“Dry” NiCo2O4 Nanorods for Electrochemical Non-enzymatic Glucose Sensing
Feng-cho Sun,Jing-tong Zhng,Ho Ren?,Shu-to Wng,Yn Zhou?,Jun Zhng
a.College of Science,China University of Petroleum(East China),Qingdao 266580,China
b.College of Chemical Engineering,China University of Petroleum(East China),Qingdao 266580,China
A rod-like NiCo2O4 modified glassy carbon electrode was fabricated and used for nonenzymatic glucose sensing.The NiCo2O4 was prepared by a facile hydrothermal reaction and subsequently treated in a commercial microwave oven to eliminate the residual water introduced during the hydrothermal procedure.Structural analysis showed that there was no significant structural alteration before and after microwave treatment.The elimination of water residuals was confirmed by the stoichiometric ratio change by using element analysis.The microwave treated NiCo2O4 (M-NiCo2O4 )showed excellent performance as a glucose sensor(sensitivity 431.29 μA·mmol/L?1·cm?2).The sensing performance decreases dramatically by soaking the M-NiCo2O4 in water.This result indicates that the introduction of residual water during hydrothermal process strongly aflects the electrochemical performance and microwave pre-treatment is crucial for better sensory performance.
Key words:Glucose sensor,Electrochemical,NiCo2O4 ,Hydrothermal
I.INTRODUCTION
Glucose sensing is a great demand for modern living due to the significant growing population of diabetics.In 2015,1.6 million deaths were caused by diabetes according to World Health Organization(WHO)data[1].Since the first generation of enzymatic glucose electrochemical biosensor was introduced by Clark et al.[2],there have been tremendous eflorts on the development of efficient and accurate enzymatic glucose sensors[3].The enzyme-based electrochemical sensor oflers high sensitivity and selectivity,as well as low detection limit,they still have some serious drawbacks such as low thermal stability,low chemical stability,and limited working conditions(temperature,pH value,oxygen partial pressure,etc.)[4].Therefore,the enzymefree electrode is considered as an alternative solution to solve the drawbacks of enzymatic sensors.
Similar to the traditional enzymatic electrochemical sensor,enzyme-free sensors also rely on the glucose oxidation reaction promoted by electrocatalysis at the nonenzymatic electrode surface.The catalytic glucose oxidation on the electrode surface could cause increased Faradic current that should be detectable by the sensor.This working mechanism requires the electrode to be high conductive for efficient sensing.Transition metal oxide based non-enzymatic electrodes are the most intensively investigated electrocatalysts for electrochemical glucose sensing,as a consequence of the low costs,ease of fabrication,high abundance,and high electrochemical catalytic activities[5].For instance,transition metal oxide based electrocatalysts,such as Co3O4[6],CuO[7,8],Cu2O[9],NiO[10,11],ZnO[12,13],MnO2[14,15],Fe2O3[16]etc.,have been intensively studied and demonstrated excellent catalytic activities for glucose oxidation.Recently,bimetallic oxides,such as spinel NiCo2O4[17?25],outperformed the NiO or Co3O4based electrocatalysts due to their enhanced conductivity[26].In addition,rational design of the material structure can also boost the catalytic performance of NiCo2O4,such as nanosheets[19,5,23],hollow spheres[25,26],and nanocages[17].The high surface atom ratios lead to larger active surface areas and stronger current signals.In this context,lower dimensional rod-like structures would deliver higher surface atom ratios,and might further enhance the electrochemical performance for glucose oxidation.
Hydrothermal synthesis is one of the most commonly used methods to fabricate metal oxides[27].This synthetic protocol oflers numerous advantages such as fast reaction,facile preparation,and high product yields[28]. In this work,we fabricated rod-like NiCo2O4by using the hydrothermal method.However,the hydrothermal based synthesis usually results in crystallized water residuals even after the annealing process.In order to eliminate the residual water in the asprepared NiCo2O4,microwave post-processing was per-formed for the NiCo2O4catalyst prior to glucose electrochemical sensing.We found that the residual water dramatically hindered the electrochemical catalytic process.This result explains the performance decrease of metal oxide based glucose sensors work in environments with high humidity.
II.EXPERIMENTS
A.Synthesis of NiCo2O4 NRs
We used all reagents as received. The mixture of 0.3 mmol nickel(II)acetate tetrahydrate,0.6 mmol cobalt acetate tetrahydrate,and 4 mmol urea were dissolved in 30 mL deionized water and transferred into a 50 mL stainless steel autoclave with Teflon coating.The autoclave was heated for 6 h at 150?C.The product was then flushed with deionized water and ethanol after cooling.The obtained Ni-Co precursor was then annealed in air for 3 h at 300?C with a heating rate of 1?/min and the black NiCo2O4powder was obtained.
