Leukocyte cell-derived chemotaxin 2 inhibits development of atherosclerosis in mice
Wen-Ming He,Ting Dai,Jiong chen,Jian-An Wang
1 Department of Cardiology of the Second Affiliated Hospital,Zhejiang University Scho ol of Medicine,Hangzhou Zhejiang 310009,China
2 Department of Cardiology,the Affiliated Hospital of Medical School of Ningbo University,Ningbo Zhejiang 315010,China
3 Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Meishan Campus, Ningbo University, Ningbo Zhejiang 315832,China
ABSTRACT Leukocyte cell-derived chemotaxin 2 (LEcT2), a multifunctional hepatokine, is involved in many pathological conditions. However, its role in atherosclerosis remains undefined. In this study, we administered vehicle or LEcT2 to male Apoe-/- mice fed a Western diet for 15 weeks. Atherosclerotic lesions were visualized and quantified with Oil-red O and hematoxylin staining. The mRNA expression levels of McP-1, MMP-1, IL-8, IL-1 β, and TNF-α were analyzed by quantitative real-time polymerase chain reaction. Serum TNF-α, IL-1 β, IL-8, McP-1,and MMP-1 concentrations were measured by enzyme-linked immunosorbent assay. cD68, cD31,and α-SMA, markers of macrophages, endothelial cells, and smooth muscle cells, respectively, were detected by immunostaining. Results showed that LEcT2 reduced total cholesterol and low-density lipoprotein concentrations in serum and inhibited the development of atherosclerotic lesions, accompanied by reductions in inflammatory cytokines and lower McP-1, MMP-1, TNF-α, IL-8, and IL-1 β mRNA abundance. Furthermore, LEcT2 decreased cD68,but increased α-SMA in atherosclerotic lesions,suggesting an increase in smooth muscle cells and reduction in macrophages. In summary, LEcT2 inhibited the development of atherosclerosis in mice,accompanied by reduced serum total cholesterol concentration and lower inflammatory responses.
Keywords: Leukocyte cell-derived chemotaxin 2(LEcT2); Atherosclerosis; Inflammation; Lipid metabolism
INTRODUCTION
cardiovascular diseases are the leading cause of mortality worldwide. With ageing populations and increasing exposure to metabolic risks, deaths due to cardiovascular diseases will increase to approximately 23 million by 2030 (GBD 2017 Risk Factor collaborators, 2018). Atherosclerosis is an important underlying cause of many clinical cardiovascular events, and is a systemic disease characterized by fatty deposits, in flammation, cell death, fibrosis, and scar tissue build up within the walls of arteries (Mozaffarian et al., 2016). In the early stages of atherosclerosis, a large amount of lipids and proinflammatory mediators accumulate in macrophages within the arterial wall, which become foam cells (Lim & Park, 2014).As the disease advances, vascular smooth muscle cells(VSMcs) migrate from the media to intima, and then proliferate and produce extracellular matrix to form a fibrous cap that covers atherosclerotic plaque (Allahverdian et al.,2018). During this stage, the secretion of many proinflammatory cytokines is increased, including interleukin(IL)-8, IL-1 β, monocyte chemoattractant protein-1 (McP-1),and tumor necrosis factor (TNF)-α (chistiakov et al., 2015). In the advanced stage, macrophages induce VSMc apoptosis via the TNF-α/NO signaling pathway (Boyle et al., 2003) and reduce collagen synthesis by secretion of matrix metalloproteinases (MMP) (Müller et al., 2014). Although the precise pathogenesis of atherosclerosis has not been clarified, it is now widely accepted that inflammation and lipid disorder both play crucial roles(Ference et al.,2017;Hansson&Libby,2006).
