accumulated more phosphate in shoots under low phosphorus and showed increased tiller number and grain yield, suggesting that MPT3 modulates plant phosphate transport[15,16].
The CRISPR/Cas9 system offers potential for development of high-yielding crop cultivars by improving stress tolerance via gene editing, and has been applied in Arabidopsis, maize,wheat, and rice [17]. Mutagenesis of OsPPa6 by the CRISPR/Cas9 system markedly lowered alkaline stress tolerance in rice [18]. The MPT-modulated osmotic response mechanism in plants subjected to abiotic stresses is unknown. The modification of rice OsMPT genes has not been reported.
In the present study, two rice MPTs, OsMPT3;1 and OsMPT3;2,that are induced by salt stress were simultaneously edited with the CRISPR/Cas9 system, and two mutants with base deletions or insertions,mpt33 and mpt30,were generated.Their physiological responses to osmotic stress were investigated by systematically evaluating the changes in the ATP,phosphate accumulation, compatible metabolites, osmotic potentials, the transcripts of differentially expressed genes,the accumulation and fluxes of the Na,K,and Ca ions in rice.We are expected that this study will provide insight into the regulatory pathway of the mitochondrial phosphate transporters in plants in response to salt stress.
2. Materials and methods
2.1. Vector construction and plant transformation
The NCBI nucleotide database(http://www.ncbi.nlm.nih.gov/)was used to select two MPT genes in Oryza sativa L.japonica cv.Kitaake, OsMPT3;1 (Loc4335983) and OsMPT3;2 (Loc4340459),which share high identity.Two target sequences upstream of the coding regions: 5′-GAAGATTGAGATGTACTCGC CGG-3′ for OsMPT3;1 and 5′-TTCTACGCCGCCTGCACGGC CGG-3′ for OsMPT3;2, were designed with the online guide design tool(http://crispr.dbcls.jp/). The underlined CGGs represent PAM(protospacer adjacent motif)sequences.The target sequences were connected with two adapters: 5′-GGCA-3′ at the 5′terminus and 5′-AAAC-3′ at the 3′ terminus. Construction of an expression vector and Agrobacterium-mediated transformation followed Wang et al. [18]. Seeds of the T0generation were grown in field soil under natural conditions, and leaf genomic DNA was extracted with a DNA extraction kit(EasyPure Genomic DNA Kit, TransGen Biotech, Beijing). Both strands of PCR fragments were sequenced to identify transformants.
2.2. Identification of mutants
To determine whether gene editing produced off-target effects, NCBI-Blast-Primer Blast (the method of designing primers) was used to align PCR product sequences and PCR was performed with specific primers (Table S1). Sequencing PCR products showed no off-target effect in genes sharing high identity with the target sequences in both OsMPT3;1 and OsMPT3;2.Two mutants,mpt33 with a deletion of a single base in OsMPT3;1 and mpt30 with an insertion of a single base in OsMPT3;2, were selected and used for subsequent experiments.
2.3. Plant culture and treatment
Seeds of the wild type(WT)and the T2mutants were sterilized and sown on MS solid medium with 120 mmol L?1NaCl and grown in a growth chamber for two weeks under a relative humidity of 75%,temperature of 28°C(day)/22°C(night),and photoperiod of 16 h light/8 h dark. Both hydroponic and potculture experiments were performed. Seeds were placed in water in an incubator at 30 °C in the dark for three days. The germinating seedlings were transferred into Hoagland solution (pH 5.5-5.8) containing 120 mmol L?1NaCl and further cultured hydroponically for 10 days in the presence or absence of 20 μmol L?1ATP. The culture solutions were renewed every three days with Hoagland solution.Alternatively, germinating seedlings were transferred to pots with peat soil, grown for four weeks in a greenhouse at 26-30°C under an illumination intensity of 8000-10,000 lx for 12 h with relative humidity of 75%, and then cultured for seven days in Hoagland solution containing 120 mmol L?1of NaCl and sprayed three times with 20 μmol L?1ATP solution.Leaves of each treatment with three biological replicates were collected for physiological measurements and transcriptome sequencing.
2.4. RNA isolation and PCR analyses
Total RNA from rice leaves from hydroponic culture was prepared with an RNA Easy Kit (Takara Biotech, Dalian,China). A total of 1 μg RNA was used for cDNA synthesis by reverse transcription-polymerase chain reaction (RT-PCR)using one-step gDNA removal and a cDNA synthesis SUPERMIX (Vazyme Biotech, Nanjing, China). A total of 2 μL cDNA was added to a 20 μL of reaction solution containing 10 μL of Top Green qPCR Supermix and 0.2 μmol L?1of specific primers (Table S2) and real-time quantitative polymerase chain reaction (RT-qPCR) was performed with three independent replicates with a light cycler system (7500ABI, Applied Biosystems, Foster City, USA) under the following conditions:95°C for 30 s,40 cycles of 95°C for 10 s,and 60°C for 30 s.The expression fold differences were calculated with the 2?ΔΔCtformula [19] using β-actin as reference gene (NCBI accession ID:XM_015774830).
2.5. Determination of ATP content in rice leaves
ATP detection followed Wu et al. [20] with modification.Approximately 0.5 g of fresh leaves was extracted from hydroponically grown plants with 5 mL of ultrapure water at 100 °C for 10 min to obtain ATP extracts of ATP and then cooled to room temperature and filtered through a 0.22 μm filter (Millipore, USA). The filtrate was subjected to highperformance liquid chromatography (UltiMate 3000 BioRS,Thermo Fisher, Germany). An injection volume of 20 μL and column temperature of 30 °C were used to detect the peak absorbance at 254 nm. An Atlantis T3 column (5.0 μm,4.6 mm × 250.0 mm, Waters, Milford, USA) was used for separation.The mobile phase was phosphate buffer(20 mmol L?1KH2PO4; 20 mmol L?1Na2HPO4, pH 7.0) at a flow rate of 1.0 mL min?1.