Forthemicrowavetreatment,theas-prepared NiCo2O4was treated in a commercial microwave oven at a power of 700 W for 1 min.The microwave-treated NiCo2O4is referred as M-NiCo2O4hereafter.
B.Instrumentation
The X-ray diflraction(XRD)data were collected on a Philips X’Pert diflractometer with a Cu Kα source worked at 40 kV and 40 mA.High resolution transmission electron microscopic(HRTEM)measurements were performed by using a JEM-2100UHR transmission microscope(JEOL,Japan)at 200 kV.Scanning electron microscopy(SEM)measurements were performed on an S-4800 microscope(Hitachi).Elemental analysis was performed on an energy-dispersive X-ray spectroscopy(EDS Inca X-Max,UK).The X-ray photoelectron spectra(XPS)were characterized by using a VG ESCALABMK II spectrometer with an Al Kα(1486.6 eV)source.
C.Preparation of NiCo2O4 /GCE and M-NiCo2O4 /GCE
4 mg of either NiCo2O4or M-NiCo2O4were mixed with 10%Nafion and drop-casted on a carefully polished glassy carbon electrode(GCE).
D.Electrochemical measurement
A CHI 660E electrochemical workstation(CH Instruments,Shanghai)was used for electrochemical measurements with a standard three-electrode setup.The GCE and NiCo2O4or M-NiCo2O4modified GCE(5 mm in diameter)were used as the working electrode,while using Hg/HgO and a Pt plate as the reference and the counter electrodes,respectively.All the electrochemical tests were performed in 0.1 mol/L NaOH solution.
III.RESULTS AND DISCUSSION
A.Structural characterizations
Since the microwave processing of the samples would eliminate the residual water in the NiCo2O4samples,we performed SEM,HRTEM,XRD,and EDS characterizations for the NiCo2O4and M-NiCo2O4samples to examine the variations in the morphologies,the crystalline symmetries,and the stoichiometric contents.All these measurements can be compared as depicted in FIG.1.As shown in FIG.1(a)and(b),the SEM images of NiCo2O4and M-NiCo2O4both show a long and thin rod-like structure,and there is no significant diflerence in the morphologies before and after microwave treatment.This result indicates that microwave pretreatment does not aflect the microscopic structure of the NiCo2O4samples.The NiCo2O4nanorods ofler high surface atom ratio and provide a large surface area,which might result in stronger redox current signals as the glucose oxidation occurs.The HRTEM image of the NiCo2O4nanorods after microwave treatment is depicted in FIG.1(c).It is clear that the crystal lattice distances of NiCo2O4are 0.25,0.48,and 0.30 nm,and these d-spacings originate from the(311),(111)and(220)crystal plane indices,respectively.These results agree well with the XRD data shown in FIG.1(d),where all the peaks of the NiCo2O4and the M-NiCo2O4samples match with the NiCo2O4reference data(PDF:No.20-0781).These characterizations indicate that the microwave treatment does not vary the crystal structure of NiCo2O4nanorods.FIG.1(e)and(f)depict the EDS patterns of NiCo2O4before and after the microwave treatment,respectively.The stoichiometric ratio between Ni,Co,and O changes from approximately 1:2:5 to 1:2:4 via microwave treatment.The loss of O content can be ascribed to the elimination of H2O.After the microwave treatment,the stoichiometric ratio coincides with the NiCo2O4formula. This suggests that NiCo2O4was successfully synthesized using the facile hydrothermal synthetic procedure followed by microwave treatment.Moreover,the EDS mapping of NiCo2O4confirms the co-existence of nickel,cobalt and oxygen elements in NiCo2O4and they are uniformly distributed(FIG.S1 in supplementary materials).

FIG.1 SEM images of the NiCo2O4 before(a)and after(b)microwave treatment.(c)HRTEM image of the NiCo2O4 after microwave treatment.(d)XRD analysis of NiCo2O4 before and after microwave treatment.EDS patterns of NiCo2O4 before(e)and after(f)microwave treatment.

FIG.2 XPS spectra of M-NiCo2O4 nanorods.(a)full spectrum,and in the Ni 2p(b),Co 2p(c),and O 1s(d)regions.
