Rod-Shaped Metal Organic Framework Structured PCN-222(Cu)/TiO2 Composites for Efficient Photocatalytic CO2 Reduction
Shuhua Duan , Shufeng Wu , Lei Wang ,*, Houde She , Jingwei Huang , Qizhao Wang ,*
1 College of Chemistry and Chemical Engineering, Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Northwest Normal University, Lanzhou 730070, P. R. China.2 State Key Laboratory of Petroleum and Petrochemical Pollution Control and Processing, Lanzhou Petrochemical Research Center,PetroChina, Lanzhou 730060, P. R. China.
Abstract: The photocatalytic reduction of CO2 has attracted considerable attention owing to the dual suppression of environmental pollution and energy shortage. The technology uses solar energy to convert carbon dioxide into hydrocarbon fuel, which is of great significance for achieving the carbon cycle. The development of low-cost photocatalytic materials is critical to achieving efficient solar energy to fuels conversion. One of the most commonly employed photocatalysts is TiO2. However, it suffers from broad band gap as well as the recombination of photo-excited holes and electron.Hence, in this work, we report the photochemical reduction of CO2 using rodlike PCN-222(Cu)/TiO2 composites as photocatalyst through a simple hydrothermal method, in which TiO2 nanoparticles are anchored at the interface of the SiC rod PCN-222(Cu). Multiple characterization techniques were used to analyze the structure, morphology,and properties of the PCN-222(Cu)/TiO2 composite. A series of characterizations including X-ray diffraction (XRD),scanning electron microscopy (SEM), diffuse reflectance spectroscopy (DRS), Fourier-transform infrared spectroscopy,photo-electrochemical, and photoluminescence (PL) confirm the successful preparation of PCN-222(Cu)/TiO2 composites.SEM reveals that the TiO2 nanoparticles are uniformly distributed on the surface of the rod-shaped PCN-222(Cu)/TiO2.XRD results show that PCN-222(Cu) and PCN-222(Cu)/TiO2 composite photocatalysts with good crystal structure were successfully synthesized. According to the DRS results, the prepared PCN-222(Cu)/TiO2 composite samples exhibit characteristic absorption peaks of metalloporphyrins in the visible region. PL spectroscopy, transient photocurrent response,and electrochemical impedance spectroscopy further confirm that the rod-like PCN-222(Cu)/TiO2 samples have high electron-hole pair separation efficiency. By controlling the mass ratio of PCN-222(Cu) and TiO2, the photocatalytic CO2 reduction performance test shows that the 10% PCN-222(Cu)/TiO2 composite achieves optimal catalytic performance,yielding 13.24 μmol·g-1·h-1 CO and 1.73 μmol·g-1·h-1 CH4, respectively. All the rod-like PCN-222(Cu)/TiO2 composites exhibit better photocatalytic CO2 activity than that of TiO2 nanoparticles or PCN-222(Cu) under the illumination of xenon lamps, which is attributed to charge transport and electron-hole separation capabilities. After three test cycles, the catalytic activity of PCN-222(Cu)/TiO2 photocatalyst was virtually unchanged. The reduction yield of the catalyst increased for 8 h under continuous illumination, indicating that PCN-222(Cu)/TiO2 composites have acceptablestability. The estimation of the band gap curve and the Mote-Schottky curve test show that the lowest unoccupied molecular orbital position of PCN-222(Cu) is more negative than the TiO2 of the conduction band; hence, a possible photocatalytic reaction mechanism of the PCN-222(Cu)/TiO2 composite is proposed. This study provides a new strategy for the integration of metal-organic frameworks and oxide semiconductors to construct efficient photocatalytic systems.
Key Words: Composite; Metal-organic frameworks; PCN-222(Cu); Photocatalytic CO2 reduction; TiO2
1 Introduction
A large amount of fossil fuel causes the concentration of carbon dioxide in the atmosphere to increase, and subsequently bring up a series of disasters such as global climate change and greenhouse effect1,2. In recent decades, a number of technologies have been used for the recovery of carbon dioxide.In particular, the photocatalytic technology not only abates the concentration of carbon dioxide in the atmosphere, but alleviates the energy shortage, which has attracted wide attention of researchers3. Photocatalytic carbon dioxide reduction technology employing carbon dioxide as raw material to produce valuable chemical products and fuels is one of the most ideal ways to achieve carbon neutral circulation, displaying great significance for the protection of the natural environment for sustainable development4,5. Since the semiconductor is sensitive to light, it is used as a catalyst for photocatalytic reduction reaction to accelerate the photocatalytic reaction process. At present, common semiconductor materials such as TiO26-9, g-C3N410-12, CdS13-15and BiVO416,etc. have been used in photocatalytic reaction technology.