2.6. Physiological measurements
Physiological measurements were performed with potcultured plants. A total of 0.5 g dried leaves was digested with 98% H2SO4and 30% H2O2and the digest solution was used for determining the phosphate content by the vanadatemolybdate-yellow colorimetric method using a Continuous Flowing Analyzer (SEAL Analytical AA3, Norderstedt, Germany) [18], and measurements of Na and K ions were performed by atomic absorption(ZEEnit 700P,Jena,Germany).Frozen leaves at ?80 °C were compressed in a 25-mL syringe tube and the expressed leaf sap was used to determine osmotic potential with an osmotic pressure dew point meter(Wescor 5520,Logan,UT,USA).A total of 0.5 g fresh leaves was used to isolate the extracts for measuring soluble sugar or proline or malondialdehyde(MDA),respectively[21].
2.7. Transcriptome and metabolome sequencing
Leaves from both mpt30 and the wild type grown in soil culture were separately collected in three biological replicates and used for total RNA isolation and cDNA library construction. Transcriptome sequencing followed He et al. [22]. For metabolome analysis,50 mg of frozen leaves from pot-culture rice were prepared in six biological replicates,placed in a 1 mL mixture of methanol/acetonitrile/water (2:2:1, v:v:v) containing 20 μL of telmisartan (internal standard), vortexed for 30 s,and fragmented with a grinder (45 Hz) for 4 min and ultrasonicated for 5 min, and then incubated at ?20 °C for 1 h. Finally, the isolates were centrifuged at 14,500 ×g at 4 °C for 15 min, and 800 μL of supernatant was transferred to a fresh tube and dried in a freezing vacuum concentrator(LNGT98, Hualida, Nanjing, China). Extraction solution (acetonitrile:water 1:1, 150 μL) was added to the dry metabolites, the same vortexing, ultrasonication, and centrifugation were performed, and 60 μL of extract was used for metabolite detection by UHPLC/MS (Ultra high-performance liquid chromatography/Mass Spectrometry)(1290,Agilent Technologies).
2.8. Flux measurement of Na and K ions in roots
Seeds of both the mutants and the wild type were germinated in water at 30 °C in dark for 3 days and then transferred to Hoagland solution and cultured for 14 days.The two-week-old seedlings were placed for 24 h in Hoagland solution containing 120 mmol L?1NaCl and used to determine net fluxes of Na and K ions in the root elongation zone(500 μm proximal to theroot tip). Calibrated electrodes were shifted on the surface of the root tips at 0.3-0.5 Hz for 8 min with non-invasive microtest technology (NMT) (Xuyue Sci. & Tech. Co. Limited.,Beijing,China),and the net Na and K fluxes were determined with Mage Flux software[21].
3. Results
3.1. OsMPT3;1 and OsMPT3;2 are homologs
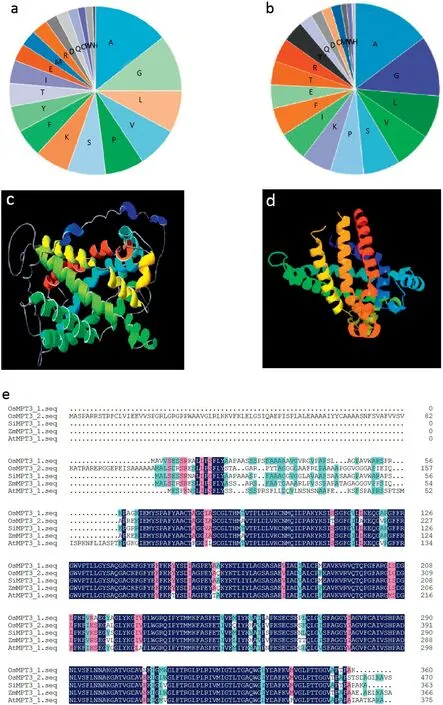
The OsMPT3;1 cDNA encoding 360 amino acids and OsMPT3;2 cDNA encoding 471 amino acids have respective molecular weights of 37.97 and 49.43 kDa. The isoelectric point of OsMPT3;1 is 9.68 and that of OsMPT3;2 is 9.72. Both OsMPT3;1 and OsMPT3;2 proteins are composed mainly of hydrophobic amino acids, including alanine, leucine, valine,and proline (Fig. 1-a, b). Prediction of PHYRE2 (Protein Homology/analogy Recognition Engine V 2.0) shows that the OsMPT3s contain six transmembrane helices and show typical structural characteristics of the mitochondrial phosphate transporter family (Fig. 1-c, d). Multiple alignment shows that OsMPT3;1 shared more than 80%identity with OsMPT3;2 and other MPT3;1 member from the plants Setaria italica,Zea mays,and Arabidopsis thaliana (Fig. 1-e), suggesting that specific structural characteristics of the OsMPT3s might function in transporting Pi from the cytoplasm into the mitochondrial matrix.
3.2. The generated mutants show successful gene editing
PCR amplification showed that both the mpt33 and mpt30 mutants were correctly modified at the target sites.As shown in Fig.2,the mpt33 mutant shows a deletion of thymine(T)at the 195th position of OsMPT3;1 (Fig. S1-a, b) and the mpt30 mutant shows an insertion of a T at the 225th position of OsMPT3;2 (Fig. S1-c, d). This insertion or deletion of a single base in the encoding region resulted in early termination of translation in both genes (Fig. S1-e, f). The absence of offtarget events was demonstrated as described in Methods.