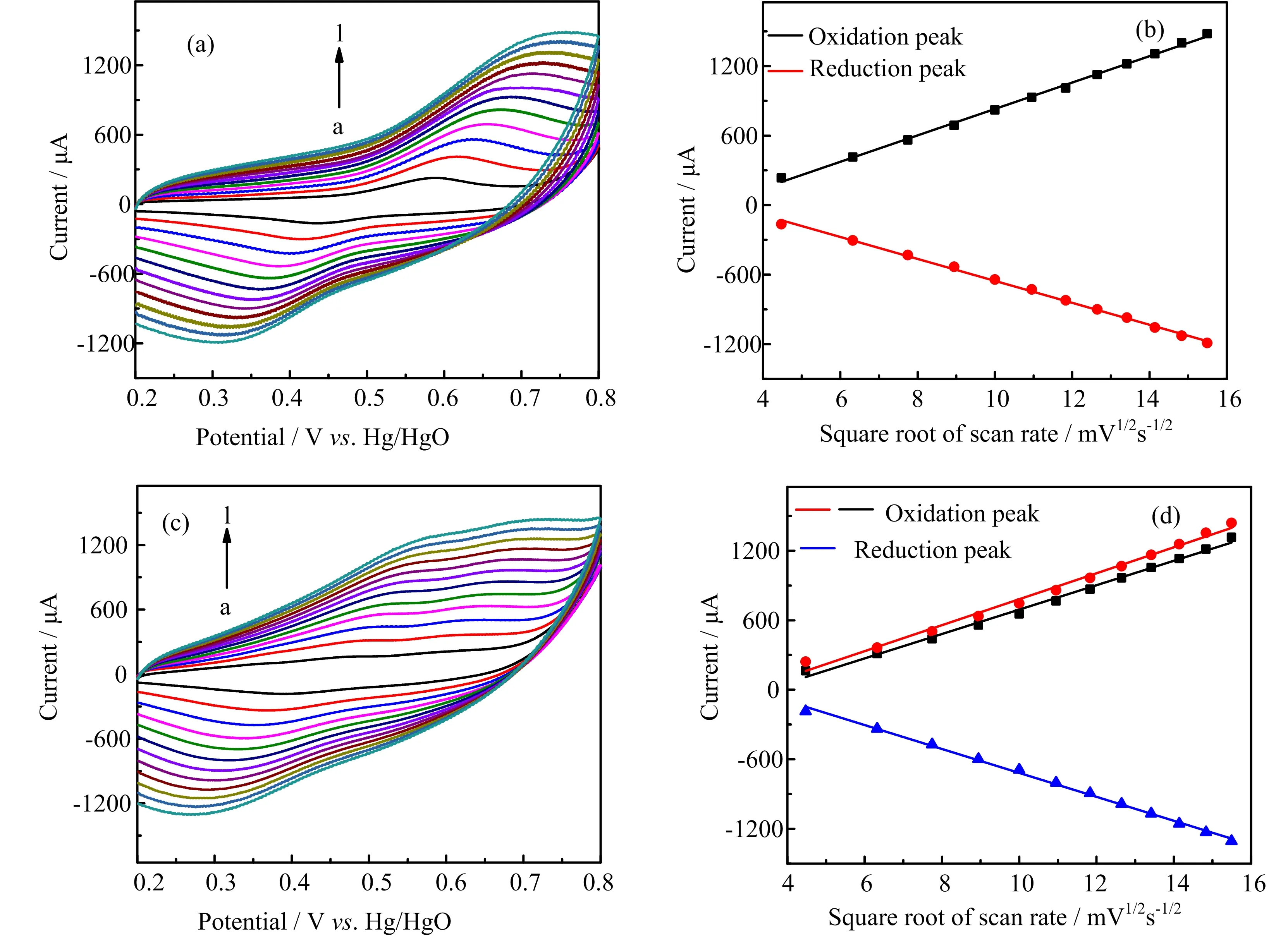
FIG.3 Cyclic voltammetry curves of(a)NiCo2O4 and(c)M-NiCo2O4 modified GCE(0.1 mol/L NaOH)and their respective Randles-Sevick plots(b,d).