Recent studies have demonstrated that a set of predominantly liver-derived proteins can directly affect the progression of atherosclerosis (Yoo & choi, 2015). Leukocyte cell-derived chemotaxin 2 (LEcT2) is a 16 kDa secretory protein first isolated from cultured supernatants of phytohemagglutinin-activated human T-cell leukemia SKW-3 cells. It is predominantly secreted by hepatocytes (Yamagoe et al., 1998) and is generally expressed in vascular cells,endothelial cells, and VSMcs (Slowik &Apte, 2017). Previous studies have reported that LEcT2 has an inhibitory effect on inflammation. In mice, LEcT2 deficiency can cause severe arthritis, with reductions in the production of cytokines and chemokines, e.g., IL-1 β , IL-6, TNF-α, and McP-1, after exogenous LEcT2 injection (Okumura et al., 2008). We recently reported that LEcT2 can protect mice against bacterial sepsis by enhancing phagocytosis and bacterial killing of macrophages via cD209a (Lu et al., 2013) and can also induce hematopoietic stem cell expansion and mobilization (Lu et al., 2016). In human hepatocellular carcinoma, low LEcT2 expression is correlated with advanced histological grade and inflammatory infiltrates. L'Hermitte et al.(2019) showed that LEcT2-deficient hepatocellular carcinoma cells can secrete chemotactic signals, resulting in amplification of inflammatory monocytes, which harbor an immature phenotype with immunosuppressive capacities and tumor-promoting potential. In addition, Zhang et al. (2018)discovered that circulating LEcT2 concentrations are significantly higher in newly diagnosed type-2 diabetic patients, especially those that are obese, and that concentrations of LEcT2 are negatively associated with highdensity lipoprotein-cholesterol levels. Researchers have also found that LEcT2 is involved in many other pathological conditions, such as obesity (Sargeant et al., 2018), skeletal muscle insulin resistance (Jung et al., 2018), non-alcoholic fatty liver disease, and metabolic syndrome (Yoo et al., 2017),thus suggesting the potential role of LEcT2 in atherosclerosis.However, such roles remain undefined. The purpose of this study was to investigate the effects of LEcT2 on atherosclerosis in Apoe-/-mice.
MATERIALS AND METHODS
Animals
Male Apoe-/-and c57BL/6 wild-type mice (6 weeks old, 20±2 g) were purchased from Beijing Vital River Laboratory Animal Technology (china). The c57BL/6 mice were fed a normal laboratory diet and used as blank controls, whereas the Apoe-/-mice were fed a Western diet (0.15% w/w cholesterol, 40 kcal% butter fat, Beijing Biotech-HD) for 15 weeks as an atherosclerosis model.All mice were housed in a constant temperature (21±2 °c) room, under a 12 h dark/12 h light cycle in a pathogen-free environment in the Animal care Facility of Ningbo University Medical School according to the institutional guidelines.
In vivo application of LECT2
Mice were randomly divided into three groups: i.e., (1) control group (c57BL/6 mice, PBS 100 μL, subcutaneous (sc), n=8),(2) atherosclerosis (AS) group (Apoe-/-mice, PBS 100 μL, sc,n=8), and (3) LEcT2 group (Apoe-/-mice, LEcT2 0.2 mg/kg,sc, n=8). Each mouse received a sc injection every 2 d from 6 to 21 weeks of age. LEcT2 was dissolved in PBS and kept at 4 °c for 2 d. Two days after the last injection, mice were euthanized to analyze and characterize atherosclerosis.
Recombinant LECT2 protein
Recombinant mouse LEcT2 proteins (purity: 96.20%) were produced from cHO cells, as described in our previous research(Lu et al.,2013,2016).
Measurement of lipid parameters
Two days after final administration, all mice were sacrificed after retro-orbital bleeding.All blood samples were centrifuged at 3 000 r/min for 15 min at room temperature to collect serum. commercially available kits were then used to measure total cholesterol (Tc), total triglyceride (TG), highdensity lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) concentrations according to the manufacturer’s protocols.
LECT2 detection
The ELISA system used one antibody as the capture antibody(rabbit anti-LEcT2, c-terminal; Santa cruz, cA, USA.) and another for detection (goat anti-LEcT2, N-terminal; Santa cruz,cA,USA.).
ELISA for cytokines
Serum concentrations of inflammatory cytokines, TNF-α(MTA00B, R&D Systems , Minneapolis, MN, USA ), IL-1β(MLB00c, R&D Systems , Minneapolis, MN, USA), IL-8 (SBJM0010, Senbeijia Bio. co, Nanjing, china), MMP-1 (cSBE07417m, cUSABIO, china), and McP-1 (cSB-E07430m,cUSABIO, china) were measured with commercial ELISA kits according to the manufacturer’s instructions.Absorbance was measured using a Multiskan Ascent plate reader (Thermo Electron corporation,Waltham,Massachusetts,USA).
