Investigational growth factors utilized in animal models of spinal fusion:Systematic review
Ethan Cottrill,A Karim Ahmed,Noah Lessing,Zachary Pennington,Wataru Ishida,Alexander Perdomo-Pantoja,Sheng-fu Lo,Elizabeth Howell,Christina Holmes,C Rory Goodwin,Nicholas Theodore,Daniel M Sciubba,Timothy F Witham
Abstract
Key words: Spinal fusion;Growth factor;Pseudoarthrosis;Systematic review
INTRODUCTION
Over 400000 Americans undergo spinal fusion surgeries each year,with the number increasing yearly alongside a growing and aging population[1-3].However,pseudoarthrosis,or failed fusion,rates are reported to be as high as 40% in primary spinal fusion surgery and up to 60% in revision cases,even when the “gold standard”treatment of grafting bone from the patient’s own iliac crest is used[4,5].When this happens,patients often suffer from significant pain and disability,and remaining treatment options are limited.
Tissue engineering has the potential to solve the problem of pseudoarthrosis by promoting site-specific de novo bone generation.Classically,tissue engineering involves using a scaffold,cells,and growth factors to generate living tissues.At present,the only tissue engineered product involving a growth factor that is FDAapproved for spinal fusion is a collagen sponge delivered with recombinant human bone morphogenetic protein 2 (rhBMP-2) (INFUSE Bone Graft,Medtronic).However,this product is associated with significant complications[6,7],which are thought to arise from the supraphysiologic therapeutic dose of rhBMP-2 required for effective bone formation[8].In addition to rhBMP-2,recombinant human parathyroid hormone(rhPTH) and recombinant human BMP-7 (rhBMP-7) have been studied clinically in spinal fusion[9-11].
Given the potential impact of tissue engineering to advance spinal fusion,many biomaterials and bioactive agents have been investigated.However,no study to date has systematically reviewed the experimental growth factors investigated for spinal fusion in preclinical animal models.Considering the efficacy and widespread use of recombinant growth factors (i.e.,rhBMP-2 and rhPTH) to optimize spinal fusion,a broad assessment of experimental growth factors is essential to inform future work and clinical potential in this area[9,12].The present study aims to systematically review all published translational animal models assessing investigational growth factors for spinal fusion and identify promising agents for translation.
MATERIALS AND METHODS
Electronic literature search
A systematic review of the literature using PubMed,Embase,Cochrane Library,and Web of Science databases was performed with searches run on May 29th,2018,along with a review of the bibliographies of the examined articles.The search query was designed to include all non-human,preclinical animal spinal fusion models reported in the literature without a timespan limit (Table 1).PRISMA guidelines were followed for this systematic review[13].
Inclusion/Exclusion criteria
Inclusion criteria were original studies involving the implantation/administration of one or more identifiable,quantifiable,experimental growth factors (i.e.,not BMPs or PTH) in an animal model of spinal fusion in the English language.Growth factors were defined as peptide-based molecules that function to regulate cell division/survival.
Our exclusion criteria were studies that involved the implantation of (1) scaffolds without growth factors;(2) BMPs or PTH;(3) non-peptide-based agents;and (4) cells,platelet-rich plasma,or other processed blood products that could confound effects of the growth factors.
All potentially eligible studies meeting the inclusion criteria were determined by 2 reviewers (Cottrill E and Lessing N).A third reviewer (Ahmed AK) served as a referee,resolving any discrepancies.Articles that met predetermined criteria for exclusion were not included in the study.
Data extraction
Extracted data for each animal model included surgical approach (e.g.,posterolateral or anterior),level of fusion,animal species and breed,animal age and sex,and any other relevant characteristics of the animals (e.g.,ovariectomized or genetically mutated).For each animal group studied,dosages/sizes of all implant materials,including growth factor and scaffold,were recorded.Spinal fusion rates as assessed by manual palpation,the “gold standard” technique[14,15],or alternatively other methods (e.g.,micro- computed tomography (CT),plain radiographs,and histological analysis),were extracted,along with the associated follow-up time points.
RESULTS
The literature search identified 4806 total articles.Following the predetermined exclusion criteria,26 articles assessing experimental growth factors in vertebrate animal models of spinal fusion were included in this review (Figure 1).Among the included studies,14 experimental growth factors have been described:AB204 (n= 1);angiopoietin 1 (n= 1);calcitonin (n= 3);erythropoietin (EPO) (n= 1);basic fibroblast growth factor (bFGF) (n= 1);growth differentiation factor 5 (GDF-5) (n= 4),combined insulin-like growth factor 1 (IGF-1) + transforming growth factor beta (TGF- β) (n= 4);insulin (n= 1);NELL-1 (n= 5);noggin (n= 1);P-15 (n= 1);peptide B2A (n= 2);and secreted phosphoprotein 24 (SPP24) (n= 1).Descriptions of the growth factors are provided in Table 2[16-31].The demographic characteristics for all included studies are provided in Table 3,and the rates of spinal fusion for all experimental groups are summarized in Table 4.Detailed information of each study is provided in Supplemental Table 1.All the included studies were preclinical animal studies (level of evidence of V).