The detailed chemical composition and corresponding electronic structures were analyzed with the help of XPS measurements.FIG.2(a)displays a complete survey of the XPS spectrum of the as-prepared MNiCo2O4,where all the element features can be clearly observed.The deconvoluted spectra in the Ni 2p region are shown in FIG.2(b),where the peaks consisting of binding energies 853.9 and 871.6 eV are ascribed to the Ni2+oxidation state and those at 855.4 and 873.1 eV originate from the Ni3+state.FIG.2(c)shows the Co 2p spectrum at a higher resolution with the spinorbit doublets has Co3+and Co2+characteristics and each is followed by a satellite feature.The peaks of 2p3/2and 2p1/2states of Co3+are shown at 779.7 and 794.9 eV and the Co2+2p3/2and 2p1/2signals appear at 781.4 and 796.8 eV.These results indicate that the as-synthesized M-NiCo2O4has mixed oxidation states both for the Co and the Ni elements,which agrees well with previous reports[29].Moreover,the deconvoluted O 1s spectrum shown in FIG.2(d)reveals three oxygen components at 529.4,531.0,and 533.4 eV,these features can be attributed to the metal-bonding oxygen,hydroxyl oxygen and oxygen appearing in moisture adsorptions,respectively[30].We also note that the XPS spectrum of NiCo2O4before microwave treatment shows almost identical features with that of the M-NiCo2O4(FIG.S2 in supplementary materials),indicating that microwave treatment does not alter the oxidation states and elemental compositions of NiCo2O4.
B.Electrochemical characterization of NiCo2O4 modified electrode
We first investigated the redox process of NiCo2O4in 0.1 mol/L NaOH with cyclic voltammetry(CV)scanning. As depicted in FIG.3(a),before microwave treatment,we observed the oxidation and cathodic peaks appear at+0.58 V and+0.43 V vs.Hg/HgO,respectively. The corresponding Randles-Sevick plot(FIG.3(b))shows that the peak current is linearly correlated with the square root of the scan rate.This result indicates that the electrode processes are diflusion controlled.In comparison,after microwave treatment,the CV curves of M-NiCo2O4show two anodic peaks at+0.51 and+0.63 V and one cathodic peak at+0.35 V.The anodic peak at+0.51 V can be attributed to the redox process of Ni2+/Ni3+,whereas the oxidation peak at+0.63 V originates from the redox process of Co2+/Co3+.The corresponding Randles-Sevick plot also indicates a diflusion controlled electrode process.The fact that only one oxidation peak was observed before microwave treatment suggests that the residual water in NiCo2O4might hinder the redox kinetics of Ni2+/Ni3+.It is worth noting that for both of NiCo2O4samples,the increasing scan rates leads to apparent shifting in peak potentials,indicating rather a sluggish electrode kinetics.This suggests a rather slow surface diflusion process of OH?or the semiconducting behavior of NiCo2O4.
FIG.4 displays the CV curves of both NiCo2O4and M-NiCo2O4modified GCE in NaOH solution with a scan rate of 40 mV/s.Upon the addition of a diflerent amount of glucose,the oxidation peak current for both electrode materials increases,suggesting both the NiCo2O4coatings are responsive toward the detection of glucose.The redox-catalyzed electrode process can be summarized as follows:
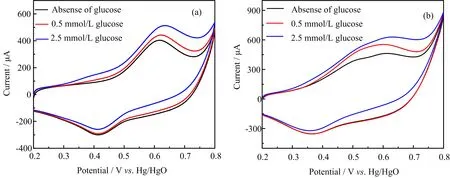
FIG.4 CV curves of(a)NiCo2O4 and(b)M-NiCo2O4 modified GCE at various glucose concentrations with scan rate 40 mV/s.

It is obvious that the catalyzed oxidation process on M-NiCo2O4modified electrode requires lower potential than that of the NiCo2O4case.The lower catalytic oxidation potential is beneficial for sensitive glucose sensing at low operating potentials.
The sensing performance of the NiCo2O4modified electrodes was evaluated by performing amperometric studies towards glucose detection by successively adding glucose withholding the NiCo2O4electrode at a constant potential.The amperometric responses of the M-NiCo2O4modified GCE at+0.5,+0.6,and+0.7 V vs.Hg/HgO are shown in FIG.5(a).With the increase of the glucose concentration,the current increases for all three measurements.The corresponding calibration graph is shown in FIG.5(b)?(d),with the linear region shown as inserts. The potential of+0.6 V is found to be optimal,with the highest sensitivity of 431.29 μA·mmol/L?1·cm?2.The limit of detection for the+0.6 V operating potential is 0.0126 mmol/L(S/N=3)and the recovery percentage of glucose lies between 95.83%(at glucose concentration of 0.24 mmol/L)and 83.73%(5.47 mmol/L)(see Table S1 in supplementary materials).The complete analytical data of the three diflerent operating potentials are shown in Table S2 in supplementary materials.In comparison,NiCo2O4was also used to modify the GCE surface followed by amperometric measurements.As shown in FIG.S3 in supplementary materials,similar to that of M-NiCo2O4modified GCE,NiCo2O4also responds to the successive addition of glucose.However,the sensitivity of the electrode modified by NiCo2O4is much lower than that of the M-NiCo2O4case.Again,the+0.6 V electrode potential oflers the highest sensitivity for the NiCo2O4modified electrode,but only 193.92 μA·mmol/L?1·cm?2is achieved.The recovery percentage of NiCo2O4modified GCE was found to be much lower than that of M-NiCo2O4,with only 66.67%recovery when glucose concentration was 0.24 mmol/L and 48.99%at 5.47 mmol/L(Table S1 in supplementary materials).Obviously,M-NiCo2O4oflers much better electrochemical performance than NiCo2O4,which confi rms the assumption that the residual water hinders the electrochemical catalytic activity.
