Protective roles of trehalose in Pleurotus pulmonarius during heat stress response
LlU Xiu-ming, WU Xiang-li, GAO Wei, QU Ji-bin, CHEN Qiang, HUANG Chen-yang, ZHANG Jin-xia
Key Laboratory of Microbial Resources, Ministry of Agriculture and Rural Affaris/Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, P.R.China
Abstract High temperature is one of the major abiotic stresses that limit edible mushroom growth and development. The understanding of physiological alterations in response to heat stress and the corresponding mechanisms involved is vital for the breeding of heat-resistant edible mushroom strains. Although trehalose functions as a protectant against abiotic stresses in fungi,the putative role of trehalose in thermotolerance remains to be elucidated. In this study, we found heat stress inhibited the growth of two Pleurotus pulmonarius strains, heat-sensitive and less-sensitive, and the inhibition was more significant for the sensitive strain. Heat stress leads to the increase of lipid peroxidation and intracellular trehalose accumulation, with a higher level in the heat-sensitive strain, and this effect is independent of exogenous trehalose application. In addition, a lower concentration of exogenous trehalose application in sensitive strain than in less-sensitive strain was found to alleviate the inhibition of mycelium growth and further increase the intracellular trehalose concentration by heat stress. Thus, the protective effects of trehalose were more remarkable in the sensitive strain. The activities of intracellular trehalose metabolic enzymes, i.e., trehalose-6-phosphate synthase, trehalose phosphorylase and neutral trehalase, were determined, and our data indicated that the changes of these enzymes activities in the sensitive strain were more bene ficial to accumulate trehalose than that in the less-sensitive strain.
Keywords: edible mushroom, heat stress, Pleurotus pulmonarius, thermotolerance, trehalose
1. lntroduction
Temperature is an important environmental factor that in fluences edible mushrooms mycelial growth, fruiting formation, spore and spawn production (Chang and Miles 2004; Kashangura 2008; Hoa and Wang 2015; Zhang R Yet al. 2016). Many physiological and biochemical injury cases could be involved in heat stress. For example,heat stress increases anaerobic respiration (Zhang R Yet al. 2016), activates apoptosis-like cell death (Songet al. 2014) and damages proteins and cell membranes(Ancín-Azpilicuetaet al. 2012). Accumulation or exogenous application of osmoprotectants, such as proline, glycine betaine (Asthir 2015) and trehalose (Ribeiroet al. 1999;Luoet al. 2008; Viannaet al. 2008; Konget al. 2012a) have been found effective in mitigating heat stress.
Trehalose, a nonreducing disaccharide of glucose, which plays an important physiological role not only as a reserve carbohydrate, but also a thermoprotectant in yeasts and many other organisms (Elbeinet al. 2003; Gancedo and Flores 2004). Trehalose protects proteins and cellular membranes from inactivation or denaturation under different stress conditions (Elbeinet al. 2003). Mahmudet al. (2010)and Caoet al. (2014) thought that an increase in trehalose content was correlated with the increase in stress tolerance inSaccharomyces cerevisiae. However, different strains of the same edible mushroom species have different sensitivities to heat stress in production, whether this is due to the difference in the synthesis of trehalose is unclear.
Pleurotuspulmonarius(Fries) Quélet also known asP.sajor-caju(Fr.) Singer (Corrêaet al. 2016), one of the most importantPleurotusspecies, has been rapidly increasing in production worldwidely because of its broad temperatural adaptability during the growth of both mycelia and fruiting body from 10 to 32°C, ease of cultivation with high yield potential, and high nutritional value (Rajarathnam and Bano 1987; Li and Yao 2005; Ruanet al. 2005).However, the cultivationofP.pulmonariusis always affected by high temperaute in China and poor tropical countries,where the traditional non-industrial cultivation mode without temperature-controlled system is commonly used.
Two representativeP.pulmonariusstrains, which are widely cultivated in China and showed different tolerance to high enviromental temperatures during cultivation were used as materials in the present study. The possible roles of trehalose in the heat resistance were investigated through comparison and analysis of effect of exogenous trehalose,the relationship between trehalose biosynthesis and thermotolerance. This information will also provide the basis for studing the heat-tolerance mechanism of other fungi.