Carbon dioxide is a linear carbon with significantly thermodynamical stability. The valence of carbon is the highest oxidation state, chemically inert and difficult to be activated. The adsorption and activation of carbon dioxide molecules in photocatalysis is a key step in the catalytic reaction17-19. TiO2has been widely used as a catalyst for photocatalytic reaction because of its good chemical stability, non-toxicity and low cost.However, because the photogenerated electron and holes over TiO2are easily recombined, their photocatalytic reactivity is highly affected. Therefore, in order to improve the catalytic activity of the catalyst, the common methods include ion doping,noble metal deposition, dye sensitization, semiconductor material recombinationetc.20-27. The metal organic frameworks(MOFs) is a crystalline porous material bearing a periodic structure connected by metal cluster and an organic linker through a coordinate bond28. The MOF material holds the advantages of adjustable structure, high surface area and high selectivity, and extensively explored as a catalyst for photocatalytic reaction. Among them, metalloporphyrin-based PCN-222 possesses high light-capturing ability, and the porous structure that contributes to CO2absorption. With broadened light-havesting as well enriched CO2molecules around the catalytic center Zr6, its photocatalytic efficiency is impressive rather than many other MOFs29-32. Also, studies have shown that the electron trap state exhibited in PCN-222 can effectively suppress electron-hole recombination17,33-35.
In present work, we design and construct a novel nanorod PCN-222(Cu)/TiO2viaa hydrothermal method. The PCN-222(Cu)/TiO2nanostructures show more excellent photocatalytic performance than TiO2under illumination of 300 W Xe lamp, which can be attributed a significantly advantageous effect on the charge separation efficiency and light absorption ability. This study provides new insights into MOF/TiO2nanomaterials with different morphologies for photocatalytic CO2reduction. In addition, a possible photocatalytic CO2reduction mechanism and the electrons migration path of the PCN-222(Cu)/TiO2composite are also proposed.
2 Experimental and computational section
2.1 Material synthesis
Pyrrole (C4H5N, 99.0%), 4-formylbenzoic acid (C8H6O3,99.0%) and propionic acid (C3H4O2, 99.0%) were purchased from Damas-beta. Benzoic acid (C7H6O2, 99.9%), copper (II)chloride dihydrate (CuCl2·2H2O, 98%), zirconium (IV) chloride(ZrCl4, 98%), terephthalic acid (C8H6O4, 98%) and titanium butoxide (C16H36O4Ti, 98%) were all purchased from Sinopharm Chemical Reagent Co. Ltd. All reagents were analytical grade and no further purification was required except for pyrrole.
2.1.1 Synthesis of pure PCN-222 (Cu)
The synthesis of copper porphyrins (CuTCPP) is based on methods already reported in the laboratory36. 10 mg of zirconium tetrachloride (ZrCl4), a volume ratio of benzoic acid and distilled water, and 10 mg of CuTCPP were sequentially added to a beaker containing 2 mL of DMF solution, and stirring for 30 min. The mixture was transferred to a 50 mL teflon-lined steel autoclave and heated at 120 °C for 24 h. After cooling to room temperature, it was centrifuged to give a red solid. The solid was washed several times with DMF and ethanol, and then dried in an oven at 80 °C for 12 h.
2.1.2 Synthesis of PCN-222(Cu)/TiO2
The synthesis of TiO2is based on methods according to our previous method37. PCN-222(Cu)/TiO2composites were prepared by hydrothermal method, as shown in Fig. 1. First, 0.1 g of TiO2nanoparticles were dissolved in 5 mL of N, Ndimethylformamide (DMF) solution and continuously stirring,followed by addition of 0.005 g, 0.01 g and 0.015 g of PCN-222(Cu). The mixture kept stirring at room temperature for 30 min,and then ultrasonicated for 10 min. The resulting solution was placed in a 50 mL polytetrafluoroethylene lining, sealed in a steel autoclave and heated in an oven at 120 °C for 24 h. The final product was collected by natural cooling to room temperature, washed several times and dried in an oven at 80 °C for 12 h. The obtained PCN-222(Cu)/TiO2samples of different mass ratios were labeled as 5%, 10%, and 15% PCN-222(Cu)/TiO2according to the mass ratio of PCN-222(Cu).
