Simultaneous determination of indapamide,perindopril and perindoprilat in human plasma or whole blood by UPLC-MS/MS and its pharmacokinetic application
Yi To,Sheng Wng,Lei Wng,Min Song,Tijun Hng,*
aDepartment of Pharmaceutical Analysis,China Pharmaceutical University,Nanjing 210009,China
bJiangsu Jiayi Pharmaceutical Co.,Ltd.,Nanjing 210000,China
Keywords:Indapamide Perindopril Perindoprilat Pharmacokinetics LC-MS/MS
A B S T R A C T Simple and sensitive methods were developed for the determination of indapamide,perindopril and its active metabolite perindoprilat in human plasma or whole blood by hyphenated ultra-performance liquid chromatography-mass spectrometry(UPLC-MS/MS).Indapamide-d3,perindopril-d4 and perindoprilat-d4 were used as the internal standards.The separation was performed on a Thermo BDS Hypersil C18column(4.6 mm × 100mm,2.4 μm)for indapamide and perindopril simultaneously following a protein precipitation pretreatment of the biosamples.The separation of perindoprilat was achieved independently on a phenomenex PFP column(4.6 mm × 150 mm,5μm).All the analytes were quantitated with positive electrospray ionization and multiple reactions monitoring mode.The assay exhibited a linear range of 1–250 ng/mL for indapamide,0.4–100ng/mL for perindopril and 0.2–20 ng/mL for perindoprilat.The methods were fully validated to meet the requirements for bioassay in accuracy,precision,recovery,reproducibility,stabilities and matrix effects,and successfully applied to the pharmacokinetic study of perindopril tert-butylamine/indapamide compound tablets in Chinese healthy volunteers and the comparative pharmacokinetic study between plasma and whole blood.
1.Introduction
Perindopril(Fig.1),a non-sulfhydryl angiotensin-converting enzyme inhibitor(ACEI),is prescribed for the treatment of hypertension and heart failure.It is hydrolysed to the active metabolite perindoprilat(Fig.1)in vivo,which can selectively inhibit the activity of angiotensinconverting enzyme(ACE)and reduce the level of angiotensinⅡ[1,2].Indapamide(Fig.1)is a thiazide-type diuretic commonly used to treat mild to moderate hypertension by diuretic effect and calcium antagonist activity.As an antihypertensive drug proved to produce a significant and sustained reduction in blood pressure,indapamide works on sodium and chloride excretion and it has been reported to reduce the vascular reactivity to pressor amines[3–5].
The combination of perindopril and indapamide is suggested as one of the antihypertensive combinations of priority use by the last update of European Hypertension Guidelines[5,6].Due to their synergistic mechanisms of actions,this combination has shown a higher antihypertensive effect with fewer side effects[7,8]. Usually perindopril tert-butylamine/indapamide is administered in the form of tablets containing 2/0.625,4/1.25 or 8/2.5 mg of the active pharmaceutical ingredients.
Several LC-MS/MS methods have been reported for the pharmacokinetic studies on perindopril and perindoprilat or indapamide individually in biological samples[9–14].However,only a few pharmacokinetic studies on perindopril tert-butylamine/indapamide compound tablets have been reported[15,16].And until now,there have not been any reports for comparative pharmacokinetic studies between plasma and whole blood.This paper describes a sensitive and reproducible LC-MS/MS method for the simultaneous determination of indapamide and perindopril in both human plasma and whole blood and an independent method for perindoprilat.The methods have been fully validated and applied to the pharmacokinetic study of perindopril tert-butylamine/indapamide compound tablets in Chinese healthy volunteers and the comparative pharmacokinetic study between plasma and whole blood.