Table1 Search terms across 4 databases to identify experimental growth factors in animal models of spinal fusion
AB204
Zhenget al[32]compared the fusion rates between AB204 and rhBMP-2 in a beagle posterolateral lumbar (L1-L2 and L4-L5) model.Investigating biphasic calcium phosphate (BCP),rhBMP-2 + BCP,and AB204 + BCP,they reported that the AB204 group showed a significantly higher fusion rate (90%) compared to the rhBMP-2 group (15%) and the BCP-only group (6.3%) as assessed by manual palpation at 8 wk postoperatively.
Angiopoietin 1
Parket al[33]investigated the effects of COMP-Ang-1 on spinal fusion in a Sprague-Dawley rat posterolateral lumbar (L3-L5) model.Investigating iliac bone allograft(Allo),bovine serum albumin (BSA)-impregnated absorbable collagen sponge + Allo,and COMP-Ang-1-impregnated absorbable collagen sponge + Allo,they reported that the COMP-Ang-1 group showed a significantly higher fusion rate (89.5%) compared to the BSA group (42.1%) and the Allo-only group (38.9%) as assessed by manual palpation at 6 wk postoperatively.
Calcitonin
Babatet al[34]investigated the effects of calcitonin (postoperatively) and pamidronate(pre- and postoperatively) on spinal fusion in a New Zealand White rabbit posterolateral lumbar (L5-L6) model.Fusion rates were determined for each treatment group:autologous iliac crest bone graft (autograft) alone (56%),autograft + calcitonin(68%),and autograft + pamidronate (37%).Fisher exact test showed no significant differences between groups at 5 wk postoperatively.
In addition,Liuet al[35]investigated the effect of daily post-operative calcitonin administration on spinal fusion in a New Zealand White rabbit posterolateral lumbar(L4-5,without wire fixation of the spinous processes;and L6-L7,with wire fixation of the spinous processes) model.With both fixation and without fixation,the bone grafts receiving calcitonin had a higher fusion rate (100%vs75%),higher histological score,and increased expression of pro-osteogenic and pro-angiogenic genes [i.e.,Col I and BMP-2,IGF-1 and vascular endothelial growth factor (VEGF)] at 8 wk postoperatively.
Additionally,Liuet al[36]investigated the effects of calcitonin (postoperatively) on spinal fusion in an ovariectomized/normal Sprague-Dawley posterolateral lumbar(L4-5,with wire fixation of spinous processes) model.They found significantly enhanced fusion mass,bone mineral density,and microstructural parameters in calcitonin-treated ovariectomized animals at 12 wk postoperatively compared to nontreated ovariectomized animals as assessedviaradiographs,micro-CT,and histologic analysis.Fusion rate was not reported.
EPO
Rolfinget al[37]investigated the effects of EPO (daily subcutaneous injection) on spinal fusion in a New Zealand White rabbit posterolateral lumbar (L5-L6) fusion model.At 6 wk postoperatively,the fusion rate was 86% in the EPO treated group + autograft and 71% in the autograft-alone group as assessedviamanual palpation.Additionally,the bone fusion volume (micro-CT) and angiogenesis (actin stained blood vessels)were both significantly greater in the EPO treatment group.

Figure1 Consolidated standards of reporting trials diagram for article selection.GF:Growth factor.
bFGF
Inoueet al[38]investigated the effects of an engineered bFGF on spinal fusion in a Sprague-Dawley rat posterolateral lumbar (L4-L5) model.Two fusion groups were studied:Femoral freeze-dried bone allograft incubated with an engineered bFGF or with phosphate buffered saline.They found that the bFGF group had a significantly higher mean grafted bone volume (radiography) as well as significantly greater new bone formation on the surface of the laminae and spinous processes (micro-CT)compared to the control group 14 d postoperatively.Fusion rate was not reported.
GDF-5
Spiroet al[39]investigated the effects of rh-GDF-5 on spinal fusion in a female baboon posterolateral lumbar (L4-L5) model.They investigated four groups:Healos?with or without rh-GDF-5 or iliac crest autograft.The fusion rate,as assessedviaradiographic/CT analysis,was 44% for the 500 micrograms rh-GDF-5/cm3Healos?group,11% for the 1500 micrograms rh-GDF-5/cm3Healos?group,22% for the autograft group,and 0% for the Healos?alone group at 20 wk postoperatively.
In addition,Spiroet al[40]investigated the effects of rh-GDF-5 in several different formulations with collagen matrices in a New Zealand White rabbit posterolateral lumbar (L5-L6) model.They found that rh-GDF-5 added to non-crosslinked mineralized Type I bovine collagen strips resulted in a fusion rate,as assessed histologically,of 75% at a concentration of 0.1 mg growth factor/cm3collagen and 80% at a concentration of 1.0 mg growth factor/cm3collagen at 12 wk postoperatively.This compared to 33% for iliac crest autograft,0% for the collagen strips alone,and 0%for the collagen strips with bone marrow aspirate from the iliac crest.