2. Materials and methods
2.1. Strains and growth conditions
TheP.pulmonariusstrains (CCMSSC 00494 and CCMSSC 00499) were provided by the China Center for Mushroom Spawn Standards and Control (CCMSSC, Beijing, China).The mycelia were grown on PDA (DifcoTMPotato Dextrose Agar, Becton, Dickinson and Company, USA) plates for 6 days at the optimal temperature (28°C). For further studies,mycelial dishes (diam. 5 mm) were removed with a cork borer from marginal growth of fresh PDA cultures and placed with the mycelial side down on the center of the Petri dish(diam. 90 mm) containing 25 mL medium.
2.2. The in fluences of temperatures on the mycial growth
The diameter of the mycelial colony was measured after 6 days’ incubation at 10 temperatures (20–38°C with 2°C intervals). Growth rate (mm d–1)=Average colony diameter/6 d.Growth inhibition rate (%)=(1–Average colony diameter of treated group/Average colony diameter of 28°C group)×100.
2.3. Assay of intracellular trehalose content
The mycelia were gently removed from cellophane on Petri dishes, washed with de-ionized water and blotted on filter papers. Then the mycelia were ground in liquid nitrogen and extracted in 10 mL water per gram of fresh mycelial weight for 10 min at 100°C. The extract was centrifuged at 10 000×g for 10 min, and the supernatant was filtered through a 0.45-μm filter unit. The trehalose content was determined by HPLC with a CarboPac PA-10 column(4 nm×250 nm). Samples were eluted with 100 mmol L–1NaOH and monitored with an electrochemical detector(Dionex DC Amperometry, USA). Commercially available trehalose (Sigma, USA) was used as the standard substance.
2.4. Exogenous trehalose treatments
In order to test the effect of trehalose on mycelial growth rate during recovery for 2 days after heat stress, both strains were grown on PDA with the addition of various concentrations of trehalose at 28°C for 3 days before 12-h 40°C treatment, with free of trehalose as control. Change of colony diameter (CCD) (mm)=Average colony diameter of recovery group–Average colony diameter group before 40°C treatment. Growth promotion rate (%)=CCD of trehalose treated group/CCD of control group without trehalose×100. To determine the in fluence of exogenous trehalose on the levels of lipid peroxidation, the intracellular trehalose content, and the trehalose metabolism pathway during heat stress, the two strains were inoculated to PDA medium containing 15 g trehalose L–1and were incubated for 3 days in the dark at 28°C, followed by heat treatment at 40°C for different time durations. All the experiments were conducted three to five times independently.
2.5. Determination of lipid peroxidation
The lipid peroxidation products were examined based on the contents of thiobarbituric acid reactive substances(TBARS) resulted from the thiobarbituric acid (TBA). TBARS content was determined according to the method of Konget al. (2012b), with some modifications. Brie fly, about 0.2 g mycelia were grounded in liquid nitrogen and placed into a tube containing 200 μL of 5% trichloroacetic acid(TCA). After centrifugation at 10 000×g for 10 min, 200 μL of the supernatant were collected and mixed with 200 μL of 0.67% TBA. The mixture was heated at 95°C for 30 min.Then the resultant mixture was centrifuged at 10 000×g for 10 min. The absorbance of the supernatant was measured at 532 nm. The values were corrected for non-specific absorption by subtracting the absorbance at 600 nm.
2.6. Preparation of crude enzyme solutions
Mycelia (1 g) were grounded with liquid nitrogen and suspended in 5 mL of extract buffer containing 100 mmol L–1potassium phosphate buffer (pH 7.0), 10% (v/v) glycerol and 1 mmol L–1EDTA. After centrifugation at 12 000×g at 4°C for 15 min, the supernatant was used as the crude enzyme solutions.