2.2 Material characterization
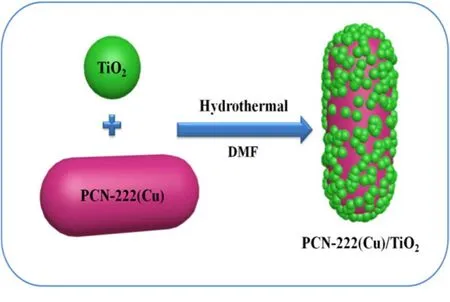
Fig. 1 Illustration of the synthesis of PCN-222(Cu)/TiO2 composites by hydrothermal method.
X-ray diffraction patterns (XRD) analysis was recorded on a Rigaku D/Max-2400/PC with scattering angle ranging from 5°to 60°. The scanning electron microscopy (JEOL,JSM-6701E)including elements mapping were performed to observe the morphology and structure of all composites. Fourier transform infrared (FT-IR) spectroscopy was performed on a Nicolet NEXUS 670 spectrometer (UnitedStates Thermo Fisher Scientific). Ultraviolet visible diffuse reflectance spectroscopy(DRS) was measured by the Beijing of China PuXinTU-1901 UV-Vis spectrophotometer equipped with an integrating sphere attachment. Photoluminescence (PL) spectroscopy of the samples was performed on a PE LS-55 fluorescence spectrophotometer (SHIMADZU).
2.3 Photoelectrochemical performance
The photoelectrochemical performance measurements were performed on CHI 660D electrochemical system with threeelectrode, which used FTO glass as working electrode, platinum as the counter electrode and Ag/AgCl as a reference electrode.0.5 mol·L-1Na2SO4was used as the electrolyte solution. All samples were backlit while measuring and the FTO glass (1 cm ×1 cm). A CEL-HXF300 xenon lamp was served as the light source for the measurement38.
2.4 Photocatalytic activity
The performance of photocatalytic CO2reduction is detected in a steel reaction system. First, 2 mL of distilled water was added to a 50 mL stainless steel reactor, and then 0.1 g of the sample was accurately weighed into a weighing bottle (40 × 25 mm) and placed in the reactor. The photocatalytic reactor was sealed and evacuated to remove internal air, followed by high purity CO2gas into the reactor. This step was repeated three times to ensure complete removal of air from the reactor to reduce experimental error. Finally, the circulating cooling water pump system was turned on, and the reactor was placed under a 300 W xenon lamp (Beijing Au light Co., Ltd. CELHXF300/CEL-HXUV300). After the photocatalytic reaction was continuously irradiated for one hour, the gas was extracted by a gas injector and injected into a gas chromatograph, and the content of the reduced products CO and CH4was quantitatively determined by a flame ionization detector (FID). During the course of the photocatalytic reaction, the reactor was kept sealing from start to end and the pressure was kept constant, and the circulating cooling system kept the reactor at room temperature.
3 Results and discussion
Fig. 2 shows the morphology and microstructure of the materials PCN-222(Cu) and PCN-222(Cu)/TiO2by scanning electron microscope (SEM). Fig. 2a is an SEM image of pure PCN-222 (Cu). It can be observed from the image that PCN-222(Cu) exhibits a more uniform rod-like morphology and a smooth surface, and its morphology is similar to that of a cylindrical magnet. Fig. 2b is a SEM image of PCN-222(Cu)/TiO2composite. TiO2is uniformly distributed on the surface of PCN-222(Cu), while partially agglomerated TiO2nanoparticles. It can be observed that the combination of PCN-222(Cu) and TiO2can effectively reduce the severe agglomeration of TiO2nanoparticles. The chemical composition of the PCN-222(Cu)/TiO2nanocomposite was determined by element mapping spectroscopy, demonstrating the presence of C, O, N,Zr, Ti and Cu in PCN-222(Cu)/TiO2nanocomposites.