3.3. Mutants showed lower tolerance to salt stress
Both mutants and WT showed normal growth on MS solid medium under favorable culture(Fig.2-a).Under salt stress,the growth of both mutants was inhibited, leading to a decrease in biomass compared with the WT,especially for the mpt30 mutant(Fig.2-b).No significant phenotypic differences between WT and mutants were observed under favorable culture, but the shoot lengths of the mutants were significantly lower than those of the WT after salt stress (Fig. 2-c, d). The mpt30 mutant showed a significant decrease in root length compared with the WT under salt stress,but there was no difference between the mpt33 mutant and the WT (Fig. 2-d). However, compared with the WT, both mean shoot and root fresh weights of the mutants were significantly decreased under both favorable culture and salt stress,but the differences in biomass between the mutants and WT were obvious under salt stress(Fig.2-e,f).Thus,the mutants with a loss of function of MPT3 genes showed lower salt tolerance than the WT.
3.4. Mutagenesis of the OsMPT3s genes down-regulated the expression of genes encoding ATPase under salt stress
RT-qPCR showed that salt stress significantly increased the transcripts of the OsMPT3;1 and OsMPT3;2 genes in the WT(Fig. S2-a). Besides these two genes, the genes encoding mitochondrial ATP synthase, ATPase1 (Os08g0250200),ATPase2 (Os12g0424180), and ATPase3 (Os12g0207500), are alsoresponsibleforcatalyzingthereactionofADP+H2O+Pi+H-+≥ATP+H+.For this reason,we performed RT-qPCR to detect the transcripts of these genes. When rice seedlings were cultured under favorable conditions, the expression level of the gene encoding ATPase1 was higher in the mutants than in the WT(Fig.S2-b),but there were no transcript differences in the genes encoding ATPase2 and ATPase3 compared to WT(Fig.S2-c,d).Salt stress increased the expression levels of the genes encoding ATPase1, ATPase2, and ATPase3, and the expression levels of these genes were significantly lower in the mutants than in the WT.
3.5. The mutants showed altered distributions of ATP, P, K+,Na+,and Ca2+ in leaves
The mutants showed decreases of 15.9%of ATP accumulation in mpt33 and 44.3% in mpt30 compared with the WT under favorable culture (Fig. 2-g). Salt stress induced synthesis of ATP in the mutants and wild-type,but the ATP contents were still significantly lower in both mpt33 and mpt30 than in the WT. In particular, when the mutants were exposed to 120 mmol L?1NaCl, the ATP contents in the mpt33 and mpt30 decreased by respectively 14.2%and 26.3%compared with the WT (Fig. 2-g). Pi contents in both mutants were decreased in comparison with the WT, but only the mpt30 mutant showed a clear difference from the WT under favorable culture.Under salt stress, both mutants showed significantly lowered accumulation of Pi in leaves compared with the WT(Fig.2-h).
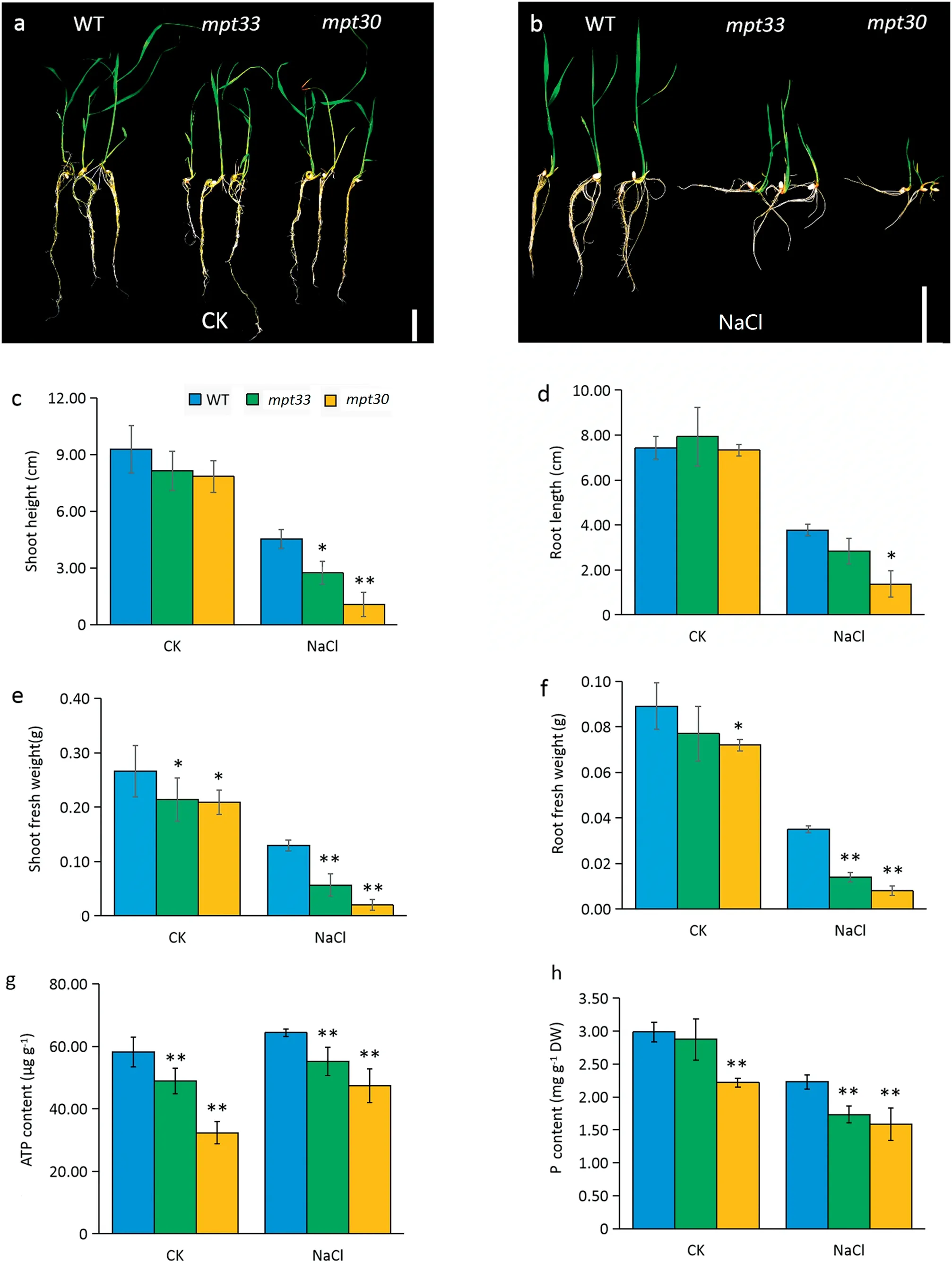
Fig.2-Phenotypic characteristics,biomass,ATP content,and phosphate content in rice under salt stress.(a)Phenotypes of wild type(WT)and mutants under favorable culture.(b)Phenotypes of WT and mutants under culture with 120 mmol L?1 NaCl solution.(c)Shoot lengths of seedlings.(d)Root lengths of seedlings.(e)Shoot fresh weights of seedlings.(f)Root fresh weights of seedlings.(g)ATP content.(h)Total phosphate content.Error bars indicate standard deviation of three biological replicates.DW,dry weight.*and**indicate significant difference between mutants and WT at P <0.05 and P <0.01,respectively(Student's t-test).