2.7. Measurement of the protein content and enzyme activities
Protein determination was done using a Bradford Protein Assay Kit (TIANDZ, Beijing, China). The trehalose-6-phosphate synthase (TPS) activity was assayed according to Hottigeret al. (1987) with some modifications. The reaction mixture with a total volume of 0.4 mL consisted of final concentrations of 50 mmol L–14-(2-hydroxyerhyl)piperazine-1-erhanesulfonic acid (HEPES) (pH 7.0),5 mmol L–1uridine diphosphate glucose (UDPG), 10 mmol L–1glucose-6-phosphate, 12.5 mmol L–1MgCl2, 2 mmol L–1EDTA-Na2and 12.5 mmol L–1KCl at final concentrations,and enzyme samples. Glucose-6-phosphatase was omitted in controls. The assay mixtures were incubated at 35°C for 30 min. The reaction was stopped by boiling for 5 min,and the samples were stored on ice for 10 min. After centrifugation (5 000×g for 10 min), the supernatants were collected for determination of uridine diphosphate(UDP). The second reaction mixture with a total volume of 0.5 mL contained 140 mmol L–1HEPES (pH 7.0), 2 mmol L–1phosphoenolpyruvate and 0.3 mmol L–1nicotinamide adenine dinucleotide (NADH) at final concentrations, and 20 U lactic dehydrogenase. The reaction was started by adding the pyruvate kinase (10 μL, 20 U) into the mixture and incubated at 35°C for 30 min. The decrease of the absorbance at 340 nm was recorded and used to calculate the concentration of UDP.
Trehalose phosphorylase (TP) activity was measured in both directions of trehalose synthesis and trehalose phosphorolysis (Saitoet al. 1998) with some modifications.In the synthesis direction, the reaction mixture consisted of 100 mmol L–1α-glucose 1-phosphate, 100 mmol L–1glucose, 100 mmol L–1HEPES buffer (pH 7.0) and enzyme sample in a total volume of 250 μL. The reaction mixture was incubated at 35°C for 3 h and boiled for 5 min to stop the reation. The produced trehalose was measured by highperformance liquid chromatography (HPLC). Whereas in the phosphorolysis direction, the reaction mixture consisted of 200 mmol L–1trehalose, 40 mmol L–1potassium phosphate buffer (pH 7.0), 10 mmol L–1glutathione, 16 mmol L–1EDTA,1 mmol L–1NADP+, 1.3 mmol L–1MgCl2·6H2O, 67 μmol L–1glucose 1,6-bisphosphate, 1.55 U PGM mL–1, 1.75 U glucose-6-phosphate dehydrogenase (G6PDH) mL–1, and enzyme sample, in a total volume of 2 mL. The formation of glucose was measured by the hexokinase/G6PDH coupled assay (Slein 1965).
The neutral trehalase (NTH) activity was measured by the method of San Miguel and Argüelles (1994), with some modifications. The reaction mixture consisted of 200 μL of 200 mmol L–1trehalose prepared in 25 mmol L–1HEPES buffer (pH 7.1), and 125 μmol L–1CaCl2( final pH 6.7). The reactions were conducted at 30°C for 30 min and terminated by heating in a water bath at 100°C for 3 min. The glucose concentration was determined in the supernatants using the glucose oxidase-peroxidase method (Kokalis-Burelle and Rodríguez-Kábana 1994).
2.8. Statistical analysis
All the experiments were conducted for at least three times,and the data presented were representative of all the results. All the data were analyzed by Microsoft Excel 2007(Microsoft Corporation, Redmond, WA, USA) and SPSS 19.0 (SPSS Inc., Chicago, IL, USA). The experimental data were assessed by a one-way analysis of variance using Duncan’s multiple range test. AP-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Two P. pulmonarius strains display distinct heat stress resistance
The mycelia growth of CCMSSC 00494 and CCMSSC 00499 on PDA were both affected by different temperatures.The growth rates for the two strains both increased at 20–28°C and decreased at 28–38°C. The optimal growing temperatures were both at 28°C. Along with the change of temperature, the growth rate of CCMSSC 00494 changed more significantly than that of CCMSSC 00499 from 20 to 38°C (Fig. 1-A). Following high temperature treatment(from 30 to 36°C), both the growth inhibition rates kept rising as the temperature increased. This meant the growth of the two strains were both inhibited. However, the growth inhibition rates of CCMSSC 00494 were significantly higher than those of CCMSSC 00499 (Fig. 1-B). From the above results, it was determined that CCMSSC 00494 was the heat-sensitive strain, and CCMSSC 00499 was the heat less-sensitive strain.