Since the sensing process would be interfered by other bio-organic molecules,we performed interference test for sensors with NiCo2O4and M-NiCo2O4modified electrodes.FIG.6 depicts the response of the current density to usual interferences,such as ascorbic acid and uric acid.These interference sources were added with the concentration of 0.1 mmol/L,which preserved the concentration ratio respect to glucose in plasma[31].It is obvious that the addition of small amount of ascorbic acid and uric acid only increases the current density slightly,whereas the addition of glucose dramatically raises the current density.This result suggests that M-NiCo2O4is a good inorganic catalytic material for glucose sensing with high robustness.
In order to examine the eflect of residual water in NiCo2O4on the sensing performance,we soaked the M-NiCo2O4in water with various durations.As shown in FIG.7,the M-NiCo2O4modified electrode shows excellent sensitivity(431.29 μA·mmol/L?1·cm?2)towards glucose sensor when it was directly applied after microwave treatment(refer to 0 h in FIG.7).After soaking the M-NiCo2O4in water for 2 h,the sensitivity dramatically decreased to 118.00 μA·mmol/L?1·cm?2,which indicates that water strongly hindered the electrochemical activity of NiCo2O4. As the soaking time increases,the sensitivity fluctuates between 100?245 μA·mmol/L?1·cm?2,which is much lower than that of the as-prepared M-NiCo2O4modified electrodes.As shown in FIG.S4(a)(supplementary materials),EDS characterization confirmed the existence of residual water in the soaked samples.In general,the residual water aflects the electrochemical performance of NiCo2O4dramatically.The sample was then treated with microwave again after being soaked in water