Highly accumulation of Na+usually hinders the absorption of K+and Ca2+,thus affecting osmotic potentials in plant cells.Therefore, we measured the contents of K+, Na+, and Ca2+in the mutants.As shown in Table 1,when plants were grown in soil watered with a solution of 0 or 120 mmol L?1of NaCl,the mutants showed increased Na+contents and lowered K+and Ca2+contents in shoots in comparison with the WT,indicating that the NaCl treatment significantly altered accumulation profiles of Na+, K+, and Ca2+in the mutants. Before NaCl treatment, the mpt33 mutant and the WT showed no significant difference in Na+accumulation, whereas the accumulation of Na+was significantly lower in the mpt30 mutant than in the WT. However, under salt stress, both the mpt30 and mpt33 mutants showed significant increases in the contents of Na+, by respectively 1.84- and 1.99-fold compared with WT. In contrast, both mutants showed significantly decreased uptake of K+compared with WT even under salt stress. Like K+, Ca2+in the mutants followed identical accumulation profiles. Although no significant differences in Na+/K+ratio were observed between the mutants and the WT under favorable culture, the mutants showed significantly increased Na+/K+ratios compared with the WT after salt stress,indicating that the mutations reduced the efflux of Na+and the uptake of K+and Ca2+.

Table 1-Contents of Na+,K+,Ca2+,and Na+/K+ratio in WT and mutants under salt stress.
3.6. The mutations affected the flux profiles of Na, K and Ca ions in rice roots
When the seedlings were grown in a favorable culture solution, the mpt30 mutant and the WT showed a similar trend of variation in the fluxes of Na ion in the elongation zone of the root tips, but the mpt30 mutant showed a lower efflux of Na ion than the WT after 8.5 min (Fig. 3-a). When seedlings were exposed to a 120 mmol L?1NaCl solution,both the mpt30 mutant and the WT showed a gradually increasing trend in the efflux of Na ion, but compared to the WT, the mutant showed a significantly decreased Na ion efflux rate in the elongation zones of the root tips (Fig. 3-b). In contrast to the Na ion, both the mutants and WT showed an influx of K ion in the elongation zones under favorable culture, and the influx flow rates of K ion were significantly higher in the mutant than in the wild type (Fig. 3-c). Under salt stress, the flux of K ion in the mpt30 mutant was converted from influx to efflux, while the influx rate of K ion in the WT gradually decreased and showed a clear efflux after 9 min (Fig. 3-d).When rice seedlings were grown in favorable solutions, both mutants and WT displayed a gradual decrease of Ca ion efflux with time, but the mpt30 mutant showed a significantly increased efflux rates of Ca ion relative to the WT (Fig. 3-e).Under salt stress,the flux profiles of Ca ion showed a similar trend in the mutant and wild type, and the efflux rates of Ca ion in both mpt30 and WT were markedly increased in comparison with favorable culture(Fig.3-f).
Under either favorable culture or salt stress, the mpt30 mutant showed significantly decreased net flux rates of Na ion in root tips compared to the WT. The mean efflux of Na ion in the mutant decreased by 76.7%under favorable culture and 60.2%under salt stress(Fig.3-g).In contrast the Na ion in root tips,the K ion showed influx,and the mean influx rate of K ion was higher in the mutant than in the WT under favorable culture.However,under salt stress,the K ion in the WT showed influx, whereas the mpt30 mutant showed efflux of K ion (Fig. 3-h). Salt stress increased the efflux of Ca ion in both the mutant and the WT;however,whether rice seedlings were cultured under favorable condition or NaCl stress, the net efflux of Ca ions in the mpt30 mutant was increased compared with the WT(Fig.3-i).