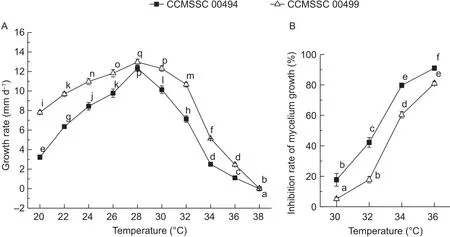
Fig. 1 In fluences of temperatures on the mycelial growth of CCMSSC 00494 (black square) and CCMSSC 00499 (white triangle).A, mycelium growth rates. B, inhibition rates of mycelium growth. Error bars represent standard deviations from 5 independent experiments. Different letters indicate statistical significances according to the Duncan’s multiple range test (P<0.05).
3.2. Heat stress induced the accumulation of intracellular trehalose
At the optimal growth temperature (28°C), there were no significant differences in trehalose content between the two strains (Fig. 2). When exposed to heat stress (40°C),both strains showed significant accumulations of trehalose compared with their respective controls. Trehalose accumulation reached the highest level at 12-h heat treatment. At this time, the intracellular trehalose content of the sensitive strain (CCMSSC 00494) was 1.06-fold of that in the less-sensitive strain (CCMSSC 00499). And after 24-h heat treatment, the ratio increased up to 1.33-fold.These results revealed that the heat stress induced the accumulation of intracellular trehalose. During the entire heat shock period, the sensitive strain (CCMSSC 00494)accumulated more trehalose than the less-sensitive strain(CCMSSC 00499).
3.3. Exogenous trehalose application can further promote the increased content of intracellular trehalose under heat stress
Exogenous trehalose (15 g trehalose L–1) lead to higher content of intracellular trehalose in the sensitive strain(CCMSSC 00494) during entire heat stress period than that in the samples with no trehalose addition (Fig. 2). While for the less-sensitive strain (CCMSSC 00499), the intracellular trehalose started to increase after 24-h heat treatment.These results suggested that exogenous trehalose addition induced the significant accumulation of intracellular trehalose for both strains under heat stress at 40°C. But the induction seemed more significant for the sensitive strain as the intracellular trehalose for the less sensitive strain accumulated only at high extent of heat stress.
3.4. Exogenous trehalose application promotes growth recovery of mycelia after heat stress
It’s well known that the extent of growth recovery was related to the resistance to stress. The effects of exogenous trehalose on growth recovery after heat stress were shown in Fig. 3. Addition of lower concentration of trehalose (5 or 10 g L–1) significantly improved mycelial recovery growth of the sensitive strain (CCMSSC 00494), but not less-sensitive strain (CCMSSC 00499). The addition of 15 g L–1trehalose significantly improved recovery growth of both strains,though the significant levels were different. At the addition of 20 or 30 g L–1trehalose, the growth promotion rates for the two strains did not differ significantly. Thus, we selected the addition of 15 g trehalose L–1treatment method for further research. These results indicated that exogenous trehalose was able to promote recovery growth after heat stress at some extent, especially in the sensitive strain.

Fig. 2 Effect of exogenous trehalose on the intracellular trehalose content level under 40°C heat stress. DW, dry weight. Error bars represent standard deviations from 3 independent experiments. Different letters indicate statistical significances according to the Duncan’s multiple range test (P<0.05).

Fig. 3 Effect of exogenous trehalose on colony diameter for CCMSSC 00494 and CCMSSC 00499 after 2 days recovery from 12-h 40°C treatment. Error bars represent standard deviations from 5 independent experiments. Different letters indicate statistical significances according to the Duncan’s multiple range test (P<0.05).
3.5. Exogenous trehalose application leads to reduction of lipid peroxidation under heat stress
To further investigate the effect of exogenous trehalose in overcoming damages by heat stress, stress parameter of lipid peroxidation was determined. Lipid peroxidation is considered a primary cause of membrane oxidative degradation under heat stress. TBARS is the product of lipid peroxidation, and is considered as an indicator of membrane damage under abiotic stresses (Nagesh and Devaraj 2008).Increased TBARS content was observed in both strains during the period of heat treatment even when exogenous trehalose was added (Fig. 4). However, exogenous trehalose significantly reduced the levels of lipid peroxidation after 12-h heat treatment. In the sensitive strain (CCMSSC 00494), the TBARS content fell by 18.59 and 23.08% at 12 and 24 h treatments, respectively, compared with that of no trehalose treatments. While in the less-sensitive strain(CCMSSC 00499), it fell by 13.80 and 24.42%, respectively.Thus, more membrane damages were induced by the heat treatment in both strains, and the exogenous trehalose can reduce the level of membrane damages.