Histological evaluation
After termination, the periaortic tissue around the aorta was cleaned, and the whole aorta from the heart to the abdominal aorta was dissected. Atherosclerotic plaque in the aortas was stained with 0.5% Oil-red O (Sigma-Aldrich) for 15 min at room temperature.After staining, the aortas were washed with 70% ethanol and then distilled water. Subsequently, the whole aorta was cut open and flattened on a black plate to take pictures. Aortic roots were embedded in optimal cutting temperature compound (Sakura, Torrance, cA, USA) and frozen in ultra-cold isopentane on dry ice. Serial cryosections(8 μm) were cut along the aortic root specimens at -20 °c using a cryotome (HM550, Thermo Scientific, Rockford, IL,USA). At least three transverse sections (spaced around 60 μm) from each aortic root were stained with hematoxylin/eosin for histology analyses. Oil-red O staining was performed to evaluate the lipid content in the plaque area. Image Pro-Plus 6.0 (Media cybernetics, USA) was used to analyze aorta images, following the recent AHA Statement (Daugherty et al.,2017).
Immunohistochemistry(IHC)
IHc staining was performed on 8 μm frozen sections of the aortic root to detect the proportion of macrophages,endothelial cells, and SMcs. Frozen sections were air-dried,fixed with cold acetone, and incubated in anti-cD31 antibody(1:50 dilution; ab28364,Abcam, USA), anti-cD68 antibody (1:50 dilution; ab125212, Abcam, USA), and anti-alpha smooth muscle actin antibody (1:200 dilution; ab5694, Abcam, USA),respectively, at 4 °c overnight. Substitution of PBS for the specific primary antibody was performed as a negative control. All steps were operated following the instruction manual of the SABc (rabbit IgG)-POD kit (Solarbio, Beijing,china). Finally, slides were stained with DAB, counterstained with hematoxylin, dehydrated, and mounted. Images were detected using a Nikon A1R confocal laser scanning microscope (Nikon, Tokyo, Japan). Image Pro-Plus 6.0 software (Media cybernetics, Rockville, USA) was used to analyze the positively stained areas. At least three sections were analyzed per aortic root.
Total RNA isolation and quantitative real-time polymerase chain reaction(qRT-PCR)
Aorta total RNA (five mice from control, AS, and LEcT2 groups) was extracted by Trizol reagent (Invitrogen, carlsbad,cA, USA) following the manufacturer’s protocols.complementary DNA (cDNA) was synthesized using a HiFiScript first-strand cDNA synthesis kit (comWin Biotech,Beijing, china) according to the manufacturer’s instructions.PcR amplification was accomplished using 1×FastStart Essential DNA Green Master (Roche, Mannheim, Germany)with a Lightcycler 480 II instrument (Roche, Switzerland). The primer sequences used included (forward and reverse): McP-1, 5'-TTAAAAAccTGGATcGGAAccAA-3' and 5'-GcATTAG cTTcAGATTTAcGGGT-3'; MMP-1, 5'-TGTTTGcAGAGcAc TAcTTGAA-3' and 5'-cAGTcAccTcTAAGccAAAGAAA-3';IL-8, 5'-TcGAGAccATTTAcTGcAAcAG-3' and 5'-cATTGcc GGTGGAAATTccTT-3'; IL-1β, 5'-AGAAGcTGTGGcAGcTA-3' and 5'-TGAGGTGcTGATGTAccA-3'; TNF-α, 5'-GAAcTGG cAGAAGAGGcAcT-3' and 5'-GGTcTGGGccATAGAAcTG A-3'; and 18S rRNA, 5'-TTTGTTGGTTTTcGGAAcTGA-3'and 5'-cGTTTATGGTcGGAAcTAcGA-3'. Data were normalized to 18S rRNA and the dosage of the target fragments was calculated using the 2-ΔΔcTmethod. Sequences were confirmed using NcBI BLAST software.
Statistical analysis
Results were presented as means±standard deviation (SD).Statistical analysis was performed using GraphPad Prism 7.0(GraphPad Software, San Diego, cA, USA). The Shapiro-Wilk test was used to check the normality of the data. If data displayed normal distribution, an unpaired Student’s t-test was used to compare two groups. If the data did not display normal distribution,the Mann-Whitney U test was performed.