3.7. The mutants showed high sensitivity to ATP
In hydroponic culture,the mutants and the wild type showed no marked differences in phenotype under favorable conditions, but salt stress reduced the growth of the mutants, a finding similar to that in culture on MS solid medium(Fig.4-a,b). However, in the presence of exogenous ATP, the mpt33 mutant showed a restoration in phenotype, while the phenotype of the mpt30 mutant was partially restored in comparison with the WT(Fig.4-c),suggesting that plants with mutagenesis of OsMPT3 are sensitive to exogenous ATP.
The mutant mpt33 showed no significant differences in osmotic potential or accumulation of soluble sugar, proline,and malondialdehyde compared with the WT under favorable culture, whereas the mpt30 mutant showed decreased osmotic potential and accumulation of proline in leaves (Fig. 4-d-g). However, both mutants showed significantly increased osmotic potentials and reduced accumulation of soluble sugar and proline in leaves in response to salt stress compared with the WT (Fig. 4-d-g). When rice seedlings were cultured in NaCl solution, the osmotic potentials of the mutants increased by respectively 16.4% and 20.0% relative to the WT (Fig. 4-d), but the soluble sugar contents in the mpt33 and mpt30 mutants were reduced by 18.1% and 29.2% relative to the WT (Fig. 4-e). Proline contents in the mpt33 and mpt30 mutants were reduced by 22.7% and 28.6% compared to the WT (Fig. 4-f), while MDA contents in the mpt33 and mpt30 mutants increased 1.45- and 1.62-fold, respectively (Fig. 4-g).
As shown in Fig. 4, exogenous ATP lowered the osmotic potentials and accumulation of MDA, but significantly increased the contents of soluble sugar and proline in WT and the mutants under salt stress. In the presence of ATP, the contents of soluble sugar in the WT, mpt33, and mpt30 mutants increased by respectively 6.6%, 18.4%, and 28.6% in comparison with the treatment without addition of ATP (Fig. 4-e). When the mutants were exposed to a solution of 120 mmol L?1NaCl in the presence of 20 μmol L?1ATP, the mean contents of proline in the WT, mpt33 and mpt30 mutants increased by respectively 17.07, 29.85, and 33.76 μg g?1, (Fig. 4-f), and the MDA contents decreased by respectively 37.50%, 39.09%, and 39.90% under salt stress in the presence of 20 μmol L?1ATP in comparison with the treatment without ATP addition (Fig. 4-g).
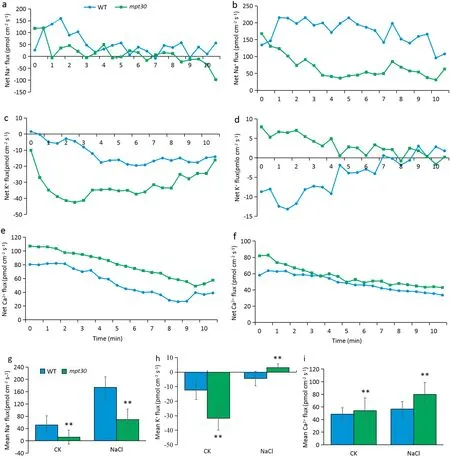
Fig. 3 - Flux profiles of Na+, K+ and Ca2+ in root tip elongation zones of 7-day-old rice seedlings. (a) Net Na+ fluxes under favorable culture. (b) Net Na+ fluxes under culture with a 120 mmol L?1 NaCl solution. (c) Net K+ fluxes under favorable culture.(d) Net K+ fluxes under salt stress. (e) Net Ca2+ fluxes under favorable culture. (f) Net Ca2+ fluxes under culture with a solution of 120 mmol·L?1 NaCl. Net effluxes are indicated by positive and net influxes by negative values. (g) Mean Na+ flux. (g) Mean K+ flux. (i) Mean Ca2+ flux. WT, wild type. Error bars indicate standard deviation of three biological replicates. * and ** indicate significant difference between WT and mutants at P < 0.05 and P < 0.01, respectively (Student's t-test).
3.8. The mutants trigger the alteration of the transcriptional levels
A total of 589 genes were significantly differentially expressed, with 405 of the up-regulated and 184 of the down-regulated genes expressed in the mpt30 mutant compared with the WT (Fig. 5-b). The GO (Gene Ontology) enrichment profiles of the differentially expressed genes (DEGs) were classified into three categories: biological process, cellular component, and molecular function. The significant GO terms in these three categories and the numbers of DEGs show that the numbers of genes involved in biological regulation of metabolic process were much higher than those in other processes, while the DEGs participating in molecular functions were grouped mainly in lyase activity and hydrolase activity (Fig. 5-c).
Differential genes were involved mainly in the synthesis of secondary metabolites, metabolism of starch and carbohydrates, glycolysis, pyruvate metabolism, tricarboxylic acid cycle, and metabolism of arginine and proline. Typically, these processes include glycolysis, pyruvate metabolism, and tricarboxylic acid cycle, which are mainly responsible for providing energy and synthesizing ATP (Fig. 5-d). Arginine and proline are required for increasing plant resistance to abiotic stress, suggesting that the tolerance response of mpt30 mutant to salt stress may be associated with the inhibition of ATP synthesis and disorders of proline metabolism.