The crystal structure of the nanocomposite was investigated using X-ray diffraction (XRD) analysis. Fig. 3a is a XRD pattern of simulated PCN-222 and PCN-222(Cu). It can be seen that the XRD diffraction peak of PCN-222 obtained by the crystal simulation software is consistent with the XRD diffraction peak of the laboratory prepared PCN-222(Cu), suggesting that PCN-222 (Cu) is successfully prepared. Fig. 3b presents the XRD pattern of TiO2and PCN-222(Cu)/TiO2composites. As can be seen from the figure, there is only a pure anatase type of TiO2. In addition to the anatase diffraction peak of TiO2anatase in the composite, the characteristic diffraction peak of PCN-222(Cu)appeared at 5°-10°. The XRD results show that PCN-222(Cu)/TiO2photocatalyst has been successfully prepared by simple hydrothermal method.
Fig. 4 presents the Fourier transform infrared (FTIR) spectra of TiO2, PCN-222(Cu) and 10% PCN-222(Cu)/TiO2,respectively. The broad peak at 3400 cm-1in all samples is attributable to the stretching vibrational peak of the hydroxyl group, which represents the presence of bound water and free water in the sample39. The presence of vibration peak of ―COOH and a vibrational peak of Cu―N at 1000 cm-1in the porphyrin structure indicates the presence of CuTCPP compound in the prepared PCN-222(Cu) structure. The vibrational peaks at 1800-1200 cm-1are attributable to the symmetrical vibration and asymmetric vibrational peaks of the carboxyl groups in the PCN-222(Cu) and PCN-222(Cu)/TiO2composites25,40. After the copper metal is coordinated in the porphyrin structure, the vibrational deformation of the nitrogen ring is enhanced, giving a Cu―N stretching vibration characteristics at 1000 cm-141. Moreover, the PCN-222(Cu)and PCN-222(Cu)/TiO2strength is enhanced at 1800-1200 cm-1, which may be attributed to the ―COOH vibration peak in the porphyrin compound, demonstrating the successful preparation of the PCN-222(Cu)/TiO2composite.