3.6. Changes in the activities of intracellular trehalose metabolic enzymes during heat stress
In general, there are three pathways for intracellular trehalose metabolism in mushrooms: TPS-TPP pathway,TP pathway and NTH pathway. Moreover, the intracellular trehalose accumulation levels were significantly different between the two strains under heat stress. Therefore,changes in the activities of intracellular trehalose metabolic enzymes in both strains were determined.
TPS-TPP pathway was also known as OtsA-OtsB pathway inEscherichia coli(Elbeinet al. 2003). The TPS activities, involved in the synthesis of trehalose from UDP,increased in myceliaof both strains under heat treatment,compared with their respective controls. Specifically,TPS activity increased markedly after 6-h treatment for both strains, while the sensitive strain (CCMSSC 00494)exhibited higher increase than the less-sensitive strain(CCMSSC 00499) (Fig. 5-A). Interestingly, after 12-h treatment, the TPS activities of both strains declined.

Fig. 4 Effect of exogenous trehalose on the lipid peroxidation level under 40°C. TBARS, thiobarbituric acid reactive substances.FW, fresh weight. Error bars represent standard deviations from 3 independent experiments. Different letters indicate statistical significances according to the Duncan’s multiple range test (P<0.05).
The activity of TP, which catalyzes the reversible phosphorolysis of trehalose into glucose-1-phosphate and glucose (Saitoet al. 1998), was highly in fluenced by heat stress (Fig. 5-B and C). The response to heat stress differed in the trehalose synthesis and breakdown activity. In the direction of trehalose synthesis, the activity of TP sharply increased at 3-h heat treatment. The highest peak occurred at 12 h, followed by a rapid decrease. In the direction of degradation, i.e., trehalose phosphorolysis, the activity of TP decreased rapidly before 6-h heat treatment and increased sharply at 12-h treatment. After 12-h treatment, the TP degradation activity didn’t change in the sensitive strain(CCMSSC 00494), whereas decreased significantly in the less-sensitive strain (CCMSSC 00499).
Under the treatment of heat stress, the activities of NTH, which related to degradation of endogenous cytosolic trehalose into two glucose molecules (Thevelein 1984)reduced (Fig. 5-D). The reduction in NTH activity of the sensitive strain (CCMSSC 00494) was greater than that of the less-sensitive strain (CCMSSC 00499) during the early treatment period. At 24-h treatment, the NTH activity slightly increased in both strains, but still lower than that of control.
All these data indicated thatP.pulmonariusaccumulated trehalose in response to heat stress (40°C) from 3 to 12 h, by increasing the activities of TPS and TP in trehalose synthesis direction to synthesize more trehalose, and reducing the activities of TP in trehalose degradation direction and NTH at the same time to relieve the degradation of trehalose.After a longer duration of heat stress (12 to 24 h), the activities of trehalose synthesis enzymes reduced and that of degradation enzymes increased, which resulted in the intracellular trehalose contents decreasing gradually.These data showed a clear response involving trehalose biosynthesis under the treatment of heat stress.
4. Discussion
Heat stress is one of the major abiotic stress factors impacting microorganisms growth and development.Thus, breeding or selecting edible mushroom cultivars adapted to high temperature can ensure fruit body quality and yield, especially for the non-industrial cultivation lack of facility to control temperature. Although the screening indexes for heat resistance have been studied in plants in detail, such as scavenging and detoxifying ROS capacity,membrane thermal stability (MTS) and photosynthetic apparatus function intact (Asthir 2015), not all clari fied concepts may apply to edible mushrooms because of different development and metabolism between mushrooms and plants. In this study, we tried to detect some indexes that referred in plants under heat stress inP.pulmonarius.In addition, exogenous trehalose or overexpression of trehalose biosynthetic genes can improve abiotic stress resistance by increased trehalose accumulation in many organisms (Sotoet al. 1999; Garget al. 2002; Cortina and Culiá?ez-Macià 2005; Konget al. 2012a; Yanget al. 2014;Rathodet al. 2016). The relationship between trehalose biosynthesis and thermotolerance, however, is not clear.Therefore, we studied the relationship inP. pulmonarius,which would provide a basis for research on the screening thermotolerance indexes for other mushrooms.