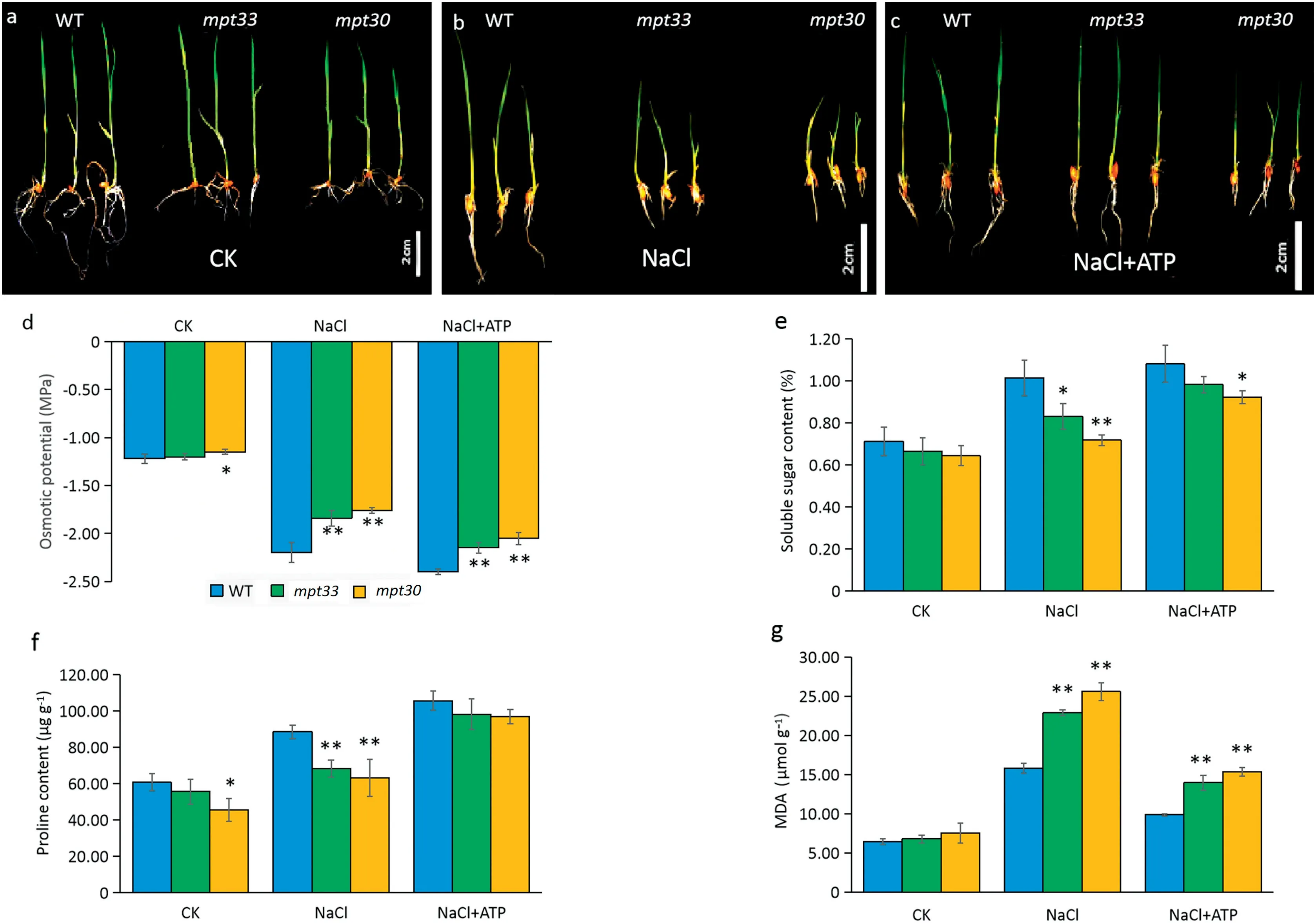
Fig.4- Phenotypic and physiological changes of WT and mutants under favorable culture,salt stress,and ATP treatment.(a)Phenotype of the WT and mutants under favorable culture.(b)Phenotype of the WT and mutants under salt stress in a 120 mmol L?1 NaCl solution.(c)Phenotype of the WT and mutants under salt stress with ATP treatment.(d)Osmotic potential change.(e)Soluble sugar content.(f)Proline content.(g) MDA content.Error bars indicate the standard deviation of three biological replicates.*and **indicate significant difference between mutants and WT at P <0.05 and P <0.01,respectively(Student's t-test).
For further profiling the expressions of the differentially expressed genes, 10 DEGs possibly involved in salt stress were presented by transcriptome sequencing (Table 2). They included three down-regulated and seven up-regulated genes. The RT-qPCR results revealed expression profiles identical with those from transcriptome sequencing (Fig. S3). As presented in Table 2, the Os07g0677200 gene encodes a kind of peroxidase (GO: 0044744) and the Os07g0152700 gene encodes a kind of glutathione transferase (GO: 0006749). As proline dehydrogenase, the Os10g0550900 gene catalyzes the conversion of proline to delta-1-pyrroline-5-carboxylate (GO:0010133), while the P5CS2 (Os01g0848200) gene encodes a delta-1-pyrroline-5-carboxylate synthase 2, one of the key enzymes for catalyzing the synthesis of proline(GO:0006561).The Os01g0118000 gene functions in catalyzing the conversion of β-D-fructose 1,6-bisphosphate to D-glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, an important step in the glycolysis process (GO: 0006096). Pyruvate kinase(Os01g0660300) could catalyze dihydroxyacetone phosphate into pyruvate and release ATP (GO: 0006096). The Os06g0246500 and the Os04g0119400 genes are two subunits encoding pyruvate dehydrogenase (GO: 0004739), while the Os08g0431300 gene encodes a dihydrolipoic acid transacetylase (GO: 0016746), and these DEGs are responsible for translating the pyruvate into acetyl CoA which finally is accumulated as tricarboxylic acid. The Os02g0625500 gene encoding adenosine kinase (GO: 0004001) could catalyze the transfer of a phosphate group to adenosine when the ATP is catalyzed to produce AMP and ADP.