2.Experimental
2.1.Chemicals and reagents
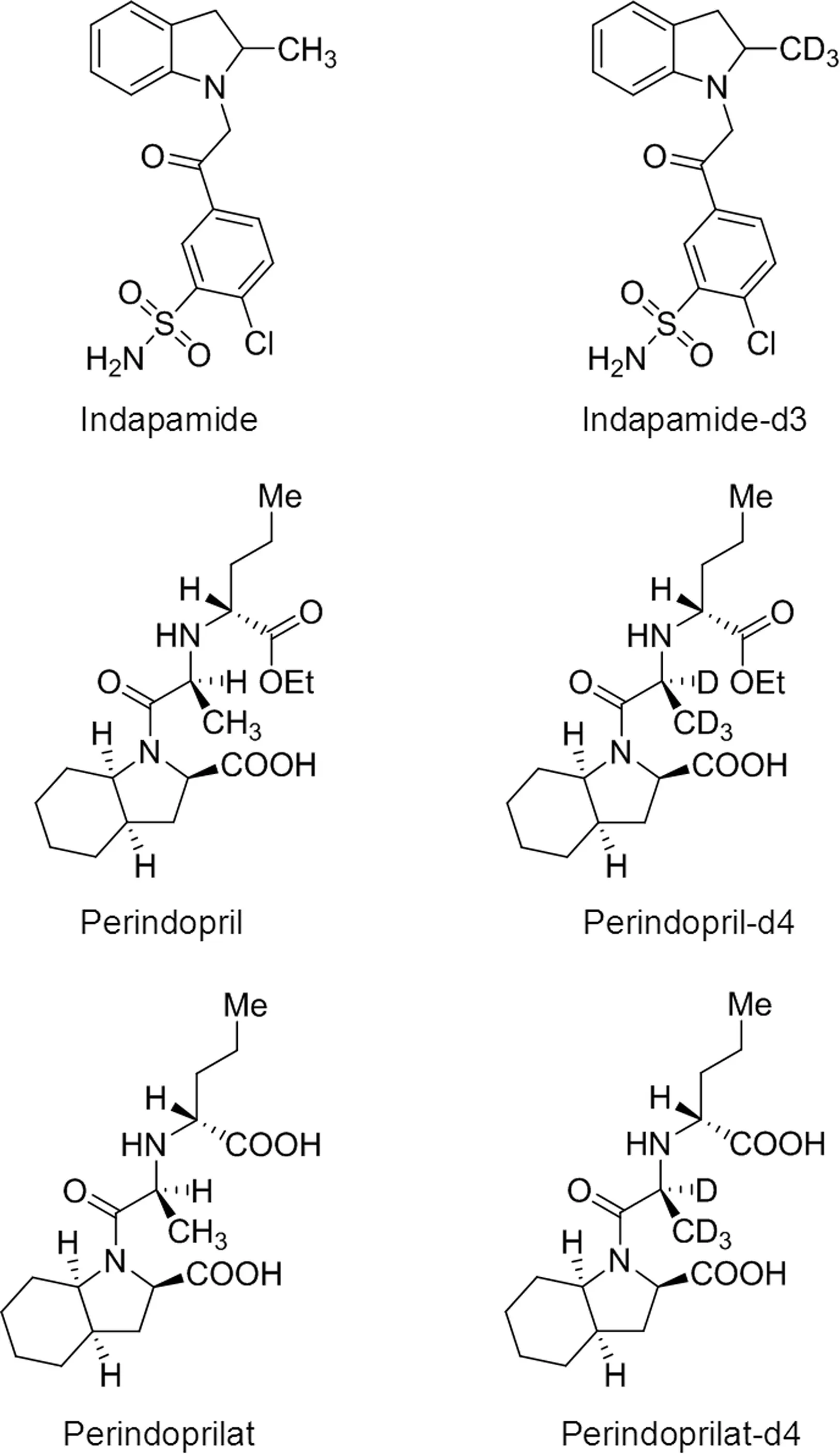
Fig.1.Chemical structures of indapamide,perindopril,perindoprilat,indapamided3(IS),perindopril-d4(IS)and perindoprilat-d4(IS).
The reference standards of indapamide(99.5%),perindopril tert-butylamine(99.2%)and perindoprilat(99.2%)were obtained from National Institutes for Food and Drug Control(Beijing,China).Indapamide-d3(97%),perindopril-d4(98%)and perindoprilat-d4(98%)reference standards employed as the internal standards(ISs)for the bioassays were obtained from Toronto Research Chemicals(North York,Canada).Chemical structures of these compounds are presented in Fig.1.Perindopril tert-butylamine/indapamide compound tablets(Batch No:607903)were purchased from Servier Pharmaceutical Co.,Ltd.(Tianjin,China).HPLC grade methanol,acetonitrile and formic acid were purchased from Tedia(Ohio,USA).Guaranteed grade perchloric acid was purchased from Sinopharm Chemical Reagent Co.,Ltd(Shanghai,China).Analytical grade ammonium acetate was purchased from Nanjing Chemical Reagent Co.,Ltd.(Nanjing,China).Water was purified with a Millipore Milli Q-Plus system(Millipore,MA,USA).