Additionally,Jahnget al[41]investigated the effects of Healos?with rh-GDF-5 in a sheep endoscopic instrumented (pedicle screws and plate) posterolateral lumbar (L4-L5) model.They found that at 4 and 6 mo 100% fusion was observed in both autograft and bone graft substitute groups,and no significant differences were observed between the groupsviahistological assessment,including the formation of vascular elements.
Magitet al[42]investigated the effects of rh-GDF-5 in New Zealand White rabbitposterolateral lumbar (L5-L6) model.The authors found that fusion rates,as assessed via manual palpation,were 38% in the autograft group,0% in the Healos?alone group,and 100% in each of the experimental Healos?/rhGDF-5 groups at 8 wk of follow-up.Further,viamicro-CT analysis,bone formation in the experimental rhGDF-5 groups were observed to be significantly greater than in the other study groups.

Table2 Normal biological activity of each included growth factor
IGF-1/TGF- β
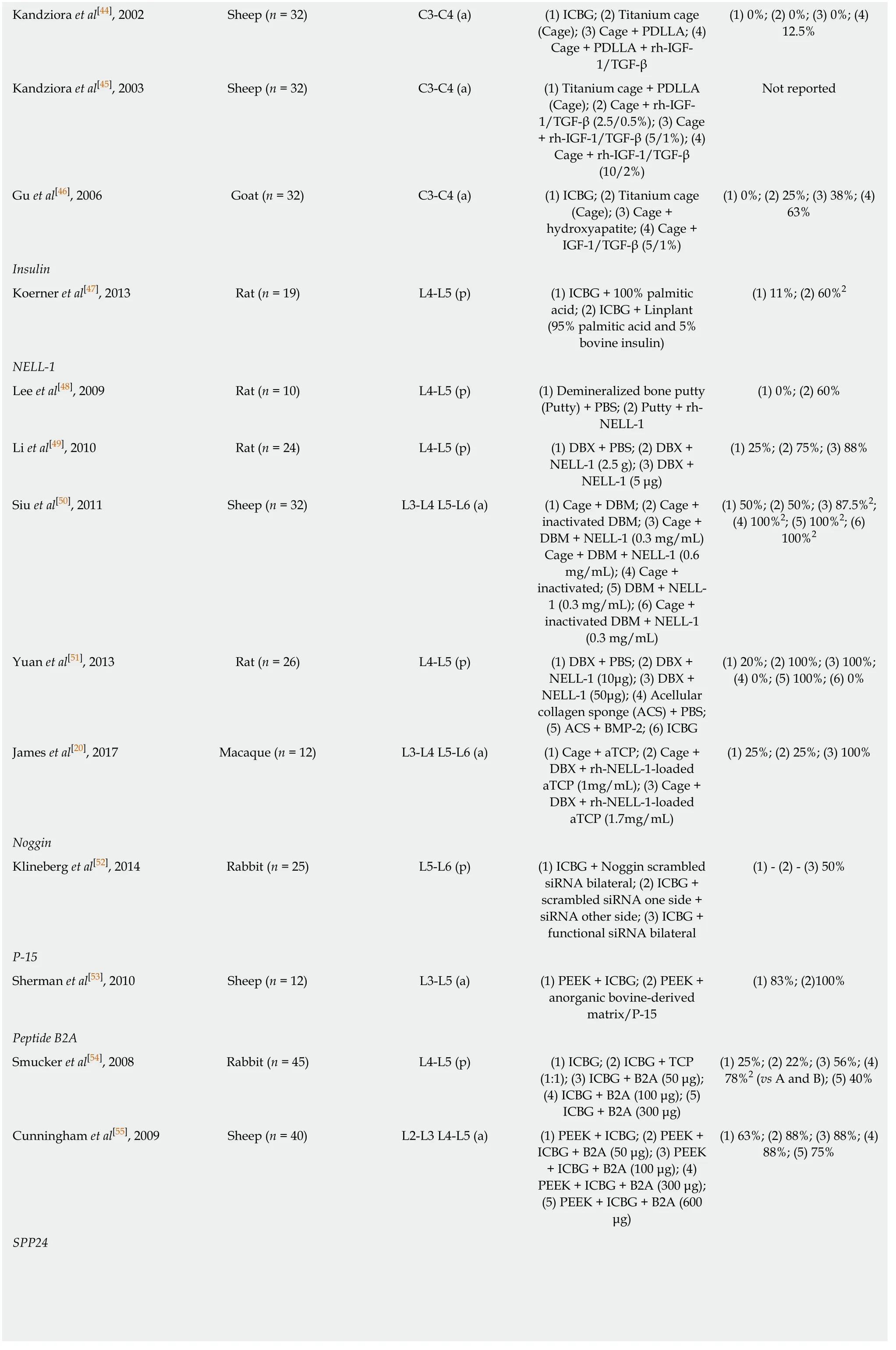
Kandzioraet al[43]investigated the effects of combined IGF-1/TGF-beta-1 on spinal fusion in a sheep anterolateral cervical (C3/4) interbody model.They investigated using a titanium cage alone,titanium cage filled with autologous iliac crest bone graft,titanium cage coated with a biodegradable poly-(D,L-lactide) (PDLLA) carrier including rh-BMP-2,and titanium cage coated with a biodegradable PDLLA carrier including rh-IGF-1 (5% w/w) and rh-TGF-beta-1 (1% w/w).As assessedviaCT,the BMP-2 and IGF-1/TGF-beta-1 groups led to intervertebral masses with a maximum intervertebral gap in the craniocaudal direction of less than 5 mm or complete fusion in 75% of animals,compared to 50% for the autograft group and 25% for the cagealone group at 12-wk postoperatively.