RESULTS
LECT2 inhibited development of atherosclerotic lesions in Apoe-/-mice
We analyzed the levels of LEcT2 in the AS and control groups to examine the relationship between serum LEcT2 levels and atherosclerosis. Results showed that the LEcT2 levels in the AS group (33.37±2.85 ng/mL, n=6) were significantly lower than that in the control group (43.62±2.20 ng/mL,n=6;P<0.05). Oil-red O staining showed no obvious atherosclerotic plaque in the control group aortas, but apparent plaque in the aortas of the AS group. The LEcT2 group had less atherosclerotic plaque in the ascending aortic arch and thoracic regions compared with the AS group (P<0.001)(Figure 1A). consistently, hematoxylin and Oil-red O staining of the aortic sinus for atherosclerotic lesions also showed reduced lipid accumulation in the LEcT2 group compared with the AS group(P<0.001)(Figure 1B).
LECT2 reduced serum lipids in Apoe-/-mice
There is a close connection between dyslipidemia and atherosclerosis (Wang et al., 2018). compared with the AS group, LcET2 administration reduced the serum total cholesterol(927±160 mg/dL,768±79 mg/dL,P<0.05),TG(57±16 mg/dL,33±11 mg/dL,P<0.01),HDL-c(123±10 mg/dL,111±7 mg/dL, P<0.05), and LDL-c concentrations (452±81 mg/dL,374±37 mg/dL,P<0.05)(Figure 2).
LECT2 reduced mRNA abundance of inflammatory cytokines and chemokines
We performed qRT-PcR to confirm the effect of LEcT2 on McP-1, MMP-1, TNF-α, IL-1β, and IL-8 mRNA expression.consistent with the systemic inflammation findings, the mRNA expression levels of these inflammatory cytokines and chemokines in the whole aorta were lower in the LEcT2 group than that in the AS group (Figure 3A-E). Furthermore, results showed a dose-dependent relationship, whereby the inflammatory cytokines and chemokines decreased with the increase in LEcT2 concentration(data not shown).
LECT2 led to lower serum inflammatory cytokine concentrations in Apoe-/-mice
Inflammation is a critical biological process in atherosclerosis(Wolf & Ley, 2019). compared with the control group, serum concentrations of inflammatory cytokines and chemokines,including TNF-α(P<0.05),IL-1β(P<0.01),IL-8(P<0.01),McP-1 (P<0.001), and MMP-1 (P<0.001), were higher in the AS group. Administration of LcET2 led to reductions of these inflammatory cytokines(Figure 3F-J).
LECT2 changed atherosclerotic plaque composition
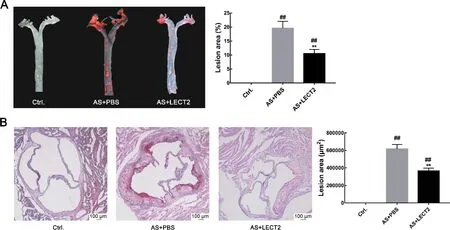
Figure 1 LECT2 attenuated development of atherosclerotic lesions in Apoe-/-mice
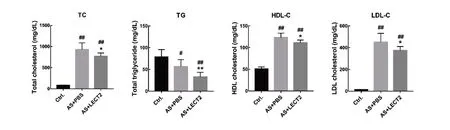
Figure 2 Effects of LECT2 on lipid profile in serum
We assessed the proportion of smooth muscle cells,macrophages, and endothelial cells by IHc staining to determine whether LEcT2 affected the composition of atherosclerotic plaque. compared with the AS group, LEcT2 administration increased the number of smooth muscle cells(Figure 4A) and reduced the proportion of cD68 macrophages(Figure 4B) but had no influence on the proportion of cD31 endothelial cells in the lesions(Figure 4c).
DISCUSSION
In the present study, we investigated the effects of LEcT2 on the development of atherosclerosis in mice. Results demonstrated that LEcT2 exhibited an anti-atherosclerotic function, accompanied by a reduction in serum total cholesterol concentration and lower inflammatory responses.