Fig. 5 Changes in the enzyme activities of intracellular trehalose metabolism for CCMSSC 00494 and CCMSSC 00499 by heat stress. TPS, trehalose-6-phosphate synthase; TP, trehalose phosphorylase; NTH, neutral trehalase. Error bars represent standard deviations from 3 independent experiments. Different letters indicate statistical significances according to the Duncan’s multiple range test (P<0.05).
In this study, intracellular trehalose contents for both the twoP.pulmonariusstrains tended to rise at first and then decline, but they were still significantly higher than the control group throughout the entire heat treatment period(Fig. 2). The changing trend of trehalose content was similar in bothPleurotus eryngiivar.tuoliensisandFlammulina velutipesduring the heat shock (Konget al. 2012a; Liuet al. 2016). It is well known that heat stress inhibited mycelial growth or decreased biomass production (Ordazet al. 2012; Zhang Xet al. 2016). And the thermotolerance was related with the recovery growth ability (Rangelet al. 2010). In this study, addition of trehalose into PDA medium promoted mycelial growth recovery after heat stress (Fig. 3). Similar results were also found in other studies: Luoet al. (2014) reported that trehalose pretreated wheat seedlings ameliorated some adverse effects and exogenous trehalose partially promoted recovery from heat stress; likewise, Nounjan and Theerakulpisut (2012)described that the supplement of exogenous trehalose to NaCl-stressed rice showed markedly higher percentage of growth recovery than those treated with NaCl only. We further con firmed that exogenous trehalose treatment not only significantly promoted the increase of intracellular trehalose concentrations, but also promoted the decrease of lipid peroxidation levels in twoP.pulmonariusstrains under heat stress (Figs. 2 and 4). Several studies showed that exogenous or intracellular trehalose has the ability to reduce ROS and lipid peroxidation levels resulted from stress (Benaroudjet al. 2001; Luoet al. 2008; Liet al. 2010).Thus, endogenous and exogenous trehalose both played protective effects against heat stress inP.pulmonarius.
Although extensive work regarding trehalose accumulation under heat stress has been done, it still remains to be investigated that how enzymes or genes in trehalose metabolism pathways are regulated and how they play different roles under stress. InF.velutipes, trehalose accumulation induced by heat stress was regulated by TPSTPP pathway, the TP pathway and NTH pathway together(Liuet al. 2016). However, Booet al. (2013) suggested that the trehalose accumulation under thermal stress condition inGlaciozyma antarcticawas possibly caused by the repression ofnth1but was not due to over-expression of thetps1andtps2genes. In deep contrast with these results, studies of Winkleret al. (1991) reported a dramatic increase in the concentration of the substrates (UDP-glucose and glucose-6-phosphate) for TPS, which caused an increase in trehalose concentration under heat stress.
In this study, similar toF.velutipes, TPS activities increased and NTH activities decreased for trehalose accumulation,TP has a reversible reaction activity in the biosynthesis and phosphorolysis of trehalose during the period of heat stress (Fig. 5). Moreover, there were significant positive correlations between the trehalose concentrations and TPS activities in both sensitive strains (r=0.942,P<0.05) and less-sensitive strain (r=0.872,P<0.05) (data now shown).Thus, TPS-TPP pathway may play a pivotal role in the heat resistance inP.pulmonarius.
The different germplasms have distinct abilities in the heat resistance. The present results showed that exogenous trehalose was more effective in promoting mycelial growth recovery in sensitive strain than that in less-sensitive strain when the lower concentration of trehalose was added(Fig. 3). The sensitive strain produced more trehalose(Fig. 2) and activated the trehalose metabolic enzymes more quickly and stronger than the less-sensitive strain (Fig. 5).These results suggested that protective effects of trehalose were more pronounced in sensitive strain than that in lesssensitive strain ofP.pulmonarius. These results were in accordance with the reports by Theerakulpisut and Gunnula(2012) and Nounjan and Theerakulpisut (2012). Trehalose itself alone cannot explain the difference in thermotolerance between sensitive strain and less-sensitive strain, although the heat resistance was both enhanced by exogenous or endogenous accumulated trehalose differently inP.pulmonarius.