In addition,Kandzioraet al[44]investigated the effects of IGF-1/TGF-beta-1 in a sheep anterolateral cervical (C3-C4) interbody model using autologous tricortical iliac crest bone graft,a titanium cage alone,titanium cage with a PDLLA carrier,and a titanium cage with a PDLLA carrier including IGF-F and TGF-beta-1.The authors observed a fusion rate of 0% in the autograft,cage-alone,and cage plus PDLLA groups and 12.5% in the IGF-1/TGF-beta-1 group at 12 weeks postoperatively.
Additionally,Kandzioraet al[45]investigated the effects of IGF-1/TGF-beta-1 in asheep anterolateral (C3-C4) interbody model using a titanium cage coated with a PDLLA carrier including no growth factors,as well as with different concentrations of IGF-1 and TGF-beta-1.They authors concluded that the application of IGF-1 and TGFbeta-1 by a PDLLA-coated cage significantly improves interbody bone formation in a dose dependent manner,as assessed via micro-CT and histomorphometrical analysis,at 12 wk postoperatively.Fusion rate was not reported.

Table3 Demographic characteristics for all included studies
Further,Guet al[46]investigated the effects of IGF-1 and TGF-beta-1 in a goat cervical (C3-C4) interbody model.Four groups were studied:autologous iliac crest bone graft,a hat-shaped titanium cage,a hat-shaped cage coated with hydroxyapatite,and a hat-shaped cage coated with hydroxyapatite plus IGF-1 and TGF-beta-1.As assessedviahistomorphologic examination,the IGF-1 and TGF-beta-1 group led to fusion in 63% of animals,compared to 38% for the hydroxyapatite group,25% for the cage alone group,and 0% for the autograft group at 12-wk postoperatively.
Insulin
Koerneret al[47]investigated the effects of time-released insulin on spinal fusion in a Sprague-Dawley posterolateral lumbar (L4-L5) model.The authors used iliac crest autograft plus either Linplant (95% micro-recrystallized palmitic acid and 5% bovine insulin) or a sham implant (100% palmitic acid).At 8 wk postoperatively,the fusion rate was 60% in the Linplant group compared to 11% in the control group,as assessed by manual palpation.In addition,half the animals in each group were euthanized on postoperative day 4 and analyzed for growth factors:IGF-I (but not TGF-beta-1,PDGF-AB,or VEGF) was significantly higher in the Linplant group.
Neural EGFL Like 1 (NELL-1)
Leeet al[48]investigated the effects of NELL-1 on spinal fusion in a male athymic ratposterolateral lumbar (L4-L5) model.They found that rh-NELL-1 lyophilized onto apatite-coated alginate/chitosan microparticles and mixed with demineralized bone matrix (DBM) led to a fusion rate of 60% at 4 wk postoperatively,compared to a fusion rate of 0% using PBS instead of rh-NELL-1.

