Trophic Interaction in a Portunus rituberculatus Polyculture Ecosystem Based on Carbon and Nitrogen Stable Isotope Analysis
FENG Jie, TIAN Xiangli, *, DONG Shuanglin, LI Da, HE Ruipeng, ZHANG Kai, ZHANG Dongxu, and ZHANG Qingqi
?
Trophic Interaction in aPolyculture Ecosystem Based on Carbon and Nitrogen Stable Isotope Analysis
FENG Jie1), TIAN Xiangli1), *, DONG Shuanglin1), LI Da3), HE Ruipeng1), ZHANG Kai1), ZHANG Dongxu1), and ZHANG Qingqi2)
1),,,266003,2),222100,3),276800,
Trophic interaction among various biomass groups in a swimming crabpolyculture pond was investigated using carbon and nitrogen stable isotope analysis. The polycultured animal species also included white shrimp, short-necked clam, and redlip mullet. The mean δ13C value for all the biomass groups in polyculture ecosystem ranged from ?25.61‰ to ?16.60‰, and the mean δ15N value ranged from 6.80‰ to 13.09‰. Significant difference in the δ13C value was found between particulate organic matter (POM) and sediment organic matter (SOM) (<0.05), indicating that these two organic matter pools have different material sources. Assuming that a13C-enrichment factor of 1.00‰ and a15N-enrichment factor of 2.70‰ existed between consumer and prey, diets of the four cultured animals were estimated using a stable isotope mixing model. The estimated model results indicated thatmainly feed on;mainly feed on shrimp feed; while,andmainly feed on POM. Shrimp feed was also an important food source ofand. The diets of,,, andshowed complementary effects in this polyculture ecosystem. Our finding indicated that the polyculture of these four organisms with suitable farming density could make an effective use of most of the food sources, which can make a highly efficient polyculture ecosystem.
trophic interaction;; polyculture; stable isotope; diet composition
1 Introduction
The swimming crabis one of China’s three major crab species with high economic and nutritive values (Su., 1997; Song., 2006), which is widely cultured on the coast of China with the main culture mode of pond culture (Shi., 2010). As an om- nivorous species,cultured in pond was often fed with the low-valued fish, shrimp, and shellfish (Li, 2005). Because the monoculture ofin pond usually can not utilize the imported food efficiently and has a low production,was more often polycultured with other ecologically beneficial organisms, which can also increase the economic and ecological benefits (Ding and Liu, 2009; Wang., 2009). Therefore, the polyculture mode ofwithand,andhas been considered as one of the effective polyculture modes (Ban., 2016). The small particles of the imported foods (.., the residue of imported fish and shellfish) which can’t be consumed byis a very good food for. Mean- while,inhabits the bottom of a pond,inhabits the upper part of the water column. Both of these two species mainly feed on phytoplankton and detritus particles (Xu., 1987; Zhang., 2016). As a result, the polyculture of these four culture organisms at suitable farming densities may facilitate the effective use of both food and space resources in the pond ecosystem.
A food web expresses the pattern of energy or materials flow in an ecosystem where the predator-prey relationships exist (Cohen, 1993). The construction of aquatic ecosystem food webs can be a useful solution for answering fundamental scientific questions about biomass community structure, and settling practical problem associated with environment pollution (Cohen, 1993). Tradi- tionally, the food web of ecosystems is investigated by gut content analysis, which provides diet information of an organism immediately before it is captured. However, the result of the organism’s diet information from this method may be incorrect if the gut content was not completely absorbed by the organism (Kling., 1992), and, in addition, the diet composition provided by the gut content analysis only reflects the organism’s instantaneous food information when it was captured. The stable isotope approach performs better than the gut content analysis because it evaluates the food source of an organism by measuring the stable isotope of its assimilated matter, and provides integrated food information over a long time period corresponding to the organism’s tissue turnover time (Hobson and Welch, 1992). As a result, the stable isotope approach has been widely applied to investigate the trophic relationship and material flow among various aquatic ecosystems, such as ponds, lakes, lagoons, and ocean (Fry and Sherr, 1984; Yokoyama., 2002; Khar- lamenko., 2008; Quan., 2010).
To date, a lot of studies have applied stable isotope analysis to investigate the trophic interaction and food webs of the aquatic aquaculture ecosystems (Jin, 2010; Feng., 2014; Guo., 2014; Mahmood., 2016; Guo., 2016; Xu., 2017), which help to investigate the usage of food resources and solve problems of practical aquaculture management (Watanabe., 2013; Sun., 2013). However, the food sources and the trophic interaction in theaquaculture pond ecosystems have been seldom studied. Even though the benefits of many of the polyculture modes associated withhave been stated with the improved production and decreased waste (Zhou., 2010; Dong., 2013; Zhang., 2015). The explicit food composition of the cultured organisms and the material flow of the polyculture ecosystem remained unknown.
In this study, the polyculture system of,,andwas developed. The stable isotope analysis was used to investigate the polyculture pond ecosystem so as to quantify the potential food sources of the cultured organisms and construct the ecosystem food web. The results were expected to give an explanation of the benefits of the polyculture ofwith,and.
2 Materials and Methods
2.1 Pond and Enclosures
The experiment was carried out using the land-based experimental enclosures in a pond located in Ganyu County, Jiangsu Province, China (34?58′N, 119?20′E). The pond was about 2×104m2in size, with a water depth of 1.6 to 1.7m. Four land-based enclosures, representing four replicates of the same size (length×width×depth=5×5×2m), were established in the pond, and were lined with polyethylene (water-proofing materials) and supported with wood poles. At the bottom, the square walls of the enclosure were covered with mud from a pond, and supported by posts at 2.5-m intervals. The structure of the enclosures is described in detail by Tian. (2001) and Wang. (1998).
2.2 Cultured Animals
The cultured animals including,,andwere stocked in the enclosures at 6indm?2, 45indm?2, 3indm?2and 30indm?2, respectively. The initial individual wet weight of,,, andwas 0.57g±0.13g, 0.045g±0.01g, 0.67g±0.09g, and 0.92g±0.11g, respectively. The crabs were fed the blue clamtwice a day (06:00 and 18:00). The amount ofsupplied to the crab changed from 80.00% to 2.00% of the crab’s body weight at different stages of development (Zhou., 2010). The shrimps were fed a commercial pellet produced by Lianyungang Chia Tai Feed Co., Ltd. (Lian- yungang, Jiangsu, China). The shrimp was also fed twice a day (06:00 and 18:00). The entire experiment was carried out for 90 days from July 13 to October 13, 2014. During the whole culture period, the polyculture ecosystem was kept in a relatively stable state, with no outbreak of disease being documented.