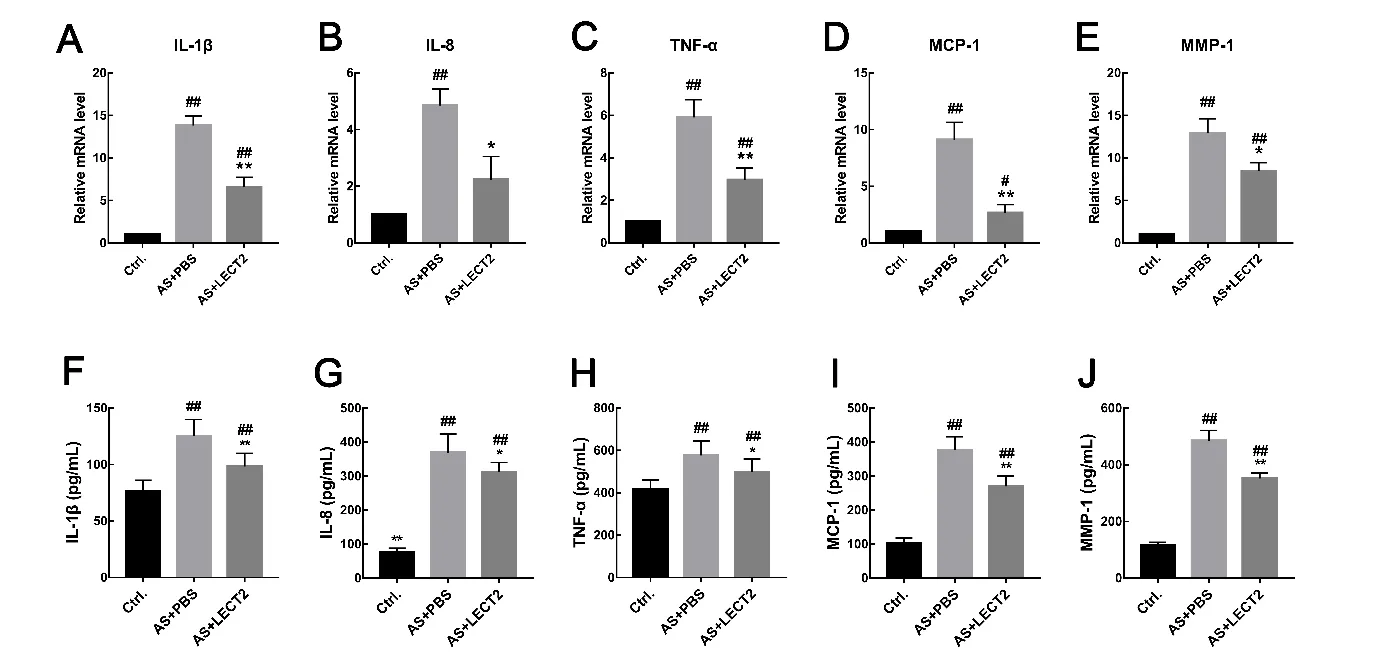
Figure 3 LECT2 reduced mRNA expression and serum concentrations of inflammatory cytokines and chemokines in Apoe-/-mice
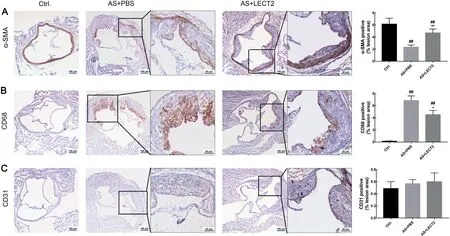
Figure 4 LECT2 changed atherosclerotic plaque composition
We first measured atherosclerotic lesions in the aortas of Apoe-/-mice and found that LEcT2 inhibited atherosclerosis,resulting in a 46% reduction in total plaque size. In addition to the observed reduction in plaque, LEcT2 also had an effect on the serum lipid profile. The liver plays a central role in regulating whole body cholesterol homeostasis. Hepatocytes maintain cellular cholesterol homeostasis by controlling several cholesterol input and elimination pathways. These input pathways are primarily regulated by the sterol regulatory element-binding protein (SREBP)-2-mediated cholesterol sensing mechanism (Ye & DeBose-Boyd, 2011). In earlier studies, Hwang et al. (2015) showed that LEcT2 increases mTOR phosphorylation and SREBP-1 cleavage in hepatocytes, leading to hepatic lipid accumulation; however,the study did not show serum lipid levels or SREBP-2 expression. Mammalian cells produce three SREBP isoforms,which control the anabolic pathways of cholesterol, free fat acids, and triglycerides (Engelking et al., 2018). SREBP-1a and SREBP-1c are active in driving the transcription of genes involved in fatty acid synthesis, whereas SREBP-2 is more active in stimulating transcription of genes involved in cholesterol biosynthesis (Ye & DeBose-Boyd, 2011). Based on our results, we hypothesize that LEcT2 may reduce serum TG, Tc, and LDL-c concentrations and contribute to its antiatherosclerotic effects by impacting the SREBP pathways.However, this needs to be corroborated by subsequent studies.