Table4 Experimental models and rates of bony fusion for each included study
Kandziora et al[44],2002 Sheep (n = 32) C3-C4 (a) (1) ICBG;(2) Titanium cage(Cage);(3) Cage + PDLLA;(4)Cage + PDLLA + rh-IGF-1/TGF-β(1) 0%;(2) 0%;(3) 0%;(4)12.5%Kandziora et al[45],2003 Sheep (n = 32) C3-C4 (a) (1) Titanium cage + PDLLA(Cage);(2) Cage + rh-IGF-1/TGF-β (2.5/0.5%);(3) Cage+ rh-IGF-1/TGF-β (5/1%);(4)Cage + rh-IGF-1/TGF-β(10/2%)Not reported Gu et al[46],2006 Goat (n = 32) C3-C4 (a) (1) ICBG;(2) Titanium cage(Cage);(3) Cage +hydroxyapatite;(4) Cage +IGF-1/TGF-β (5/1%)(1) 0%;(2) 25%;(3) 38%;(4)63%Insulin Koerner et al[47],2013 Rat (n = 19) L4-L5 (p) (1) ICBG + 100% palmitic acid;(2) ICBG + Linplant(95% palmitic acid and 5%bovine insulin)(1) 11%;(2) 60%2 NELL-1 Lee et al[48],2009 Rat (n = 10) L4-L5 (p) (1) Demineralized bone putty(Putty) + PBS;(2) Putty + rh-NELL-1(1) 0%;(2) 60%Li et al[49],2010 Rat (n = 24) L4-L5 (p) (1) DBX + PBS;(2) DBX +NELL-1 (2.5 g);(3) DBX +NELL-1 (5 μg)(1) 25%;(2) 75%;(3) 88%Siu et al[50],2011 Sheep (n = 32) L3-L4 L5-L6 (a) (1) Cage + DBM;(2) Cage +inactivated DBM;(3) Cage +DBM + NELL-1 (0.3 mg/mL)Cage + DBM + NELL-1 (0.6 mg/mL);(4) Cage +inactivated;(5) DBM + NELL-1 (0.3 mg/mL);(6) Cage +inactivated DBM + NELL-1(0.3 mg/mL)(1) 50%;(2) 50%;(3) 87.5%2;(4) 100%2;(5) 100%2;(6)100%2 Yuan et al[51],2013 Rat (n = 26) L4-L5 (p) (1) DBX + PBS;(2) DBX +NELL-1 (10μg);(3) DBX +NELL-1 (50μg);(4) Acellular collagen sponge (ACS) + PBS;(5) ACS + BMP-2;(6) ICBG(1) 20%;(2) 100%;(3) 100%;(4) 0%;(5) 100%;(6) 0%James et al[20],2017 Macaque (n = 12) L3-L4 L5-L6 (a) (1) Cage + aTCP;(2) Cage +DBX + rh-NELL-1-loaded aTCP (1mg/mL);(3) Cage +DBX + rh-NELL-1-loaded aTCP (1.7mg/mL)(1) 25%;(2) 25%;(3) 100%Noggin Klineberg et al[52],2014 Rabbit (n = 25) L5-L6 (p) (1) ICBG + Noggin scrambled siRNA bilateral;(2) ICBG +scrambled siRNA one side +siRNA other side;(3) ICBG +functional siRNA bilateral(1) - (2) - (3) 50%P-15 Sherman et al[53],2010 Sheep (n = 12) L3-L5 (a) (1) PEEK + ICBG;(2) PEEK +anorganic bovine-derived matrix/P-15(1) 83%;(2)100%Peptide B2A Smucker et al[54],2008 Rabbit (n = 45) L4-L5 (p) (1) ICBG;(2) ICBG + TCP(1:1);(3) ICBG + B2A (50 μg);(4) ICBG + B2A (100 μg);(5)ICBG + B2A (300 μg)(1) 25%;(2) 22%;(3) 56%;(4)78%2 (vs A and B);(5) 40%Cunningham et al[55],2009 Sheep (n = 40) L2-L3 L4-L5 (a) (1) PEEK + ICBG;(2) PEEK +ICBG + B2A (50 μg);(3) PEEK+ ICBG + B2A (100 μg);(4)PEEK + ICBG + B2A (300 μg);(5) PEEK + ICBG + B2A (600 μg)(1) 63%;(2) 88%;(3) 88%;(4)88%;(5) 75%SPP24

1(a/p):anterior/posterolateral surgical approach;N2:Statistically significant compared to all other experimental groups.ICBG:Iliac crest bone graft.BCP:Biphasic calcium phosphate;rhBMP-2:Recombinant human bone morphogenetic protein 2;bFGF:Basic fibroblast growth factor;GDF-5:Growth differentiation factor 5;PDLLA:Poly-D,L-lactide;TGF:Transforming growth factor;IGF:Insulin-like growth factor.
Similarly,Liet al[49]investigated the effects of NELL-1 on spinal fusion in an athymic rat posterolateral lumbar (L4-5) model.The authors found that NELL-1 lyophilized onto β-tricalcium phosphate (TCP) microparticles and mixed with DBX (a type of DBM) led to a fusion rate of 75% (2.5 micrograms NELL-1) and 88% (5 micrograms NELL-1) at 4 wk postoperatively,compared to a fusion rate of 25% using PBS instead of NELL-1.
Siuet al[50]investigated the effects of NELL-1 on spinal fusion in a skeletally mature Rambouillet × Columbian ewe posterolateral lumbar (L3-L4 and L5-L6) model.Six groups with different implant compositions were studied:DBM alone or mixed with NELL-1 (0.3 or 0.6 mg/mL) and heat-inactivated DBM (inDBM) alone or mixed with NELL-1 (0.3 or 0.6 mg/mL).At 3 mo postoperatively,the fusion rates were 88% for DBM + 0.3 mg/mL NELL-1,100% for DBM + 0.6 mg/mL NELL-1,and 50% for DBM alone.At 4 mo postoperatively,the fusion rates were 100% for inDBM + 0.3 mg/mL NELL-1,100% for inDBM + 0.6mg/mL NELL-1,and 50% for inDBM alone.