for 108 h.As expected,the sensitivity recovered and reached 410.09 μA·mmol/L?1·cm?2.FIG.S4(b)in supplementary materials shows the EDS analysis of MNiCo2O4after soaking in water followed by annealing at 300?C for 2 h.The molar ratio of O,Co,and Ni preserves at about 5:2:1.This is interesting that even at a high annealing temperature of 300?C,it is still unable to eliminate the residual water unless a microwave treatment is performed.This also suggests an efficient and economic approach to recover the sensor performance.
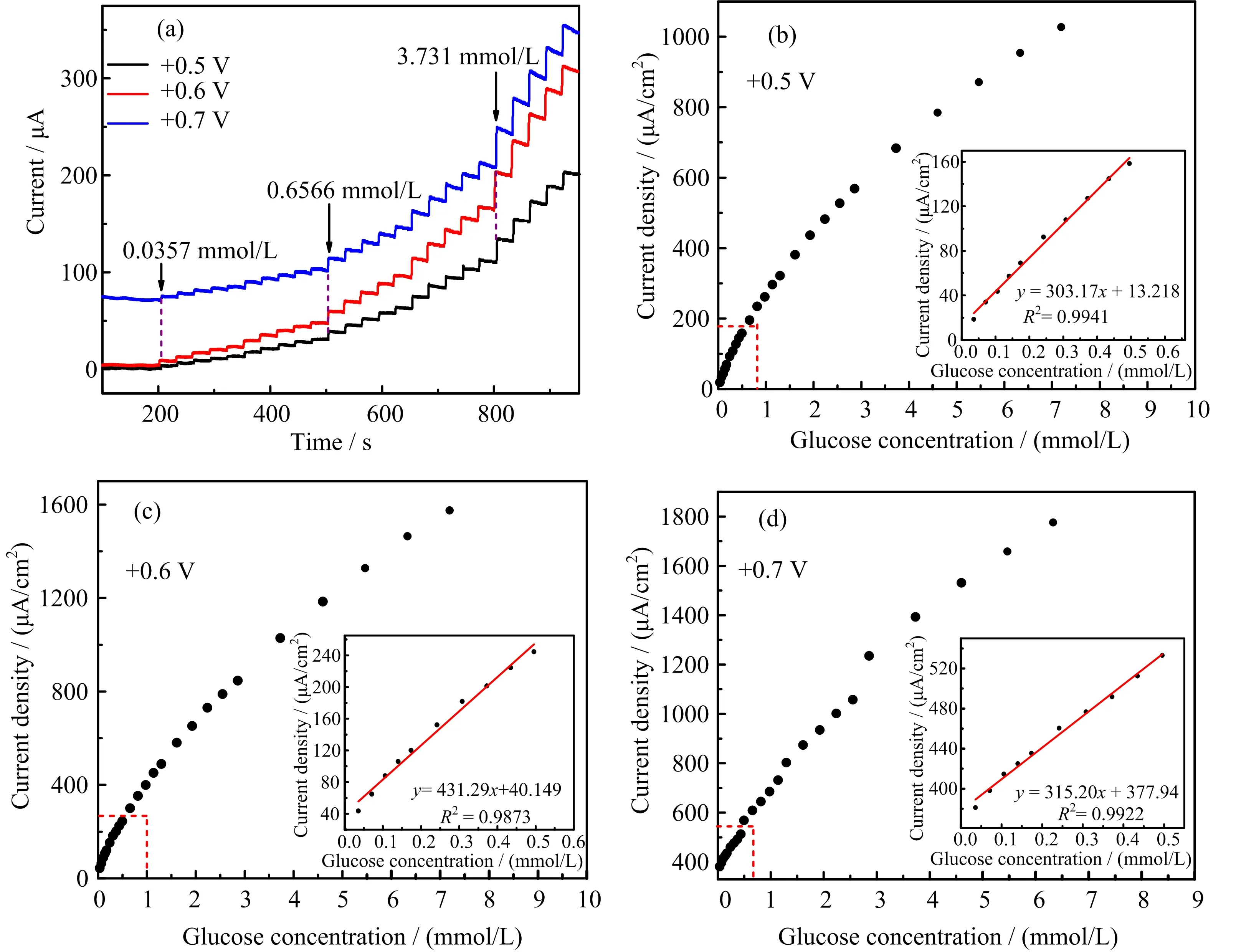
FIG.5(a)Amperometric analysis of M-NiCo2O4 modified GCE upon consecutive addition of glucose at+0.5,+0.6,and+0.7 V vs.Hg/HgO.(b?d)The linear calibration plot of(a),the insertion shows the linear region of the calibration graph.
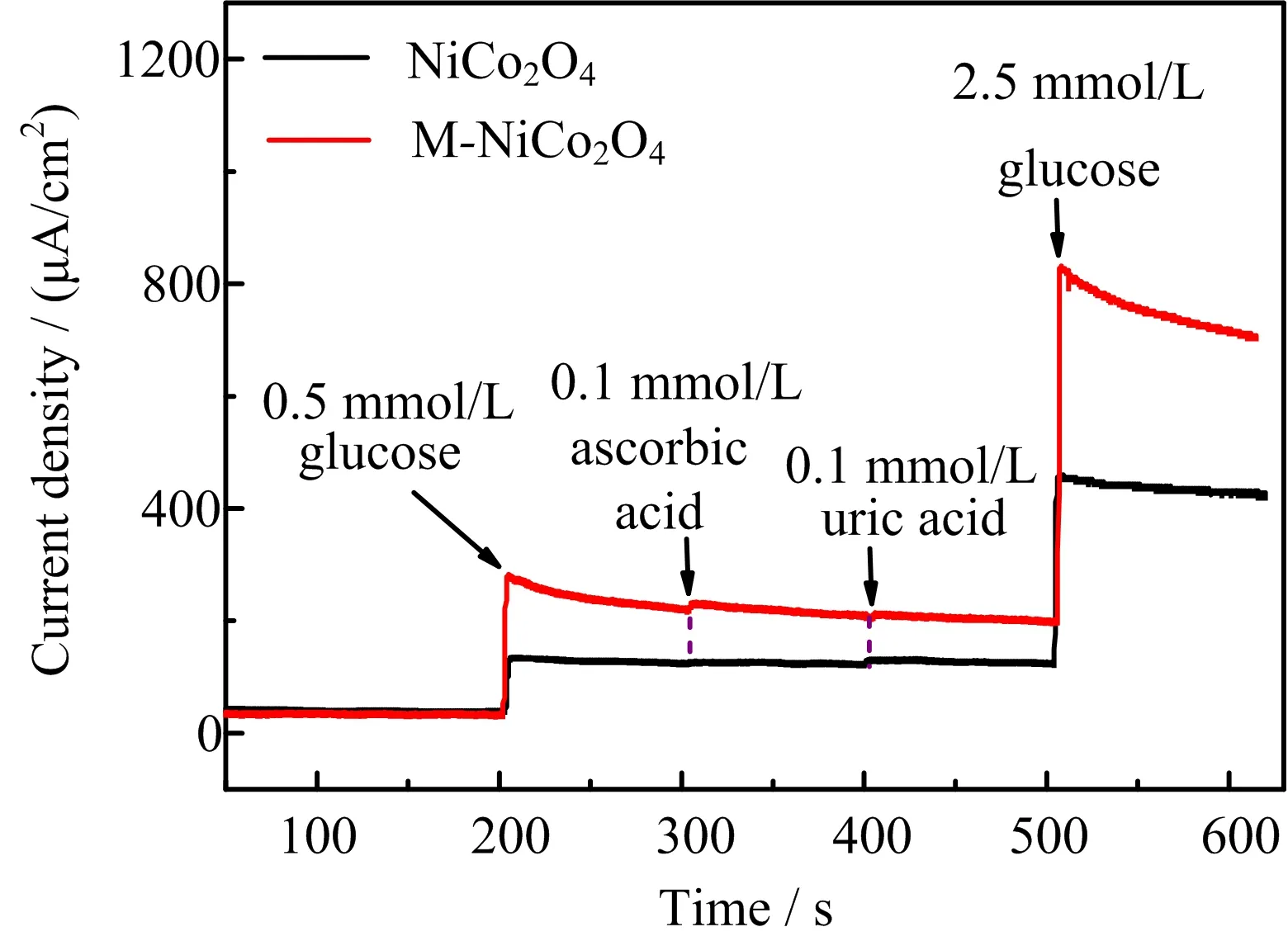
FIG.6 Interference test of NiCo2O4 and M-NiCo2O4 nanorod modified GCE in 0.1 mol/L NaOH(aq)at+0.6 V vs.Hg/HgO with 0.5 mmol/L glucose,2.5 mmol/L glucose,0.1 mmol/L ascorbic acid,and 0.1 mmol/L uric acid.

FIG.7 The glucose sensing sensitivities after soaking the M-NiCo2O4 in water.0 h denotes the M-NiCo2O4 was applied on the GCE directly after microwave-treatment,and post-108 denotes the microwave-retreated M-NiCo2O4 after soaking in water for 108 h.
IV.CONCLUSION
We synthesized NiCo2O4nanorods via hydrothermal reaction followed by annealing in air at 300?C for 3 h.The NiCo2O4was then treated in a commercial microwave oven at 700 W for 1 min to eliminate the residual water that may be introduced during the hydrothermal synthesis.The as-prepared M-NiCo2O4was drop-casted on a GCE and applied for glucose electrochemical sensing.Although there is no significant structural alteration before and