Various substances related to trehalose metabolism may involve in heat resistance. For example, T6P (trehalose-6-phosphate, an intermediate of TPS-TPP pathway) is an important sugar signaling molecule for plant stress responses. It controls glycolysis in yeasts and filamentous fungi (Eastmond and Graham 2003), and inhibits the catalytic activity of SnRK1 (sucrose non-fermenting-1-related protein kinase 1), a key transcriptional regulator that responds to carbon and energy supply (Zhanget al. 2009;Paulet al. 2010). Moreover, a new conclusion was recently reported that yeast tolerance to various stresses relies on the TPS protein, not on trehalose (Petitjeanet al. 2015). And the new functions of TPS protein as a key pro-survival factor during growth, chronological ageing, and apoptotic stress in yeast have been reported (Petitjeanet al. 2017). The synthesises of HSPs are also key processes in response to heat stress. Almost all HSPs functions as molecular chaperones, preventing deleterious protein conformations and eliminate non-native aggregations (Morimoto 1998;Feder and Hofmann 1999). The HSP104 has been shown to exhibit synergistic (Elliottet al. 1996) or complementary effect for loss of trehalose (Tapia and Koshland 2014). And the robust increase in transcription of HSPs genes by heatshock factor-1 (Hsf1) was linked to the combined effects of trehalose (Bulman and Nelson 2005; Conlin and Nelson 2007). In addition, a high trehalose level maintains Hsf1 in a highly active state, preventing the decrease in activity of Hsf1 that occurs during a sustained heat shock response(Conlin and Nelson 2007). It was also shown that signaling molecules NO can effectively protect mycelia ofP.eryngiivar.tuoliensisfrom heat stress-induced oxidative damage and is involved in the regulation of accumulation of trehalose(Konget al. 2012a, b).
Heat stress resulted in the inhibition of mycelium growth,increased lipid peroxidation level and intracellular trehalose content inP. pulmonarius. Furthermore, trehalose played a role as a thermoprotectant, because exogenous trehalose increased the intracellular trehalose content and further alleviated the negative impact on mycelial growth induced by heat stress. The intracellular trehalose concentrations in less-sensitive strain were significantly lower than that in heat-sensitive strain, and the endogenous and exogenous trehalose protective effects in the heat-sensitive strain were significantly higher than that in the less-sensitive strain under heat stress. This indicated that differences in levels of trehalose biosynthesis may not be the only reason to the difference of thermotolerance. As shown above, trehalose plays an important role in heat resistance, however, it is very complicated that the mechanism underlying the heat resistance may involve the alteration of signaling cascades and transcriptional control, increasing production of stress proteins, osmoprotectants and antioxidants (Hasanuzzamanet al. 2013). Therefore, we presume that trehalose biosynthesis is just one aspect of the heat resistance mechanisms. Further research needs to be done to clarify the mechanisms of heat resistance inP. pulmonarius.
5. Conclusion
Heat stress inhibited the mycelial growth ofPleurotus pulmonariusand accelerated the lipid peroxidation.Trehalose plays protective roles on heat resistance of the two strains and the protective effects were more remarkable in the sensitive strain. The trehalose metabolic enzymes in the sensitive strain act more positively to accumulate trehalose than those in the less-sensitive strain.
Acknowledgements
This work was supported by the National Basic Research Program of China (2014CB138303) and the earmarked fund for China Agriculture Research System (CARS20).The authors thank the Department of Quality and Safety Test Technology in the Institute of Vegetables and Flowers,Chinese Academy of Agricultural Sciences for providing the experimental facilities.
 Journal of Integrative Agriculture2019年2期
Journal of Integrative Agriculture2019年2期
- Journal of Integrative Agriculture的其它文章
- Digital mapping in agriculture and environment
- Maize production under risk: The simultaneous adoption of off-farm income diversification and agricultural credit to manage risk
- miR-34c inhibits proliferation and enhances apoptosis in immature porcine Sertoli cells by targeting the SMAD7 gene
- lnhibition of KU70 and KU80 by CRlSPR interference, not NgAgo interference, increases the ef ficiency of homologous recombination in pig fetal fibroblasts
- Kiwifruit (Actinidia chinensis) R1R2R3-MYB transcription factor AcMYB3R enhances drought and salinity tolerance in Arabidopsis thaliana
- Arbuscular mycorrhizal fungi combined with exogenous calcium improves the growth of peanut (Arachis hypogaea L.) seedlings under continuous cropping