2.3 Sample Collection and Preparation
Water samples for POM were collected after the imported food ofand shrimp feed had been supplied to the enclosure ecosystem for 24h, when the imported uneaten food could settle on the bottom of the enclosure pond completely. POM was obtained by means of filtration: about 2L seawater was filtered through a screen with mesh size of 63μm to remove large particles and zooplankton and then filtered under vacuum onto pre- combusted (450℃, 6h) and pre-weighed Whatman GF/F glass fiber filters. The distilled double deionized water was used to wash the residues on filter paper to remove the salt that might retain in the residue. Inorganic carbon compounds in POM samples for carbon stable isotope analysis was removed by acidification, while the samples for nitrogen stable isotope analysis was not conducted by such treatment.
Zooplankton water samples were obtained by horizontal towing using a 63μm plankton net, which were filtered through a 100 mesh sieve. Samples larger than 150μm were collected as macrozooplankton, and those smaller than 150μm were collected as microzooplankton (Zhang., 2011). All the zooplankton samples were held in filtered seawater overnight to allow the evacuation of gut contents. Periphyton grown on the polyvinyl plastic situ- ated 20cm, 70cm, and 120cm under the water surface was carefully scraped and rinsed with distilled water after sampling. Samples from different depths were thoroughly mixed. Both the zooplankton samples and periphyton were dried at 60℃ for 48h, processed to a fine powder using mortar and pestle, and desiccated pending isotope analysis, respectively. The shrimp feed, muscle tissues of,,,-andwere prepared for stable isotope analysis, respectively. These samples and bottom sediment were dried at 60℃ until constant weight, and ground to a fine powder, respectively. Samples for carbon stable isotope analysis were acidized for 4h with 1N HCl to remove carbonate and then dried at 60℃ for 24h. Samples for nitrogen stable isotopes analysis were directly measured without the treatment of acidification (Cui., 2012). All samples were then stored at ?20℃ prior to subsequent stable isotope analysis.
2.4 Water Quality

2.5 Measurement of Stable Isotope and Isotope Mixing Model
The carbon and nitrogen isotope ratios of the prepared samples were measured with an elemental analyzer coupled with an isotope ratio mass spectrometer (EA-IRMS, ThermoFinnigan MAT Delta-plus). Stable isotope ratios are expressed in standard δ-unit notation, which is defined as following relationship:
δ[(sample/standard)?1]×1000‰, (1)
whererepresents13C or15N, andrepresents13C:12C ratio for carbon or15N:14N ratio for nitrogen. The results were reported relative to the Vienna Pee Dee Belemnite standard (PDB) for carbon, and air N2for nitrogen. A laboratory working standard (glycine) was run for every 10 samples. Analytical precision for carbon and nitrogen measurements was ±0.01‰.
In this study, potential food sources of,,, macrozooplankton and microzooplankton were evaluated with the software ‘Isosource’ (Phillips and Gregg, 2003). For C and N isotopes, average fractionation effects of 1‰ (Peterson and Fry, 1987; McClelland and Valiela, 1998; McCutchan., 2003) and 2.7‰ (Caut., 2009; Feng., 2014) were respectively used to correct stable isotope shifts for each trophic level.
The macrobenthos were mainly composed of polychaetes, and the macrozooplankton was mainly composed of copepods and planktonic mollusks in this polyculture ecosystem (Feng., 2017). The polychaetes, copepods, and mollusks were the food sources for(Jiang., 1998; Yang, 2001). However, Feng. (2017) demonstrated that the biomass of polychaetes can only support less than 0.2% to the total consumption ofin this polyculture ecosystem. As a result, the macrobenthos was not considered as one of the food sources for. Thewas imported as food for. Consequently,and macrozooplankton were considered as the probable food sources forin this polyculture ecosystem. The Food contributions (%) ofand macrozooplankton to the food uptake ofwere calculated based on carbon stable isotope analysis.
2.6 Data Analysis
Data were presented as means and standard deviation (means±SD). The values of δ13C and δ15N of measured samples were compared with one-way ANOVA followed by Duncan multiple comparison, respectively. The conducted analyses were performed with software of SPSS 17.0 (SPSS Inc., Richmond, CA, USA) and value of<0.05 was considered significant.
3 Results
Water quality data are listed in Table 1 and Table 2. During the sampling period, salinity varied between 28.0‰ and 29.5‰, temperature varied between 23.5℃ and 26.5℃, and DO varied between 4.65mgL?1and 7.51mgL?1. The Chllevel in the enclosures was 10.5±0.5μgL?1, and the pH varied between 8.02 and 8.46. The concentration of DIC and NO3?-N were 15.21±2.10mgL?1and 0.82±0.13mgL?1, respectively. The concentration of NH4+-N increased from 0d to 45d, and then decreased to 75d. The highest NH4+-N value was 0.45±0.029mgL?1, and the lowest value was 0.05±0.0026mgL?1.
The δ13C and δ15N values of measured samples in poly- culture ecosystem were expressed in Table 3. The δ13C value ranged from ?25.61‰±0.36‰ to ?16.60‰±0.26‰. POM was the most δ13C depleted, followed by microzoopalnkton with the δ13C value of ?24.40‰±0.11‰. The periphyton was the most δ13C enriched, followed bywith the δ13C value of ?19.29‰±0.13‰. The δ15N value ranged from 6.80‰±0.05‰ to 13.09‰±0.13‰. The shrimp feed was the most δ15N depleted, followed by POM, with the δ15N value of 8.46‰±0.21‰.was the most δ15N enriched, followed byand macrozooplankton, with the δ15N value of 11.48‰±0.15‰ and 11.44‰±0.12‰, respectively.