after the microwave treatment,the elimination of residual water was confi rmed by the stoichiometric ratio change revealed by EDS analysis.The M-NiCo2O4showed excellent performance towards glucose sensoring with a sensitivity up to 431.29 μA·mmol/L?1·cm?2.By soaking the MNiCo2O4in water after various durations,the sensing performance reduced dramatically.The performance can be recovered by another microwave treatment.We found that the residual water is difficult to remove by conventional annealing process even with a temperature up to 300?C.This result showed that the introduction of residual water during the hydrothermal procedure strongly aflects the electrochemical performance.Microwave pre-treatment is crucial to eliminate the residual water and provides an efficient and economic approach to recovering the performance of the glucose sensors using NiCo2O4modified electrodes.
Supplementary materials:SEM image and EDS mapping of M-NiCo2O4,XPS analysis of microwave untreated NiCo2O4nanorods,glucose sensing tests for untreated NiCo2O4nanorods,EDS analysis of MNiCo2O4after soaking in water,recovery test data and limit of detection data are presented in Supplementary materials.
V.ACKNOWLEDGMENTS
This work was supported by the Shandong Provincial Natural Science Foundation, China(No.ZR2017QB015),theNationalNaturalScience Foundation ofChina (No.21773309),and China University of Petroleum Student’s Platform for Innovation and Entrepreneurship Training Program(No.20161449).
[1]WHO.URL:http://www.who.int/mediacentre/factshe ets/fs312/en/Accessed 2017/09/27.
[2]L.C.Clark and C.Lyons,Ann.N.Y.Acad.Sci.102,29(1962).
[3]J.Wang,Electroanalysis 13,983(2001).
[4]H.Zhu,L.Li,W.Zhou,Z.Shao,and X.Chen,J.Mater.Chem.B 4,7333(2016).