Fig. 2 SEM image of (a) PCN-222(Cu), (b) PCN-222(Cu)/TiO2 nanocomposite, and the corresponding elemental mapping images of PCN-222(Cu)/TiO2.
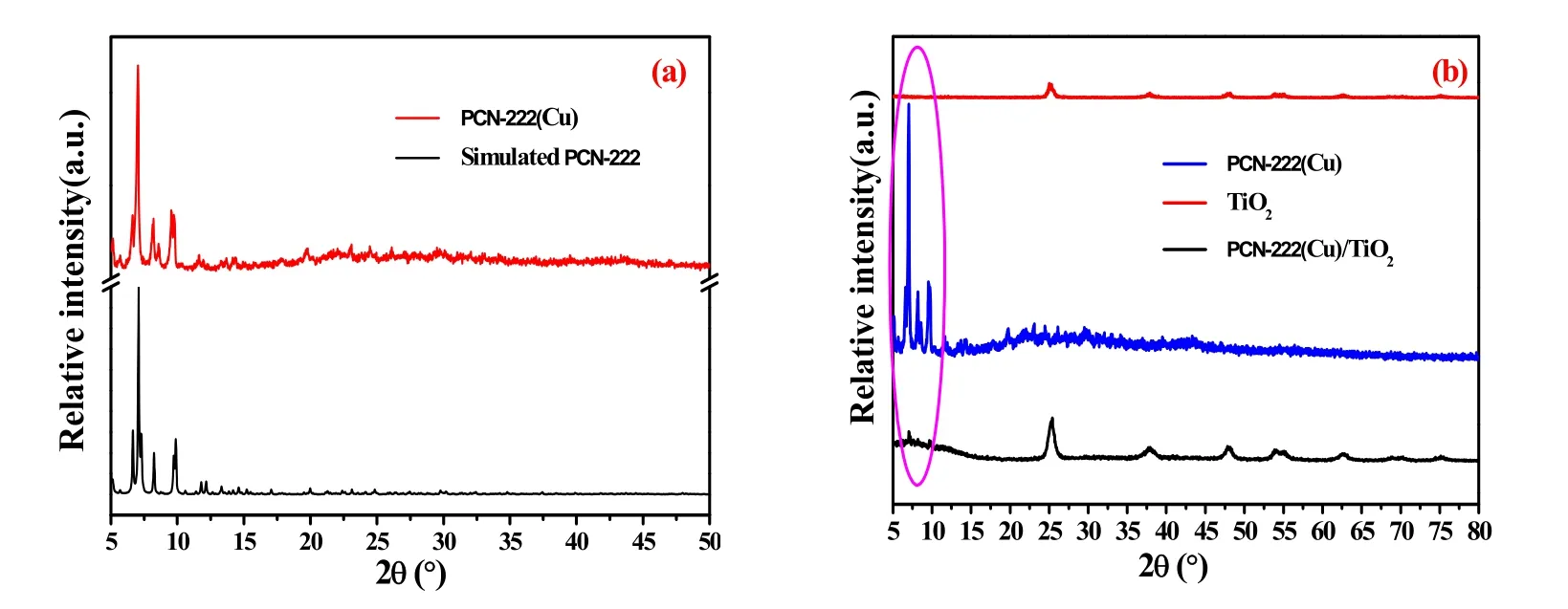
Fig. 3 XRD patterns of TiO2, PCN-222(Cu), PCN-222(Cu)/TiO2.
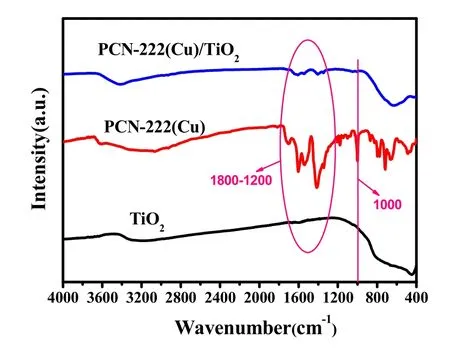
Fig. 4 FT-IR spectra the synthesis TiO2, PCN-222(Cu) and 10% PCN-222(Cu)/TiO2.
Fig. 5 shows the UV-visible diffuse reflectance (DRS) to determine the light absorption capacity of the samples.Compared with the absorption edge of TiO2, the absorption edge of PCN-222(Cu)/TiO2composites did not change, but the characteristic absorption peaks of S-band and Q-band metal porphyrin appears in the visible region, which proved PCN-222(Cu) successfully combined with TiO241,42. The potential position of PCN-222(Cu) was measured by Motty-Schottky (MS) curves (Fig. 5c). The positive slope indicates that the rod PCN-222(Cu) isn-type semiconductor. According to the M-S curve, the flat band potential of PCN-222(Cu) relative to the Ag/AgCl electrode is approximately -0.55 V. The DRS diagram exhibits the PCN-222(Cu) band gap value is 1.73 eV by intercepting the tangent of (Ahv)2and photon energy. Hence, the LUMO and HOMO positions of PCN-222(Cu) are at -0.55 V and 1.18 V, respectively.
Fig. 6a shows the Photoluminescence (PL) spectra of sample TiO2, PCN-222(Cu) and PCN-222(Cu)/TiO2. The catalyst generates photogenerated electrons and holes after being excited by light, and afterwards electrons and holes will recombine to generate fluorescence, so that the fluorescence intensity can effectively reflect the separation efficiency of electrons and holes of the photocatalyst. From the test results, the pure TiO2exhibits the strongest fluorescence intensity among the three samples. The intensity of PCN-222(Cu)/TiO2is between PCN-222(Cu) and TiO2, indicating PCN-222(Cu)/TiO2composite material can effectively improve the separation efficiency of photoinduced charges, and thereby improving the photocatalytic activity of the catalyst.