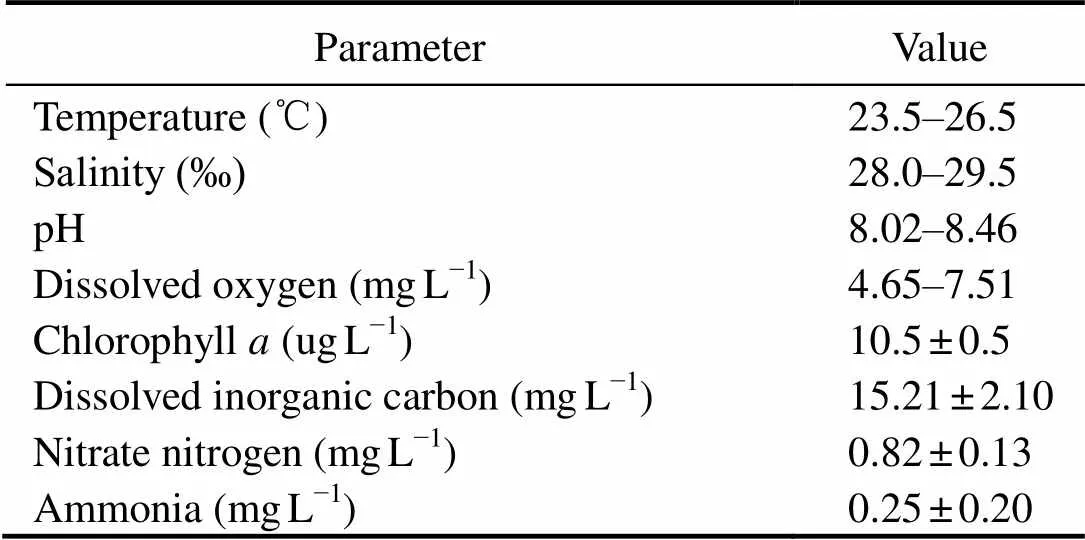
Table 1 Water quality of the polyculture pond ecosystem
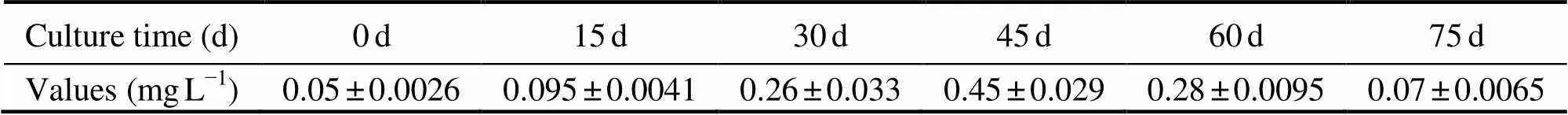
Table 2 Concentration of ammonium in the polyculture pond ecosystem during the experiment
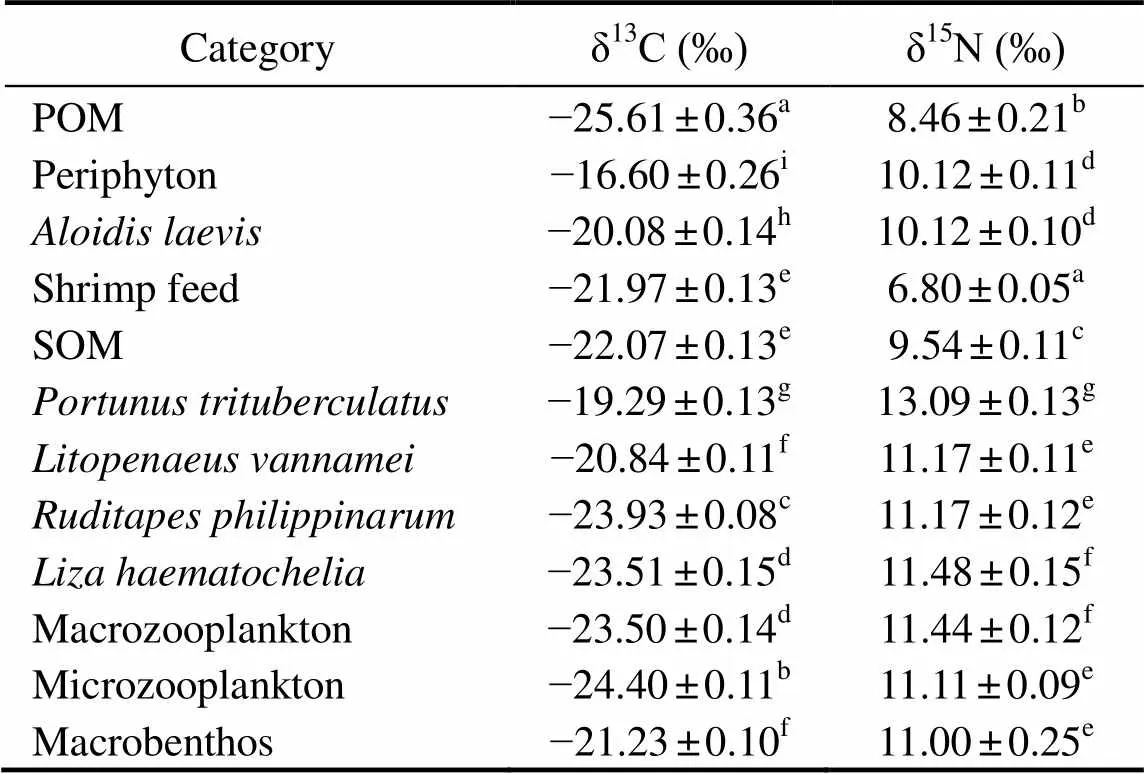
Table 3 Mean values (±SD, n=3) of δ13C and δ15N of measured samples in the polyculture pond ecosystem
Notes: POM, particulate organic matter; SOM, sediment organic matter. Different letters within the same column indicate significant differences (<0.05).
The potential food sources to diets of,,,, macrozooplankton and microzooplankton were shown in Fig.1. The relative contributions of various food sources to the total food assimilation of the consumers were showed in Table 4. Analysis of the food sources ofusing δ13C values indicated thatwas the largest component of its diet (94.00%), and the macozooplankton contributed about 6.00% to the diet ofon average. The results calculated by ‘Isosource’ indicated that the shrimp feed was the largest component in the diet of, with a relative contribution of 50.00%.and SOM were important food sources for, which contributed 28.60% and 11.27% on average to thefeeding, respectively. The POM was the dominant food source for, with the relative contribution of 66.10%. The POM was also the dominant food source for, with the relative contribution of 81.00%. Meanwhile, the shrimp feed contributed about 11.56% and 12.00% on average to the diets ofand, respectively.