Yuanet al[51]investigated the effects of NELL-1 in a male athymic rat posterolateral lumbar (L4-5) model.They found that DBX mixed with NELL-1 (10 or 50 microgram)led to a 100% fusion rate at 4 wk postoperatively,compared to fusion rates of 20%using PBS instead of NELL-1,100% using an acellular collagen sponge and BMP-2 (90 micrograms),and 0% using iliac crest autograft.
Jameset al[20]investigated the effects of NELL-1 on spinal fusion in a 5- to 7-year-old Rhesus macaque posterolateral lumbar (L3-L4 and L5-L6) model.Three groups were studied:intervertebral cage plus DBX mixed with saline- or rh-NELL-1-(1.0 or 1.7 mg/mL) loaded apatite-coated β-tricalcium phosphate (aTCP) particles.At 4 mo postoperatively,fusion rates as assessedviaCT were 100% for the higher dose of rh-NELL-1,25% for the lower dose of rh-NELL-1,and 25% for the saline control.Additionally,immunofluorescence staining showed increased Sca-1+CD31-CD45-stromal cells in the rh-NELL-1 treated groups compared to the saline control.
Noggin
Klineberget al[52]investigated the effects of noggin on spinal fusion in a skeletally mature New Zealand White rabbit posterolateral lumbar (L5-L6).Noggin siRNA was injected into the paraspinal muscles to interrupt the negative feedback loop on endogenous BMP.Autologous iliac crest bone graft with paraspinal injections of either scrambled (non-functional) siRNA bilaterally,scrambled siRNA on one side of the spine and functional noggin siRNA on the other,or functional noggin siRNA bilaterally were studied.As assessed via manual palpation,the fusion rate of the bilateral functional siRNA group was 50%,which was not significantly different compared to historical autograft-only controls from the group,despite the fact that noggin protein was successfully knocked downin vivofor the initial 7 days before returning to normal levels by 6 wk.
P-15
Shermanet al[53]investigated the effects of an organic bovine-derived hydroxyapatite matrix combined with a synthetic 15 amino acid residue (ABM/P-15) on spinal fusion in a skeletally mature ewe anterolateral lumbar (L3-L5) model.Sheep were treated with polyetheretherketone (PEEK) interbody rings filled with autologous iliac crest bone graft at one level and AMB/P-15 formulated in a carboxymethylcellulose hydrogel matrix at the other.At 6 mo postoperatively,100% of fusion sites in both groups achieved successful bony arthrodesis as assessedviaCT,and histomorphometric analysis showed no statistically significant differences in the fusion masses between these groups.
Peptide B2A
Smuckeret al[54]investigated the effects of B2A on spinal fusion in a skeletally mature New Zealand White rabbit posterolateral lumbar (L4-L5) model.The authors investigated iliac crest autograft alone and 1:1 mixtures of autograft and B2A-coated ceramic granules (CG) (0,50,100,and 300 μg B2A/mL CG).As assessedviamanual palpation at 6 wk postoperatively,the fusion rates were 25% for the autograft alone group and 22%,56%,78%,and 40% for the 0,50,100,and 300 μg B2A/mL CG groups,respectively.The newly formed bone in the B2A-treated groups appeared morphologically normal without hyperplasia.
Cunninghamet al[55]investigated the effects of B2A on spinal fusion in a 3- to 6-year-old crossbred Suffolk sheep anterolateral lumbar (L2-L3 and L4-L5) model.The sheep were treated with a PEEK interbody cage packed with 1:1 mixtures of autograft and B2A-coated ceramic granules (CG) (0,50,300,or 600 μg B2A/mL CG).As assessedviaCT at 4 mo postoperatively,the fusion rates were 63%,88%,88%,and 75% for the 0,50,300,and 600 μg B2A/mL CG groups,respectively.In biomechanical testing,no statistically significant differences were observed between any of the groups.
SPP24
Sintuuet al[56]investigated the effects of SPP24 on spinal fusion in a 6- to 8-wk-old male Lewis rat posterolateral lumbar (L3-L4) model.Bilaterally placed implant materials consisted of collagen sponges soaked in high or low dose rh-BMP-2 (1 or 10 micrograms),plus treatment:0,0.1,0.5,1.0,or 2.5 mg of either full-length SPP24 or truncated SPP18 (solely the BMP-binding region of the full peptide).As assessedviamanual palpation at 8 wk postoperatively,the fusion rate was 0% in all specimens treated with rh-BMP-2 and TR-spp18 or FL-spp24 (any concentration),compared to a fusion rate of 100% in specimens treated with rh-BMP-2 (10 micrograms) alone.Further,FL-spp24 showed a greater inhibitory impact compared to TR-spp18.
DISCUSSION
Pseudoarthrosis following spinal surgery can lead to significant patient morbidity and diminished quality of life,with unpredictable clinical outcomes following revision surgery.Optimizing the rate of spinal fusion relies on enhanced surgical technique,effective biologics (e.g.,growth factors),instrumentation,and a greater appreciation of the local physiology[57].Following FDA approval in 2002 for use in the anterior lumbar spine,rhBMP-2 revolutionized the role for growth factor adjuncts in spinal fusion[58,59],drastically increasing in use from 5.5% of all fusion cases in 2003 to 28.1% of all fusion cases in 2008 in the United States[60].However,rhBMP-2 is associated with significant complications[6,7],which has fueled the investigation of different growth factors for spinal fusion.Despite significant research interest in this area,there are no published systematic reviews summarizing the state of the art in experimental growth factors for spinal fusion.