[5]K.Naik,A.Gangan,B.Chakraborty,S.K.Nayak,and C.S.Rout,ACS Appl.Mater.Interf.9,23894(2017).
[6]L.Kang,D.He,L.Bie,and P.Jiang,Sens.Actuators.B 220,888(2015).
[7]K.Li,G.Fan,L.Yang,and F.Li,Sens.Actuators.B 199,175(2014).
[8]Y.Zhang,Y.Liu,L.Su,Z.Zhang,D.Huo,C.Hou,and Y.Lei,Sens.Actuators.B 191,86(2014).
[9]C.Dong,H.Zhong,T.Kou,J.Frenzel,G.Eggeler,and Z.Zhang,ACS Appl.Mater.Interf.7,20215(2015)
[10]Y.Ding,Y.Liu,L.Zhang,Y.Wang,M.Bellagamba,J.Parisi,C.M.Li,and Y.Lei,Electrochimi.Acta 58,209(2011).
[11]S.Ci,T.Huang,Z.Wen,S.Cui,S.Mao,D.A.Steeber,and J.Chen,Biosens.Bioelectron.54,251(2014).
[12]S.SoYoon,A.Ramadoss,B.Saravanakumar,and S.J.Kim,J.Electroanal.Chem.717,90(2014).
[13]Y.Liu,H.Pang,C.Wei,M.Hao,S.Zheng,and M.Zheng,Microchimi.Acta 181,1581(2014).
[14]C.Guo,H.Li,X.Zhang,H.Huo,and C.Xu,Sens.Actuators.B 206,407(2015).
[15]F.Xiao,Y.Li,H.Gao,S.Ge,and H.Duan,Biosens.Bioelectron.41,417(2013).
[16]X.Cao and N.Wang,Analyst 136,4241(2011).
[17]B.Xue,K.Li,L.Feng,J.Lu,and L.Zhang,Electrochimi.Acta 239,36(2017).
[18]M.U.Anu Prathap and R.Srivastava,Electrochimi.Acta 108,145(2013).
[19]G.Ma,M.Yang,C.Li,H.Tan,L.Deng,S.Xie,F.Xu,L.Wang,and Y.Song,Electrochimi.Acta 220,545(2016).
[20]S.Cui,L.Li,Y.Ding,J.Zhang,H.Yang,and Y.Wang,Talanta 164,291(2017).
[21]Z.Yu,H.Li,X.Zhang,N.Liu,W.Tan,X.Zhang,and L.Zhang,Biosens.Bioelectron.75,161(2016).
[22]J.Yang,M.Cho,and Y.Lee,Biosens.Bioelectron.75,15(2016).
[23]K.K.Naik,S.Kumar,and C.S.Rout,RSC Adv.5,74585(2015).
[24]H.Rao,Z.Zhang,H.Ge,X.Liu,P.Zou,X.Wang,and Y.Wang,New J.Chem.41,3667(2017).
[25]W.Huang,T.Lin,Y.Cao,X.Lai,J.Peng,and J.Tu,Sens 17,217(2017).
[26]W.Huang,Y.Cao,Y.Chen,J.Peng,X.Lai,and J.Tu,Appl.Surf.Sci.396,804(2017).
[27]S.Feng and R.Xu,Acc.Chem.Res.34,239(2011).
[28]Q.Yang,Z.Lu,J.Liu,X.Lei,Z.Chang,L.Luo,and X.Sun,Prog.Natl.Sci.Mater.Int.23,351(2013).
[29]P.Wu,S.Cheng,M.Yao,L.Yang,Y.Zhu,P.Liu,O.Xing,J.Zhou,M.Wang,H.Luo,and M.Liu,Adv.Func.Mater.27,1702160(2017).
[30]D.U.Lee,B.J.Kim,and Z.Chen,J.Mater.Chem.A 1,4754(2013).
[31]H.A.Krebs,Annl.Rev.Biochem.19,409(1950).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Chinese Abstracts(中文摘要)
- Intracellular Self-assembly of TPE-biotin Nanoparticles Enables Aggregation-Induced Emission Fluorescence for Cancer-Targeted Imaging
- Renewable p-Xylene Production by Co-catalytic Pyrolysis of Cellulose and Methanol
- A Facile Surface Passivation of Hematite Photoanodes with Molybdate Overlayers for Efficient PEC Water Oxidation
- Polyaniline Nanotubes Prepared by One-Step Synergistic Polymerization of Aniline and Acrylic Acid
- Using Self-referencing Interlaced Submatrices to Determine the Number of Chemical Species in a Mixture