In addition to lipid accumulation, the inflammatory mechanism is another important factor in the pathogenesis and clinical manifestation of atherosclerosis. In the process of atherosclerosis, proinflammatory factors, including IL-1β and IL-8, are expressed by ox-LDL-stimulated endothelial cells and VSMcs, which, in turn, activate macrophages, resulting in amplification of the inflammatory response (chistiakov et al.,2015; Wolf & Ley, 2019). The McP-1 chemokine, also known as cc-chemokine ligand 2, plays an important role in the recruitment of monocytes to atherosclerotic lesions, and also contributes to thrombin generation and thrombus formation by generating tissue factor (charo & Taubman, 2004). Han et al.(1998) reported that elevated plasma LDL concentrations can enhance c-c chemokine receptor type 2 (ccR2, receptor for McP-1) expression and chemotactic response. Thus, LEcT2 may reduce McP-1 expression by decreasing LDL-c concentrations. On the other hand, inflammation can increase cellular cholesterol uptake and synthesis via up-regulation of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl-coenzyme A reductase, but can decrease cholesterol efflux via down-regulation of liver X receptor alpha and ATP-binding cassette transporter A1 (Zhong et al., 2015),implying that inflammation promotes lipid accumulation and foam cell formation by disrupting cellular cholesterol homeostasis. Previous research has also shown that LEcT2 is a direct target gene of the Wnt/β-catenin signaling pathway,and β-catenin negatively regulates nuclear transcription factor(NF- κB) signaling, which plays an important role in the regulation of inflammation responses (Ovejero et al., 2004).The results of our study are consistent with the above theory.We proved that circulating LEcT2 levels were significantly lower in atherosclerotic mice, suggesting that LEcT2 has an inhibitory effect on atherosclerosis. LEcT2 mitigated the expression and secretion of IL-1 β, IL-8, TNF-α, and IL-1β(Figure 3) dose-dependently in Apoe-/-mice fed with a Western diet, suggesting that LEcT2 may inhibit atherosclerosis by alleviating inflammatory responses via Wnt/β-catenin signaling. In addition, macrophage-derived MMPs,which play a potential role in fibrous cap thinning, are thought to promote vascular smooth muscle cell growth and extracellular matrix and atherosclerotic plaque homeostasis(Johnson, 2007) via degradation of various extracellular matrix proteins, including collagens (Moore & Tabas, 2011).We found that LEcT2 also increased the content of SMcs but reduced the proportion of macrophages (Figure 4), likely reflecting the role of LEcT2 in regulating the composition of atherosclerotic plaque.
In conclusion, we showed, for the first time, that LEcT2 inhibits the development of atherosclerosis in a hypercholesterolemic mouse model, accompanied by reduced serum total cholesterol concentration and lower inflammatory responses. Our future studies will aim to define the mechanisms involved in LEcT2 prevention of atherosclerosis development.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS'CONTRIBUTIONS
W.M.H., J.A.W., and J.c. conceived and designed the experiments. W.M.H.and T.D. performed the experiments, analyzed the data, and wrote the manuscript. J.A.W. and J.c. reviewed and corrected the manuscript. All authors read and approved the final version of the manuscript.
- Zoological Research的其它文章
- Transcription profiles of skin and head kidney from goldfish suffering hemorrhagic septicemia with an emphasis on the TLR signaling pathway
- A new cave-dwelling blind loach,Тriplophysa erythraea sp.nov.(cypriniformes:Nemacheilidae),from Hunan Province,china
- Effect of temperature on antioxidant defense and innate immunity in Brandt’s voles
- Allele-specific expression and alternative splicing in horse×donkey and cattle×yak hybrids
- Natural history of Valentin’s rock lizard(Darevskia valentini)in Armenia
- On the road to Mandalay:contribution to the Microhyla Tschudi,1838(Amphibia:Anura:Microhylidae)fauna of Myanmar with description of two new species