The present systematic review,across 4 databases,resulted in the inclusion of 26 spinal fusion animal studies comprising 14 investigational growth factors.The fusion rates of the current gold standard treatment (autologous iliac crest bone graft,ICBG)and the leading clinically used growth factor (BMP-2) ranged widely in the included studies,from 0-100% for ICBG[44,46,51]and from 13%-100% for BMP-2[43,51,56].This variation reflects the unpredictable clinical outcomes following spinal fusion surgery and supports the need for efficacious materials that promote strong and reliable spinal fusion.Among the experimental growth factors,four resulted in fusion rates of 100% in some cases (Table 4):calcitonin[35],GDF-5[41,42],NELL-1[20,50,51],and P-15[53](Table 4).In addition,six growth factors resulted in significantly enhanced fusion rates compared to ICBG,BMP-2,or other internal control in some studies (Table 4):AB204vsBMP-2[32],COMP-Ang-1vsICBG[33],GDF-5vsICBG[42],insulin (as Linplant)vsinternal control (ICBG plus sham implant)[47],NELL-1vsinternal control (DBM)[50],and Peptide B2AvsICBG[54].The majority of other identified growth factors resulted in fusion rates similar to ICBG (Table 4);only SPP24 was shown to significantly decrease the rate of spinal fusion[56].Directly comparing different growth factors herein is difficult given the extensive heterogeneity in animal species,fusion method,and experimental groups and timepoints across the studies (Tables 3 and 4).Further,it is known that the scaffolds themselves affect bone formation[61,62],possibly confounding the effects of the growth factors across studies.
In addition,similar effects on spinal fusion were generally,though not always,observed when multiple studies investigated the same growth factor.For example,several groups investigated the effects of NELL-1 on spinal fusion[20,48-51].In all these experiments,NELL-1 was shown to enhance fusion rates.In contrast,for GDF-5,Magitet al[42]observed a significantly enhanced fusion rate compared to ICBG,while Jahnget al[41]observed a 100% fusion rate for both GDF-5 and ICBG groups,and Spiroet al[39]observed a non-significant decrease in fusion rate using GDF-5 (1500 micrograms/cm3) compared to ICBG.The differences in animal species (rabbit,sheep,and baboon) and surgical method (endoscopic with instrumentation and posterolateral without instrumentation) may help to account for these variations.
The successful clinical translation of any factor intended to enhance spinal fusion will depend not only on its capacity to promote strong and reliable spinal fusion in humans,but also on its safety profile (i.e.,the associated local and systemic complications).At present,the growth factors AB204,COMP-Ang-1,GDF-5,NELL-1,P-15,insulin,and Peptide B2A represent some of the most promising investigational growth factors for promoting spinal fusion,with each demonstrating fusion efficacy in preclinical studies.However,the safety profiles of these growth factors in the setting of spinal fusion are largely unknown.In our review,none of the included studies reported complications directly related to the growth factors,though this absence of evidence obviously does not mean the absence of complications,any of which could hinder or halt clinical translation.Future work investigating the efficacy and safety of these growth factors not only in larger numbers of animals but also in higher-order species will be important for informing their potential clinical translation.
Interestingly,this systematic review found that,within the inclusion/exclusion criteria of our study,relatively few (i.e.,fourteen) unique growth factors have been investigated in preclinical animal models of spinal fusion.This reveals that a relatively select group of growth factors in the overall setting of bone tissue engineering has been investigated in spinal fusion.For example,growth factors like stromal-derived growth factor 1 (SDF-1) and platelet derived growth factor (PDGF),both of which have been studied in the setting of regenerating critical sized bone defects[63,64],are notably absent in the preclinical spine fusion literature.While tissue engineering for spinal fusion is unique from other areas of bone tissue engineering in that the fusion site may be in motion during the fusion process,our review suggests potential new research strategies regarding the investigation of currently unexplored growth factors (e.g.,SDF-1 and PDGF) for spinal fusion.Lastly,it is notable that relatively few studies involved combinations of growth factors[43-46].We believe that the simultaneous or sequential delivery of multiple different growth factors may result in a synergistic enhancement in spinal fusion.We encourage future work in these areas,as well as in continued advancements in growth factor delivery methods and scaffold materials,towards the development of efficacious and safe,clinically translatable materials for spinal fusion.
Schimandleet al[65]in 1994,Sandhuet al[66]in 2002,and Drespeet al[67]in 2005 previously published reviews of animal models for spinal fusion.These reviews focused on the different species utilized,technical methodology,and representative outcomes.To the best of our knowledge,the present study is the first to systematically review investigational growth factors utilized in animal models of spinal fusion.Despite the novelty of this review,there are several limitations,including those inherent to systematic reviews.Additionally,many of the animal models differ with regard to the methodology and data collected (Tables 3 and 4).As a result of this heterogeneity,directly comparing end points (i.e.,rates of fusion) across multiple studies is not possible.Further,three studies did not report fusion rates[36,38,45],limiting the interpretability of those studies.In addition,our review excludes growth factors that have been studied clinically in spinal fusion.