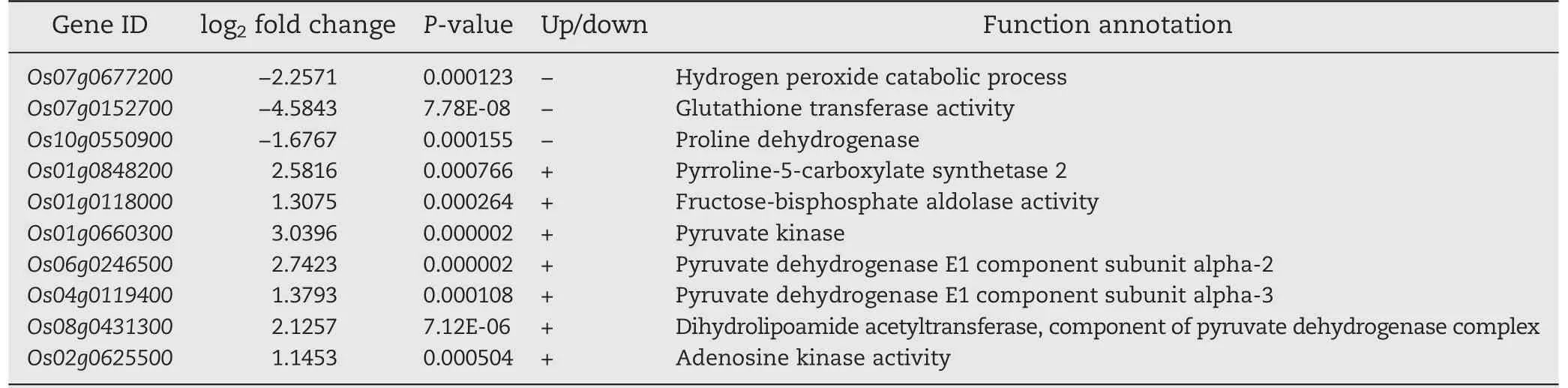
Table 2-Expressions of the partial differentially expressed genes of OsMPT3-mediated in rice under salt stress.
3.9.Mutagenesis of the OsMPT3;2 gene changed the distribution of the differential metabolites
A total of 129 cationic metabolites and 144 anionic differential metabolites were differentially expressed (Fig. 5-e, f; VIPvariable importance in the projection>1,P <0.05).Analysis of metabolic pathways of differential metabolites by KEGG(Kyoto Encyclopedia of Genes and Genomes) showed that 17 metabolic pathways were matched and 14 differential metabolites were isolated (Table 3). Glutamine is a precursor substance for protein synthesis and is involved in a variety of metabolic pathways, including pyrimidine metabolism,nitrogen metabolism, proline metabolism and purine metabolism. Under salt stress, amounts of glutamine were significantly increased in the mutant in comparison with the wild type.Like glutamine,adenosine is an important precursor for the synthesis of ATP, and was highly accumulated in the mpt30 mutant compared with the wild type after salt stress.
4. Discussion
4.1. Mitochondrial phosphate transporter functions by coordinating ATPase
A mitochondrial phosphate transporter (MPT)is a member of the phosphate transporter family participating in the exchange of phosphate (Pi) between the cytoplasm andmitochondria matrix through the Pi/H+symport pathway or Pi/OH?antiport pathway, and is essential for catalyzing the formation of ATP from ADP and Pi in plants [23]. As a major source of biochemical energy in living cells, ATP acts as a critical signal molecule modulating plant responses to abiotic stress via intercellular communication [24]. In previous studies, two mitochondrial small subunit genes encoding ATP synthase, RMtATP6 and AtMtATP6, conferred salt tolerance in Oryza sativa and Arabidopsis, respectively[25,26] More transcripts of the SpAHA1 gene encoding the plasma membrane H+-ATPase also promote salt tolerance by beneficially triggering the physiological changes in rice and transgenic Arabidopsis[27].Similarly,the activities of NHX protein(a Na+/H+exchanger)and H+/ATPase affect the transport of Na+in the vacuole in plant[28].In our study,two mutants with a loss of function of mitochondrial phosphate transporters, the mpt33 and mpt30, showed lower salt resistance than the wild type,indicating that the mutagenesis of the OsMPT3 genes likely obstructed the transport pathway of Pi from the mitochondrial outer membrane to the inner membrane and reduced ATP synthesis in the mitochondrial matrix, given that both the mpt33 and mpt30 mutants showed reduced activities of ATPase and contents of ATP and phosphate under salt stress.

Table 3-Expression profiles of differentially metabolites involving in major metabolic pathway in rice in response to salt stress.
4.2. The OsMPT3 gene is an osmotic regulator depending on ATP synthesis
Generally,ATP-mediated energy supply modulates the transport of Na+and influx of K+to alleviate salt damage to the plant[25,29].Under salt stress,the plasma membrane(PM)H+-ATPase activity is activated by J3 (DnaJ homolog3) through inhibiting PKS5 kinase activity, which is required for establishing a proton gradient across the PM and promoting the activity of the Na+/H+antiporter (SOS1) [30]. SOS3 interacts with SOS2 kinase and activates the phosphorylation of SOS2 and then the SOS3-SOS2 kinase complex mediate Na+efflux from the cytoplasm to the apoplast [31]. Two major transporters, NHX1 and NHX2, are essential for mediating K+uptake to the vacuole to maintain K+homeostasis through vacuolar H+-ATPase [6]. In our study, both the mpt33 and mpt30 mutants accumulated more Na+and lowered K+,showing that OsMPTs play an important regulatory role in maintaining osmotic potentials, which are generally related to the influx or efflux of Na and K ions in the root tips. The efflux profiles of Na and K ions in the roots suggested that the abilities to discharge Na+and accumulated K+in the mutants were significantly weakened in comparison with the WT,suggesting that the OsMPT3 genes could regulate the uptake and flux of Na+and K+to respond to salt stress.Interestingly,the phenotype and physiological metabolism of the mutants were restored in the presence of exogenous ATP, suggesting that the OsMPT3 gene might mediate rice salt tolerance through a dependent pathway of the synthesis of ATP.