Next, we conducted a series of photoelectrochemical (PEC)performance measurements to gain insight into the photocatalytic CO2reduction performance of rod-shaped PCN-222(Cu)/TiO2composites. Fig. 6b-d shows the PEC properties of TiO2and PCN-222(Cu)/TiO2to investigate the effect of the charge separation efficiency of composites. Fig. 6b shows that the transient photocurrent spectra of PCN-222(Cu)/TiO2is significantly enhanced compared with TiO2, indicating that the composite can effectively improve the separation efficiency of photogenerated electron holes. On the other hand, the electrochemical impedance spectroscopy can effectively reflect the charge carriers transport efficiency of the PCN-222(Cu)/TiO2photocatalyst, and the composite manifests the smallest arc radius in the spectrum, which is further reflected in the lowest resistance. Fig. 6b, c are electrochemical impedance spectra(EIS) of TiO2and PCN-222(Cu)/TiO2. The EIS plots show a significant decrement of electrochemical impedance value from TiO2and PCN-222(Cu)/TiO2electrode. The semicircle in the PCN-222(Cu)/TiO2is smaller than TiO2electrode in both light and dark conditions, suggesting that the PCN-222(Cu)/TiO2benefit the transfer and separation of photo-induced carriers in the interface between semiconductor and electrolyte43.Therefore, the composite material PCN-222(Cu)/TiO2can impressively reinforce photocatalytic CO2reduction performance.
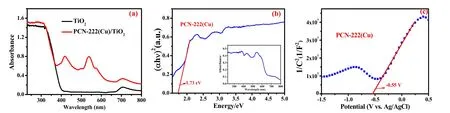
Fig. 5 (a) UV-Vis diffuse reflectance spectra of TiO2 and PCN-222(Cu)/TiO2, (b) estimation of band gap energies and UV-Vis diffuse reflectance spectra of PCN-222(Cu) (inset), (c) Mott-Schottky plot of the PCN-222(Cu) electrode (0.5 mol·L-1 Na2SO4).
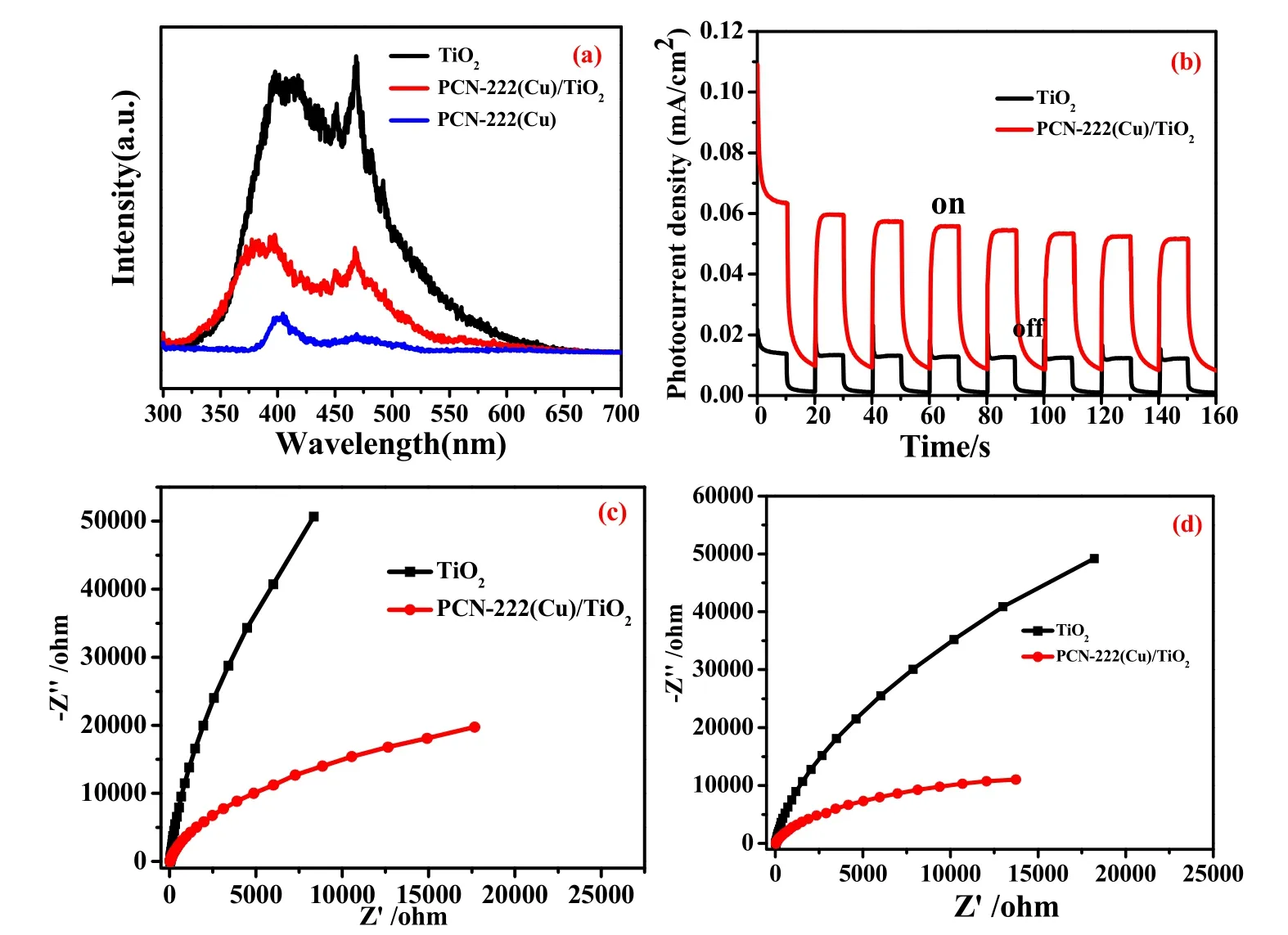
Fig. 6 (a) PL spectra of the various components, (b) the photocurrent-time (I-t) curves, Nyquist plots of EIS measurements on the TiO2 and PCN-222 (Cu)/TiO2 (a) in the dark and (b) light irradiation (0.5 mol·L-1 Na2SO4).