In conclusion,this is the first study to systematically review all the published investigational growth factors utilized in preclinical animal models for spinal fusion.Future studies aimed at directly comparing the most promising experimental growth factors identified herein -e.g.,AB204,COMP-Ang-1,GDF-5,NELL-1,P-15,insulin,Peptide B2A,and others (Table 4) - in preclinical models may inform the development of efficacious,clinically translatable materials for spinal fusion.Further,future work involving the safety and cost of production of these growth factors,in comparison to BMP-2,may support the replacement of BMP-2 for safer and more cost-effective growth factors for spinal fusion.
ARTICLE HIGHLIGHTS
Research background
Over 400000 Americans undergo spinal fusion surgeries each year,with the number increasing yearly alongside a growing and aging population.However,pseudoarthrosis,or failed fusion,rates are reported to be as high as 40% in primary spinal fusion surgery and up to 60% in revision cases,even when the "gold standard" treatment of grafting bone from the patient’s own iliac crest is used.
Research motivation
To date,no study has systematically reviewed the experimental growth factors investigated for spinal fusion in preclinical animal models.Considering the efficacy and widespread use of recombinant growth factors (i.e.,rhBMP-2 and rhPTH) to optimize spinal fusion,a broad assessment of experimental growth factors is essential to inform future work and clinical potential in this area.
Research objectives
Systematically review all published translational animal models assessing investigational growth factors for spinal fusion and identify promising agents for translation.
Research methods
A systematic review of the literature using PubMed,Embase,Cochrane Library,and Web of Science databases was performed.Inclusion criteria were original studies involving the implantation/administration of one or more identifiable,quantifiable,experimental growth factors (i.e.,not BMPs or PTH) in an animal model of spinal fusion in the English language.Exclusion criteria were studies that involved the implantation of (1) scaffolds without growth factors;(2) BMPs or PTH,(3) non-peptide-based agents,and (4) cells,platelet-rich plasma,or other processed blood products that could confound effects of the growth factors.PRISMA guidelines were followed for this systematic review.
Research results
The literature search identified 4806 total articles,from which 26 articles met the inclusion/exclusion criteria and were included in this review.Among the included studies,14 experimental growth factors were identified:AB204 (n= 1);angiopoietin 1 (n= 1);calcitonin (n=3);erythropoietin (n= 1);basic fibroblast growth factor (n= 1);growth differentiation factor 5 (n= 4),combined insulin-like growth factor 1 + transforming growth factor beta (n= 4);insulin (n=1);NELL-1 (n= 5);noggin (n= 1);P-15 (n= 1);peptide B2A (n= 2);and secreted phosphoprotein 24 (n= 1).Among the identified growth factors,calcitonin,GDF-5,NELL-1,and P-15 resulted in fusion rates of 100% in some cases.In addition,six growth factors - AB204,angiopoietin 1,GDF-5,insulin,NELL-1,and peptide B2A - resulted in significantly enhanced fusion rates compared to ICBG,BMP-2,or other internal control in some studies.Large heterogeneity in animal species,fusion method,and experimental groups and time points was observed across the included studies,limiting the direct comparison of the growth factors identified herein.
Research conclusions
This is the first study to systematically review all the published investigational growth factors utilized in preclinical animal models of spinal fusion.Future studies aimed at directly comparing the most promising investigational growth factors identified herein -e.g.,AB204,COMP-Ang-1,GDF-5,NELL-1,P-15,insulin,Peptide B2A,and others - in preclinical models may inform the development of efficacious and safe,clinically translatable materials for spinal fusion.
Research perspectives
The successful clinical translation of any factor intended to enhance spinal fusion will depend not only on its capacity to promote strong and reliable spinal fusion in humans,but also on its safety profile.Our study reveals that relatively few growth factors and delivery strategies in the overall setting of bone tissue engineering have been investigated in spinal fusion.We encourage future investigation of currently unexplored growth factors for spinal fusion,as well as continued advancements in growth factor delivery methods and scaffold materials,towards the development of efficacious and safe,clinically translatable materials for spinal fusion.
ACKNOWLEDGEMENTS
We would like to thank Ms.Carrie Price,MLS,for technical assistance with the literature search.
 World Journal of Orthopedics2019年4期
World Journal of Orthopedics2019年4期
- World Journal of Orthopedics的其它文章
- Recalcitrant distal humeral non-union following previous Leiomyosarcoma excision treated with retainment of a radiated nonangiogenic segment augmented with 20 cm free fibula composite graft:A case report
- Fracture of allograft interbody spacer resulting in post-operative radiculopathy:A case report
- Growing pains:What do we know about etiology? A systematic review