Calcium(Ca2+),an important second messenger in eukaryotic cells, is essential for increasing plant salt tolerance [32].When plants are exposed to high salinity, cytosolic Ca2+is rapidly elevated, initiating stress signal transduction pathways for plant protection [33]. Ca2+sensors bind Ca2+ to promote interaction with downstream effectors. Tang et al.[34] reported that as Ca2+sensor, both PbCaM1 and PbCaM3 were significantly up-regulated in pear in response to salt stress. The Ca2+-binding proteins SOS3 and SCaBP8 were activated by higher cytosolic Ca2+, and then activated SOS2,a serine/threonine protein kinase [35-37]. Activated SOS2 activates the plasma membrane (PM) Na+/H+antiporter SOS1, which is responsible for transporting excess Na+into the apoplast [38]. In the present study, both the mpt30 and mpt33 mutants showed significantly reduced Ca2+contents,higher Ca2+efflux rates,and lower ability of retaining calcium than the WT, indicating that intracellular levels of Ca2+in cytoplasm were regulated by controlling the influx and efflux of Ca ion. Although the influx of Ca2+to cytosol occurs in a passive form via Ca2+-channels, its efflux is an active process that is mediated by Ca2+/H+antiporters and Ca2+pumps, and the energy used in this process is provided by ATP hydrolysis and proton motive force [39]. Demidchik et al. [40] reported that increased extracellular ATP elevated the level of Ca2+in cytoplasm in Arabidopsis,thus up-regulating the transcripts of the MPK3 gene encoding a kinase involved in salt stress response. Thus, the reduced Ca2+uptake and weaker salt tolerance in the mutants may have been triggered by the loss of function of the OsMPT3 genes and a decrease in ATP.
4.3. OsMPT3s play important regulatory role in accumulation of metabolites
When plants are exposed to salt stress, cell permeability generally decreases to maintain the uptake of water,but as an indicator of stress-induced peroxidation of membrane lipids,the malondialdehyde (MDA) is increased, thus lowering the antioxidant capacity in plant cells. Maintaining cell permeability is associated with the levels of soluble sugar and proline [21,22]. In the present study, cell permeability and MDA in the mpt33 and mpt30 mutants were significantly increased, but the contents of proline and soluble sugar were lowered, suggesting that the mutagenesis of the OsMPT3 genes triggered changes in the accumulation profiles of these organic osmolytes. Proline is usually highly accumulated under salt stress, thus alleviating salt damage to the plant [41]. Li et al. [21] and He et al. [22] reported that overexpressing the ThPP1 gene and TsPIP1;1 gene increased the contents of proline to increase rice salt tolerance.However, the CRISPR/Cas9-mediated mutagenesis of the OsPPa6 gene significantly reduced the proline content and alkaline tolerance in rice [18]. As an initial substrate, proline synthesis is performed by the glutamate and ornithine pathways. Proline is first synthesized mainly from glutamate in plants, while delta-1-pyrroline-5-carboxylate synthase(P5CS) and delta-pyrroline-5-carboxylate reductase (P5CR)are the two key enzymes for proline synthesis, and P5CS exerts a strong effect on proline accumulation in the glutamate pathway compared to P5CR [42]. In the presence of ATP, P5CS catalyzes the conversion of L-glutamate to Lglutamyl 5-phosphate, a major step in proline synthesis [43].Proline dehydrogenase (ProDH) is a main rate-limiting enzyme in proline degradation[44].In this study,both the mpt33 and mpt30 mutants showed significantly reduced accumulation of proline and up-regulation of the proline synthesis gene(P5CS2-Os01g0848200) and down-regulation of the proline dehydrogenase gene (ProDH-Os10g0550900), indicating that the mutagenesis of the rice OsMPT3 gene affects the accumulation of proline.Our metabolome analysis also demonstrates an obvious up-regulation of the precursor of proline synthesis,L-glutamine,in the mutants.Usually,L-glutamine could be converted to L-glutamate by catalyzing a reaction of related enzymes, while P5CS catalyzes L-glutamate to L-glutamyl 5-phosphate through the supply pathway of ATP.In this study,an enhanced decrease in ATP content in the mpt33 and mpt30 mutants shows that the transfer process of L-glutamate into L-glutamyl 5-phosphate was impeded, leading to the upregulation of L-glutamine and decreased accumulation of proline. Thus, mutagenesis of the rice OsMPT3 gene lowered the accumulation of proline by changing the expression profiles of the P5CS2 and ProDH genes under salt stress,thereby altering osmotic potentials.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFC0501203) and the National Genetically Modified Organism Project(2016ZX08010005-9). Special thanks to Prof. Lanqin Xia for providing the pCXUN-U3 vector and Prof.Wan Jianmin's team for rice transformation.
Author contributions
Shengcai Huang performed the entire experiment and prepared the preliminary manuscript;Shichao Xin and Guoqiang Xie managed the reproduction of rice in the fields; Jiao Han constructed the transformation vector;Zhonglai Liu and Bing Wang participated in physiological measurements; Shuqing Zhang and Qingyu Wu provided guidance for the experimental design and writing; Xianguo Cheng designed the entire experiment and corrected the manuscript.
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2020.02.001.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Crop genome editing: A way to breeding by design
- Less and shrunken pollen 1 (LSP1) encodes a member of the ABC transporter family required for pollen wall development in rice (Oryza sativa L.)
- OsABA8ox2, an ABA catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response
- Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system
- Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize