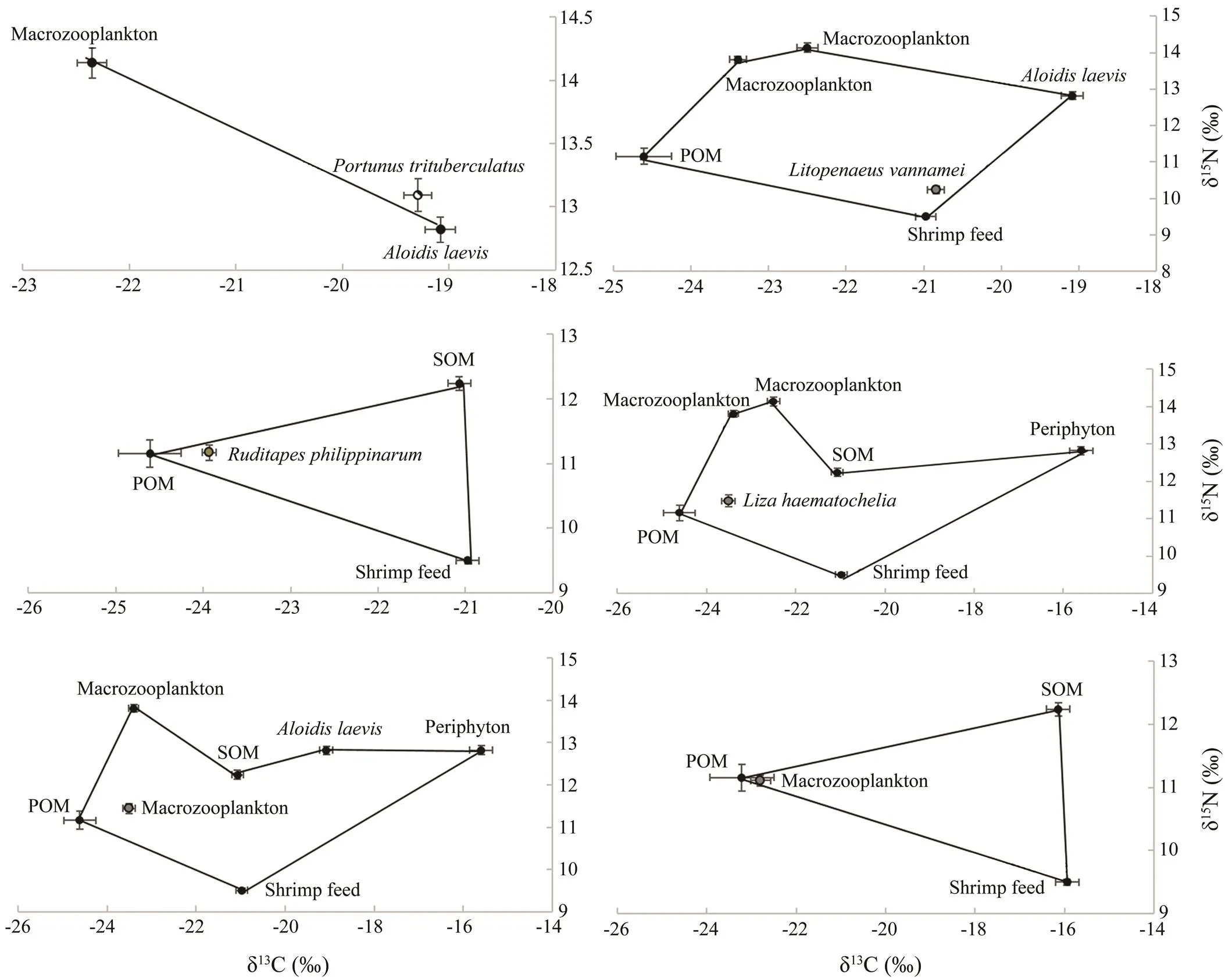
Fig.1 δ13C (‰) and δ15N (‰) values for consumers and their potential food sources in the polyculture pond ecosystem. The stable isotope ratios of food sources were correlated with average fractionation effects of 1‰ and 2.7‰ for carbon and nitrogen, respectively. POM, particulate organic matter; SOM, sediment organic matter.
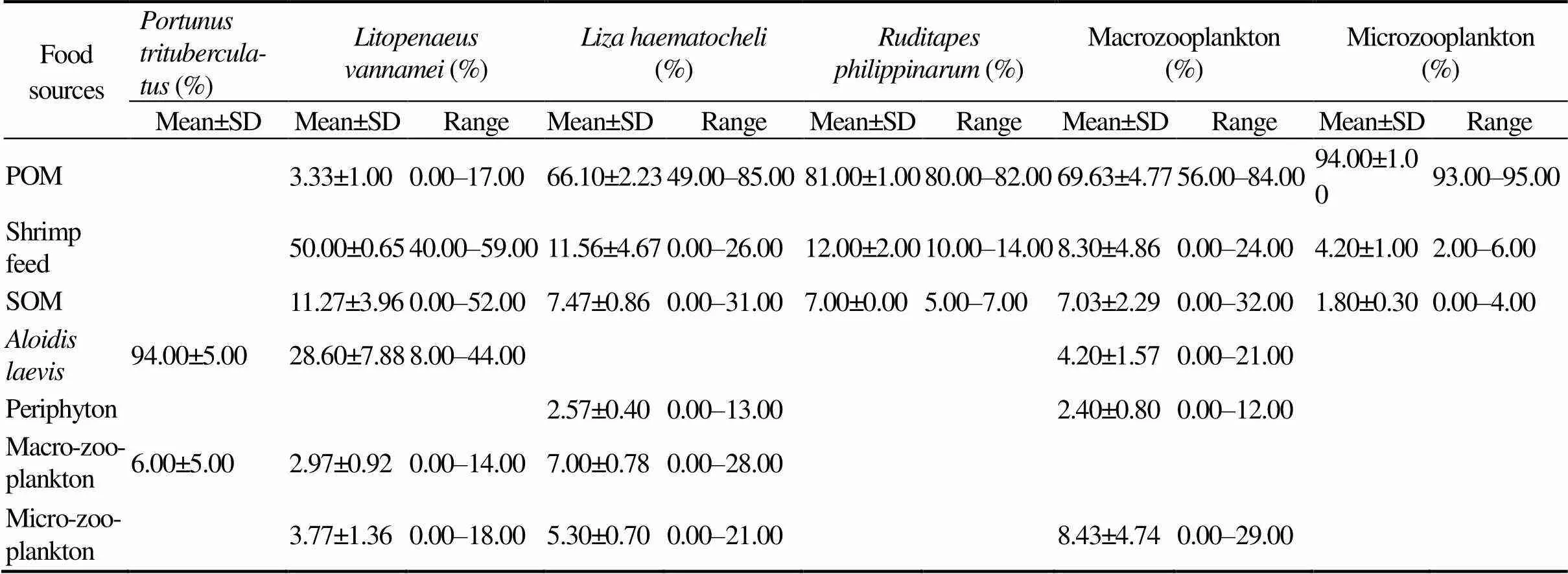
Table 4 Contributions of food sources to diets of main consumers in the polyculture pond ecosystem
Notes: POM, particulate organic matter; SOM, sediment organic matter. Average fractionation effects of 1‰ and 2.7‰ for C and N were respectively used to correct the stable isotope shifts between trophic levels. The food contributions (%) ofand macrozooplankton to the food uptake ofwere calculated based on carbon stable isotope analysis.
4 Discussion
The POM, which was mainly made up of phytoplankton (Danovaro and Fabiano, 1997; Feng., 2011), had a mean δ13C value of ?25.61‰±0.36‰ in this study. This value was lower than the values of POM in many other pond ecosystems such as the sea cucumber pond (Jin, 2010), the sea cucumber-jellyfish-Asian paddle crab polyculture pond (Feng., 2011) and the jellyfish– shellfish–fish–prawn polyculture pond (Guo., 2014). The δ13C value of phytoplankton is affected by CO2avail- ability, its isotopic content, and phytoplankton physiology (Yoshioka and Wada, 1994). The relatively low δ13C value of phytoplankton in this pond ecosystem may be ascribed to the high DIC concentration (mean: 15.21mgL?1), for DIC has been reported to be negatively correlated with δ13C value of phytoplankton in aquatic ecosystems (Leh- mann., 2004). The δ13C value of SOM in this study was ?21.95‰, which was significantly higher than that of the POM, for SOM was usually a mixture of the sediment particles of phytoplankton, feces of the cultured animals, imported artificial feeds and the benthic algae (Sara., 2007; Ren., 2013), while the POM was mainly composed of phytoplankton. The imported artificial feeds and cultured animals both had a δ13C value higher than phytoplankton in this study. Moreover, the benthic microalgae also tended to have a higher δ13C value than planktonic microalgae (France, 1995). As a result, all of these particles dedicated to a higher δ13C value of the SOM.
The δ15N value of POM (8.46‰±0.21‰) was within the range reported for marine POM (3.00‰–10.00‰) (Wada., 1991) but higher than those reported for the fish farming ecosystem (5.40‰) (Sara., 2004), shrimp pond (2.00‰) (Yokoyama., 2002), sea cucumber and prawn polyculture pond (5.70‰) (Guo., 2016) and sea cucumber pond ecosystem (5.52‰–7.52‰) (Sun., 2013). The assimilation of δ15N-enriched ammonium by algae may contribute to the high δ15N values of phytoplankton (Fogel and Cifuentes, 1993). It was observed that the ammonium had reached peak values on late August in this polyculture ecosystem. The ammonium which serves as an N source for algae may help increase δ15N values of phytoplankton of this ecosystem. Moreover, the imported food ofwhich represents the major N- input of this polyculture ecosystem (Ban, 2016) may also contribute to the high δ15N value of phytoplankton, because the importedalso has a high value of δ15N ratio (Altabet, 1988; Freudenthal., 2001; Caut., 2009).
The relative contribution of primary production and the imported food to the growth of cultured animals has been considered as a long-standing question in aquaculture pond ecosystems (Anderson., 1987). However, the measurement of stable isotope values of the cultured animals and their potential food sources may provide some clues about the food sources that are assimilated (Jin., 2013; Li., 2013; Li., 2017) as the stable isotope approach assumes a fixed isotopic trophic enrichment between the prey and predator. In the present study, δ13C trophic enrichment of 1‰ and the δ15N trophic enrichment of 2.7‰ were used to estimate the relative contribution of the food resources to their consumers (Peterson and Fry, 1987; Caut., 2009).
The relative contribution of shrimp feed to the dietary regime ofwas estimated ranging from 40.00% to 59.00% (mean: 50.00%) using an iso-source mixing model. It indicated that shrimp feed contributed about 50% to the growth carbon of. This result was higher than the previously reported while shrimp feed supplied between 23% and 47% (35%) of the growth carbon to shrimp in pond ecosystem (Anderson., 1987). However, lots of studies had reported that the artificial feeds might be inefficiently utilized by shrimp cultured in pond ecosystem (Schroeder, 1983a, b; World Bank., 2002; Burford., 2004; Silva, 2013). Large amount of shrimp feed was deposited as sediment and was inaccessible to the food chain (Silva, 2013). It was supposed that shrimp feed may also be not sufficiently consumed by shrimp in this polyculture ecosystem. Thecontributed about 8.00%–44.00% to the dietary regime of(mean: 28.60%) in this study. This demonstrated that shrimp was a suitable polyculture organism for. The small particles ofleft bywere consumed again(Dong., 2013), which could effectively increase theutilization efficiency.
The POM was estimated as the primary food source forin this stud. Marine phytoplankton and benthic microalgae, which supported primary production, were widely demonstrated as the two main energy sources for intertidal bivalves (Yokoyama and Ishihi, 2003; Riera, 2007; Watanabe., 2009; Zhao., 2012). The smaller- sized bivalves tend to derive their food mostly from dead microalgae or other detritus that settle on the bottom, and the bigger bivalves have a well-balanced food source composition and may be more dependent on food sources from water-column (Compton., 2008; Davenport., 2011; Suh and Shin, 2013). However, controversy remained regarding the relative contribution of bottom or water-column-derived POM to the diets of Manila clams with varying sizes (Watanabe., 2009). The size of the observed clams (mean: 2.66cm) in this study was similar to the size of the bigger clams in the research of Suh and Shin (2013). The diet of observed clams supported the notion that the bigger clams were more dependent on food sources from water-column. A possible explanation for the diet of clams in this study is that the benthic microalgae in this ecosystem was quite low, and the water agitation system, which stirred water and supplied oxygen into pond water, provided a convenient environment for theto feed on POM from different levels of the water column.
is a popular polyculture species for its scavenging effects (Xian., 2003). The juvenile redlip mullet has a totally carnivorous diet with the total length shorter than 15mm, an omnivorous diet with the total length between 15–45mm, and an herbivorous diet with the total length longer than 50mm (Li., 1993). Moreover, phytoplankton of diatoms accounted for the largest percentage of juvenile redlip mullet’s (longer than 50mm) diets (Zhang., 1982). Quan. (2007) reported that marsh plant () could also be a main food source for., which contributed no less than 50.00% of the organic carbon for.in Changjiang River estuary, and epibenthic microalgae constituted the main food sources for.in an artificial lagoon (Quan., 2010). In this study, the POM reached up to 49.00–85.00% (mean: 66.10%) of the diet of, and the diatom constituted the largest part of the phytoplankton biomass (Unpublished data), indicating that diatom was also the main food source for. The primary food source for.widely varied among habitat types.
In addition to the POM, the iso-source mixing model showed that the shrimp feed was also a significant part of food source for(mean: 12.00%) and.(mean: 11.56%). The important role that the shrimp feed played in polycultured species-bivalve’s diet has also been reported by Yokoyama. (2002). In their research, shrimp feed was the main food source for green mussels in a shrimp polyculture pond ecosystem. As mentioned above, there may be a part of shrimp feed imported to the aquaculture pond that had not been utilized by the shrimp, which may lead to a waste of the shrimp feed. The consumption of shrimp feed byand.would increase the utilization efficiency of the imported shrimp feed effectively in this polyculture ecosystem.
Based upon stable isotope analysis, a simplified diagram of food web in the polyculture pond was obtained and shown in Fig.2, which presented that food sources for consumers in this polyculture ecosystem mainly stem from POM,, and shrimp feed. Material flow fromto, shrimp feed to, POM to, and POM to., were the four main material flow pathways in this polyculture ecosystem. Meanwhile, material flow fromto shrimp, shrimp feed toand shrimp feed to., were important bypass flow pathways. It can be inferred that the consumption ofby, and the consumption of shrimp feed byand.would make the imported food ofand shrimp feed effectively utilized. The consumption of POM byand.would make primary production supported by phytoplankton effectively utilized. As the phytoplankton, which support primary production, and the imported food are the two primary energy sources for aquaculture pond ecosystems (Feng., 2014), poly- culture ofwith,, andwith suitable farming density can make these two primary food sources utilized with high efficiency. In addition, the consumption of POM of fecal material released by cultured animals (., fish) and other detritus particles by.andwould make the economic benefit of production and survival rate of the cultured organisms increase due to the maintenance of water quality. In summary, this polyculture mode is an ecologically efficient, product-diversified, and profitable aquaculture mode for. It is worthwhile to be conducted by more farmers.
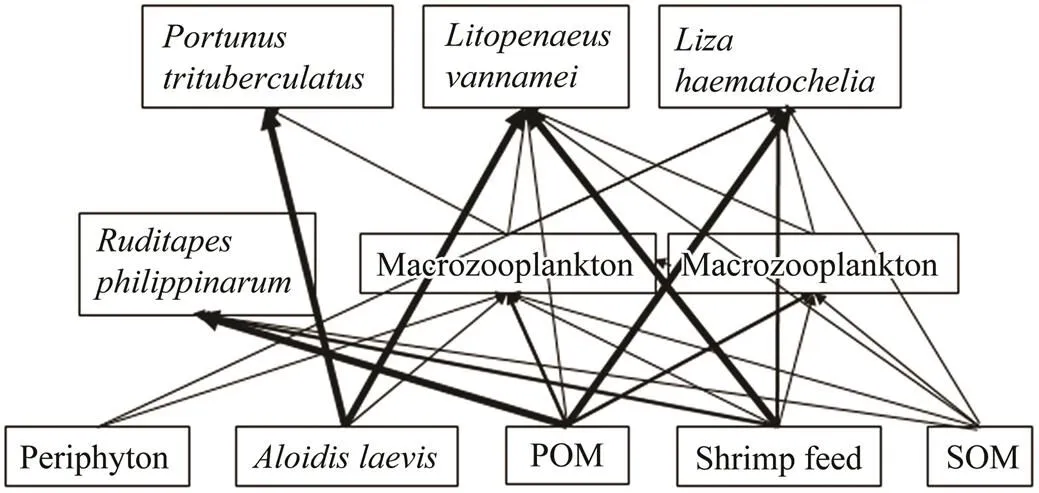
Fig.2 A simplified diagram of the polyculture pond food web. Bold arrows represent main energy flow pathways. POM, particulate organic matter; SOM, sediment organic matter.
Acknowledgements
This study was supported by the National Great Project of Scientific and Technical Supporting Programs (No. 2011BAD13B03) and the Programs for Excellent Youth Foundation of Shandong Province (No. JQ201009). We thank Drs. Mingliang Gao, Wenbo Ban, and Run Wang for helping with experimental management and sampling.
Altabet, M. A., 1988. Variations in nitrogen isotopic composition between sinking and suspended particles: Implications for nitrogen cycling and particle transformation in the open ocean., 35:535-554.
Anderson, R. K., Parker, P. L., and Lawrence, A., 1987. A13C/12C tracer study of the utilization of presented feed by a commercially important shrimpin a pond grow out system., 18 (3): 148-155.
Ban, W. B., 2016. Optimization, nitrogen and phosphorus budgets and emergy analysis of differentpolyculture systems. Master thesis. Ocean University of China, Qingdao (in Chinese with English abstract).
Burford, M. A., Preston, N. P., Minh, T. H., Hoa, T. T. T., Bunn, S. E., and Fry, V. M., 2015. Dominant sources of dietary carbon and nitrogen for shrimp reared in extensive rice-shrimp ponds., 35 (2): 194-203.
Caut, S., Angulo, E., and Courchamp, F., 2009. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction., 46: 443-453.
Cohen, J. E., Beaver, R. A., Cousins, S. H., DeAngelis, D. L., Goldwasser, L., Heong, K. L., Holt, R. D., Kohn, A. J., Lawton, J. H., Martinez, N., O’Malley, R., Page, L. M., Patten, B. C., Pimm, S. L., Polis, G. A., Rejmanek, M., Schoener, T. W., Schoenly, I. S., Sprules, W. G., Teal, J. M., Ulanowicz, R. E., Warren, P. H., Wilbur, H. M., and Yodzis, P., 1993. Improving food webs., 74 (1): 252.
Compton, T. J., Kentie, R., Storey, A. W., Veltheim, I., Pearson, G. B., and Piersma, T., 2008. Carbon isotope signatures reveal that diet is related to the relative sizes of the gills and palps in bivalves., 361: 104-110.
Cui, Y., Wu, Y., Zhang, J., and Wang, N., 2012. Potential dietary influence on the stable isotopes and fatty acid compositions of jellyfishes in the Yellow Sea.Biological Association , 92: 1325-1333.
Danovaro, R., and Fabiano, M., 1997. Seasonal changes in quality and quantity of food available for benthic suspension- feeders in the Golfo Marconi (north-western Mediterranean)., 44: 723-736.
Davenport, J., Ezgeta-Balic, D., Peharda, M., Skejic, S., Nin cevic-Gladan, Z., and Matijevic, S., 2011. Size-differential feeding inL (Mollusca: Bivalvia): Exploitation of detritus, phytoplankton and zooplankton., 92: 246-254.
Diana, J. S., Lin, C. K., and Schneeberger, P. J., 1991. Relationships among nutrient inputs, water nutrient concentrations, primary production, and yield ofin ponds., 92: 323-341.
Ding, T. B., and Liu, Z. H., 2009. Factors affecting survival rate of the pond-culturedand the resolvent method., 8: 21-22 (in Chinese with English abstract).
Dong, J., Tian, X. L., Dong, S. L., Zhang, K., Feng, J., and He, R. P., 2013. Study on nitrogen and phosphorus budget in polyculture system ofwith., 43 (12): 16-24 (in Chinese with English abstract).
Feng, J. X., Gao, Q. F., Dong, S. L., Sun, Z. L., and Zhang, K., 2014. Trophic relationships in a polyculture pond based on carbon and nitrogen stable isotope analyses: A case study in Jinghai Bay, China., 428-429 (11): 258-264.
Feng, J., Tian, X. L., Dong, S. L., He, R. P., Zhang, K., Zhang, D. X., and Zhang, Q. Q., 2017. Model-based analysis of the energy fluxes and trophic structure of apolyculture ecosystem., 9: 479-490.
Feng, J., Tian, X. L., Dong, S. L., Zhang, K., and Dong, J., 2015. Studies on the energy budget of different polyculture systems of swimming crab., 45 (3): 9-47 (in Chinese with English abstract).
Fogel, M. L., and Cifuentes, L. A., 1993... Springer, US, 73-98.
France, R., 1995. Carbon-13 enrichment in benthic compared to planktonic algae: Food web implications., 124: 307-312.
Freudenthal, T., Wagner, T., Wenzh?fer, F., Zabel, M., and Wefer, G., 2001. Early diagenesis of organic matter from sediments of the eastern subtropical Atlantic: Evidence from stable nitrogen and carbon isotopes., 65: 1795-1808.
Fry, B., and Sherr, E. B., 1984. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems., 27: 13-47.
Guo, K., Zhao, W., Wang, S., and Dong, S. L., 2014. Study of food web structure and trophic level in the sea ponds of an optimized culture model (jellyfish–shellfish–fish–prawn)., 22 (6): 1783-1791.
Guo, K., Zhao, W., Wang, S., Liu, B. Z., and Zhang, P., 2016. Food web structure and trophic levels in a saltwater pond sea cucumber and prawn polyculture system., 35 (4): 58-62.
Hobson, K. A., and Welch, H. E., 1992. Determination of trophic relationships within a high Artic marine food web using δ13C and δ15N analysis., 84: 9-18.
Jiang, W. M., Tian, X., Chen, R. S., and Wei, S., 1998. Diet of(A. Miline-Edwards) and(Miers) in the Bohai Sea., 19: 53-59 (in Chinese with English abstract).
Jin, B. C., 2010. Using stable isotope to evaluate food sources of pond-cultured sea cucumber (). PhD thesis. Ocean University of China, Qingdao (in Chinese with English abstract).
Jin, B. C., Dong, S. L., Tian, X. L., Wang, F., Gao, Q. F., Li, G. H., and Guan, J. H., 2013. Using carbon stable isotope ratio (δ~(13)C) to evaluate contribution of artificial feeds to growth of pond cultured juvenile sea cucumber(Selenka)., 37 (2): 269 (in Chinese with English abstract).
Kharlamenko, V. I., Kiyashko, S. I., Rodkina, S. A., and Imbs, A. B., 2008. Determination of food sources of marine invertebrates from a subtidal sand community using analyses of fatty acids and stable isotopes., 34: 101-109.
Kling, G. W., Fry, B., and O’Brien, W. J., 1992. Stable isotopes and planktonic trophic structure in arctic lakes., 73: 561-566.
Lehmann, M. F., Bernasconi, S. M., Mckenzie, J. A., Barbieri, A., Simona, M., and Veronesi, M., 2004. Seasonal variation of the δ13C and δ15N of particulate and dissolved carbon and nitrogen in Lake Lugano: Constraints on biogeochemical cycling in a eutrophic lake., 49 (2): 415-429.
Lei, Y. Z., 2006.. China Agriculture Press, Beijing, 49-56.
Li, D. S., 2005. A study on pond culture of., 8 (3): 12-13 (in Chinese).
Li, M. D., 1993. Researches on Chinese Mulletduring past forty-two years., 12 (6): 81-86 (in Chinese with English abstract).
Li, X. M., Zhu, Y. J., Wang, X. G., Xu, D. G., and Yang, D. G., 2017. Using a stable isotope ratio to evaluate the contribution of food to bighead carp () subjected to different breeding methods., 24 (2): 278-283.
Li, Y. M., Huang, H. X., Wang, P., Liu, M., Li, Y. J., and Li, X., 2013. Analysis on diet ofusing tracer technology of carbon stable isotope., 40 (20):125-128.
Lorenzen, C., and Jeffrey, S., 1980. Determination of chlorophyll in seawater., 35: 1-20.
Mahmood, T., Fang, J., Jiang, Z., and Zhang, J., 2016. Carbon, nitrogen flow and trophic relationship among the cultured species in an integrated multi-trophic aquaculture (IMTA) bay., 8: 207-219.
McClelland, J. W., and Valiela, I., 1998. Changes in food web structure under the influence of increased anthropogenic nitrogen inputs to estuaries., 186: 259-271.
McCutchan, J. H., Lewis, W. M., Kendall, C., and McGrath, C. C., 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur., 102: 378-390.
Peterson, B. J., and Fry, B., 1987. Stable isotopes in ecosystem studies., 18: 293- 320.
Phillips, D. L., and Gregg, J. W., 2003. Source partitioning using stable isotopes: Coping with too many sources., 136: 261-267.
Quan, W. M., Fu, C. Z., Jin, B. S., Luo, Y. Q., Li, B., Chen, J. K., and Wu, J. H., 2007. Tidal marshes as energy sources for commercially important nektonic organisms: Stable isotope analysis., 352: 89-99.
Quan, W. M., Shi, L. Y., and Chen, Y. Q., 2010. Stable isotopes in aquatic food web of an artificial lagoon in the Hangzhou Bay, China., 28 (3): 489-497.
Ren, L., Zhang, J., Fang, J., Tang, Q., Zhang, M., and Du, M., 2013. Impact of shellfish biodeposits and rotten seaweed on the sediments of Ailian Bay, China., 22 (2): 811-819.
Riera, P., 2007. Trophic subsidies of,andin the Bay of Mont Saint Michel (France): A δ13C and δ15N investigation., 72: 33-41.
Sara, G., Pirro, M. D., Romano, C., Rumolo, P., Sprovieri, M., and Mazzola, A., 2007. Sources of organic matter for intertidal consumers on Ascophyllum-shores (SW Iceland): A multi- stable isotope approach., 61 (4): 297-302.
Sara, G., Scilipoti, D., Mazzola, A., and Modica, A., 2004. Effects of fish farming waste to sedimentary and particulate organic matter in a southern Mediterranean area (Gulf of Castellammare, Sicily): A multiple stable isotope study (delta13C and delta15N)., 234 (1): 199-213.
Schroeder, G. L., 1983a. Sources of fish and prawn growth in polyculture ponds as indicated by BC analysis., 35: 29-42.
Schroeder, G. L., l983b. Stable isotope ratios as naturally occurring tracers in the aquaculture food web., 30: 203-210.
Shi, H. L., Jin, C. L., Lin, G. Z., Lou, B., and Mao, G. M., 2010. The aquaculture ofin Zhejiang Pro- vince., 7: 39-41 (in Chinese with English abstract).
Silva, C. A. R. E., 2013. Shrimp farming: Where does the carbon go?, 56 (1): 13-18.
Song, H. T., Yu, C. G., Xue, L. J., and Yao, G. Z., 2006.. Ocean Press, Beijing, 83-86 (in Chinese).
Su, X. R., Li, T. W., Ding, M. J., and Chen, P. K., 1997. Evalution on nutritive value of., 15 (2): 168-172.
Suh, Y. J., and Shin, K. H., 2013. Size-related and seasonal diet of the manila clam (), as determined using dual stable isotopes., 135 (20): 94-105.
Sun, Z. L., Gao, Q. F., Dong, S. L., Shin, P. K. S., and Wang, F., 2013. Seasonal changes in food uptake by the sea cucumberin a farm pond: Evidence from C and N stable isotopes., 12 (1): 160-168.
Tian, X. L., Li, D. S., Dong, S. L., Yan, X. Z., Qi, Z. X., Liu, G. C., and Lu, J., 2001. An experimental study on closed-poly- culture of penaeid shrimp with tilapia and constricted tagelus., 202: 57-71.
Wada, E., Mizutani, H., and Minagawa, M., 1991. The use of stable isotopes for food web analysis., 30: 361-371.
Wang, J. Q., Li, D. S., Dong, S. L., Wang, K. X., and Tian, X. L., 1998. Experimental studies on polyculture in closed shrimp ponds I. Intensive polyculture of Chinese shrimp ().with tilapia hybrids., 163: 11-27.
Wang, X. Q., Cao, M., Yan, B. L., and Zhang, Q. Q., 2009. Integrated culture of swimming crab., 28 (2): 105-108 (in Chinese with English abstract).
Watanabe, S., Katayama, S., Kodama, M., Cho, N., Nakata, K., and Fukuda, M., 2009. Small-scale variation in feeding environments for the Manila clamin a tidal flat in Tokyo Bay., 75: 937-945.
Watanabe, S., Kodama, M., and Sumbing, J. G., 2013. Lebata- Ramos MJH. Diet-tissue stable isotopic fractionation of tropical sea cucumber,., 47 (47): 127-134.
World Bank, NACA, WWF, and FAO, 2002.. Published by the Consortium, 119pp.
Xian, W., Yang, H., and Liu, R., 2003. Function of a detritivorous fish as scavenger of aquaculture environment., 13: 81-84.
Xu, G. Z., Zhen, C. W., Tang, T. D., Li, W. J., Liang, S. J., Sun, Q. H., Zhou, S. C., Chen, H. B., Pan, C. R., Cai, Z. C., Yan, Y. B., and Tian, J., 1987.. Chinese Agricultural Press, Beijing, 11-13 (in Chinese).
Xu, Q., Zhang, L. B., Zhang, T., Zhang, X. L., and Yang, H. S., 2017. Functional groupings and food web of an artificial reef used for sea cucumber aquaculture in northern China., 119: 1-7.
Yang, J. M., 2001. A study on food and trophic levels of Bohai Sea invertebrates., 16: 8-16 (in Chinese with English abstract).
Yokoyama, H., and Ishihi, Y., 2003. Feeding of the bivalveon benthic microalgae: Isotope evidence., 255: 303-309
Yokoyama, H., Higano, J., Adachi, K., Ishihi, Y., and Yamada, Y., 2002. Evaluation of shrimp polyculture system in Thailand based on stable carbon and nitrogen isotope ratios.,68: 745-750.
Yoshioka, T., and Wada, E., 1994. A stable isotope study on seasonal food web dynamics in a eutrophic lake., 75 (3): 835-846.
Zhang, K., Tian, X. L., Dong, S. L., Dong, J., Feng, J., He, R. P., and Yan, F. J., 2015. Nitrogen and phosphorus of polyculture system of,, and45 (2): 44-53 (in Chinese with English abstract).
Zhang, K., Tian, X. L., Dong, S. L., Feng, J., and He, R. P., 2016. An experimental study on the budget of organic carbon in polyculture ecosystems of swimming crab with white shrimp and short-necked clam., 451: 58-64.
Zhang, L. Y., Fan, N. C., and Huang, M. X., 1982. Study on the feeding habits of redlip mullet. In:. Barracuda and redlip mullet research group, eds., Agricultural Press, Beijing, 31-53.
Zhang, T. W., 2011. The model of carbon flux in intensive Penaeid shrimp culture and ecological cultivation. PhD thesis. Ocean University of China, Qingdao (in Chinese with English abstract).
Zhao, L. Q., Yan, X. W., Huo, Z. M., Yang, F., and Zhang, G. F., 2012. Divergent selection for shell length in the Manila clam., 43: 878-884.
Zhou, Y. G., Ma, S., Su, Y. P., Yan, B. L., Wang, X. Q., and Liu, Y. K., 2010. Studies on the effects of polyculture ofwith., 40 (3): 11-16 (in Chinese with English abstract).
September 9, 2017;
March 8, 2018;
September 13, 2018
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-532-82032117 E-mail: xianglitian@ouc.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2018年6期
Journal of Ocean University of China2018年6期
- Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Rhopilema hispidum
- The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate
- An Evaluation on the Ratio of Plant to Animal Protein in the Diet of Juvenile Sea Cucumber (Apostichopus japonicus): Growth, Nutrient Digestibility and Nonspecific Immunity
- Weighted Correlation Network Analysis (WGCNA) of Japanese Flounder (Paralichthys olivaceus) Embryo Transcriptome Provides Crucial Gene Sets for Understanding Haploid Syndrome and Rescue by Diploidization
- Lead Induces Different Responses of Two Subpopulations of Phagocytes in the Holothurian Eupentacta fraudatrix