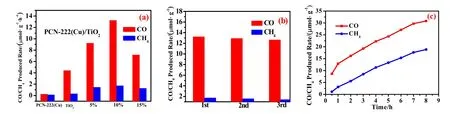
Fig. 7 (a)The dependence of TiO2 and different ratios of PCN-222(Cu)/TiO2 and Xe lamps on total CO/CH4 release in 1 h. (b) Three cycles of CO2 reduction over the 10%PCN-222(Cu)/TiO2. (c) Total CO/CH4 evolution amount of 10%PCN-222(Cu)/TiO2 with Xe lamp within 8 h.
The photocatalytic performance characterization of the prepared rod-shaped PCN-222(Cu)/TiO2composite was evaluated under irradiation with a 300 W Xe lamp. As shown in Fig. 7a, a series of different ratios of PCN-222(Cu)/TiO2composites exhibit different CO2conversion efficiencies,showing a tendency to increase first and then decrease. All PCN-222(Cu)/TiO2samples show higher CO2reduction photocatalytic activity than pure TiO2. In all prepared composite materials, the composite 10% PCN-222(Cu)/TiO2had the best catalytic rate, and the yield was 13.24 μmol·g-1·h-1CO and 1.73 μmol·g-1·h-1CH4, respectively. In terms of the yield of the reduced product CO, 10% PCN-222(Cu)/TiO2is three times that of TiO2. The high photocatalytic activity is mainly due to the combination of PCN-222(Cu) and TiO2, which improves the carrier transmission efficiency and PCN-222(Cu) has high visible light absorption capacity. In addition, the synergistic effect of the PCN-222(Cu)/TiO2composite effectively improves the dispersibility of the TiO2nanoparticles and provides more opportunities for the contacts between CO2and the heterocatalyst33. The amount of reduced product observed remains essentially unchanged after three catalytic cycles,indicating that the stability of the 10% PCN-222(Cu)/TiO2composite is better for photocatalytic CO2reduction (Fig. 7b).Fig. 7c shows the continuous 8 h light performance test on 10%PCN-222(Cu)/TiO2. The results reveals that the yields of CO and CH4increases, indicating that the photocatalyst remains catalytically active throughout the test.
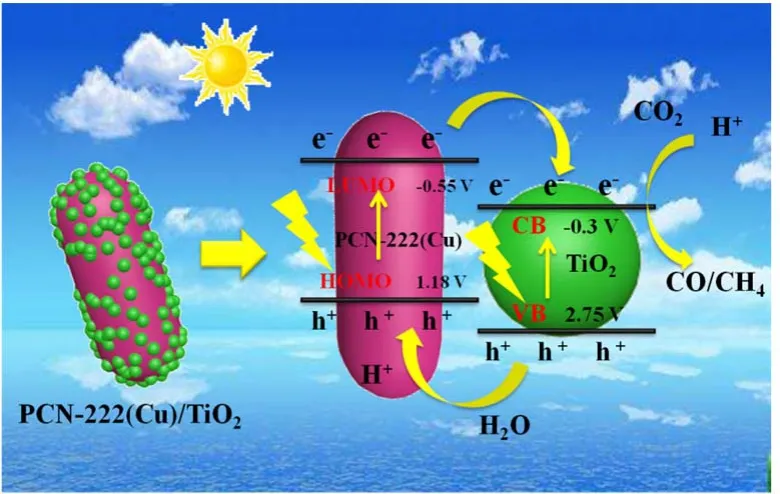
Fig. 8 The mechanism of electron/holes transfer and separation of the PCN-222(Cu)/TiO2 composite.
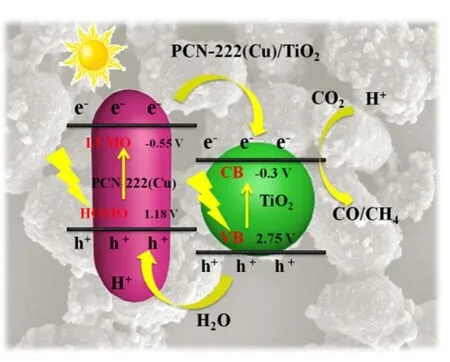
The conduction band (CB) level of TiO2is -0.3 V, while the valence band (VB) potential of TiO2is calculated as 2.75 eV according to the previous work. Based on the above characterization and photocatalytic performance test results, we proposed a possible reaction mechanism for PCN-222(Cu)/TiO2photocatalysis, as shown in Fig. 8. When 300 W Xe lamp was irradiated on the surface of the catalyst, both PCN-222(Cu) and TiO2are excited to generate electrons and holes. At the same time, since the lowest unoccupied molecular orbital (LUMO)potential of PCN-222(Cu) is more negative than the CB potential of TiO2, the electrons on the LUMO of PCN-222(Cu) are directly transferred to the CB of TiO2with initiation of reduction on the surface of TiO2. The holes on the VB of TiO2are transferred to the highest occupied molecular orbital (HOMO) position of PCN-222(Cu), which can oxidize H2O to generate ·OH radicals and release O2and H+44,45. PCN-222(Cu)/TiO2composite improves the separation efficiency of electron holes, prolongs the lifetime of photogenerated carriers, and effectively ameliorates the photocatalytic CO2reduction activity of the composites.
4 Conclusions
A series of PCN-222(Cu)/TiO2composites with different ratios of rod-like PCN-222(Cu) and TiO2were prepared by hydrothermal method. The samples were characterized by XRD,SEM, DRS, FT-IR, PEC, PL,etc., indicating that TiO2is integrated on the surface of PCN-222(Cu). The photocatalytic CO2reduction performance of PCN-222(Cu)/TiO2were tested.It is found that 10% PCN-222(Cu)/TiO2has the highest photocatalytic activity, and the photocatalytic CO2reduction product formation rate is as follows: CO yield was 13.24 μmol·g-1·h-1; CH4yield was 1.73 μmol·g-1·h-1. Cyclic stability tests show that PCN-222(Cu)/TiO2has good catalytic stability.The result of this work provides a new idea based on the complexation of rod-shaped metalloporphyrin PCN-222(Cu)with semiconductor materials, which can effectively promote the separation of photogenerated charges and enhance the photocatalytic reduction activity.
- 物理化學(xué)學(xué)報(bào)的其它文章
- 魅力光催化劑
- Cu2+ Modified g-C3N4 Photocatalysts for Visible Light Photocatalytic Properties
- Controlling Self-Assembly of 3D In2O3 Nanostructures for Boosting Photocatalytic Hydrogen Production
- 硫化鎘反蛋白石光子晶體制備及光解水制氫
- Fabrication of Z-Scheme Heterojunction of SiC/Pt/Cds Nanorod for Efficient Photocatalytic H2 Evolution
- 光催化甲烷轉(zhuǎn)化研究進(jìn)展