2.2.Instruments
Quantitative analysis was performed with a Dionex Ultimate 3000 chromatographic system(Thermo Scientific,MA,USA)coupled to a TSQ Quantum Ultra AM triple quadrupole mass spectrometer(Thermo Scientific,MA,USA)operated in positive electrospray ionization(ESI)through multiple reaction monitoring(MRM)mode.System control and data acquisitions were conducted by LCQUAN?2.9QF1(Thermo Scientific,MA,USA).
2.3.Liquid chromatographic and mass spectrometric conditions
2.3.1.Indapamide and perindopril
Simultaneously chromatographic separations of perindopril and indapamide were achieved on a Thermo BDS Hypersil C18column(4.6 mm × 100 mm,2.4 μm)maintained at 40°C through linear gradient elution at 0.65mL/min of the mobile phases consisting of A(a mixture of water and methanol(90:10,v/v)containing 0.05%ammonium acetate and 0.2%formic acid)and B(methanol containing 0.05%ammonium acetate and 0.2%formic acid)according to the following programs(A:B):0.0 min(60:40)–3.5 min(60:40)–3.6 min(5:95)–4.6 min(5:95)–4.7 min(60:40)–6.0 min(60:40).
Mass spectrometer was operated in a positive ionization mode and the analytes were detected by MRM with the transitions ofm/z366.4–132.1 for indapamide,m/z369.1–135.0 for indapamide-d3,m/z369.3–172.1 for perindopril,andm/z373.3–176.1 for perindopril-d4,respectively.The parameters were optimized as follows:spray voltage 4.5 kV,capillary temperature 350°C,the sheath gas 275 kPa,auxiliary gas 35 kPa,ion sweep gas 3.5 kPa and collision argon gas 1.6×10-6bar.The collision energy was set 12 eV for indapamide and indapamide-d3,16 eV for perindopril and perindopril-d4.
2.3.2.Perindoprilat
Chromatographic separation of perindoprilat was achieved on a Phenomenex PFP column(4.6 mm × 150 mm,5 μm)maintained at 40°C through linear gradient elution at 1mL/min of the same mobile phases consisting of A and B according to the following programs(A:B):0.0 min(70:30)–1.0 min(70:30)–1.5 min(20:80)–5.0 min(20:80)–5.1 min(70:30)–7.0 min(70:30).
Mass spectrometer was operated in a positive ionization mode and the analytes were detected by MRM with the transitions ofm/z341.2–170.1 for perindoprilat,andm/z345.2–170.1 for perindoprilat-d4.The parameters were optimized as follows:spray voltage 4.5 kV,capillary temperature 350°C,the sheath gas 275 kPa,auxiliary gas 35 kPa,ion sweep gas 3.5 kPa and collision argon gas 1.6×10-6bar.The collision energy was set 15eV for perindoprilat and perindoprilat-d4.
2.4.Preparation of calibration curve and quality control(QC)samples
Primary stock solutions of indapamide(100μg/mL)and perindopril(100μg/mL)were prepared in acetonitrile and diluted with the same solution to make a series of standard working solutions of 5,12.5,25,50,125,250,500,1000 and 1250 ng/mL for indapamide and 2,5,10,20,50,100,200,400 and 500ng/mL for perindopril.Primary stock solution of perindoprilat(100μg/mL)was prepared in a mixture of methanol and water(80:20,v/v)and diluted with the same solution to make a series of standard working solutions of 1,2.5,5,10,25,50,80 and 100 ng/mL.
The stock solutions of indapamide-d3(100μg/mL),perindopril-d4(100μg/mL)and perindoprilat-d4(100μg/mL)were prepared in acetonitrile and diluted with the same solution at concentrations of 80 ng/mL,60 ng/mL and 100 ng/mL,respectively.
Calibration standard samples were then prepared by 5-fold spiking the working solutions with blank plasma or whole blood to give respectivefinal concentrations of 1,2.5,5,10,25,50,100,200 and 250 ng/mL for indapamide,0.4,1,2,4,10,20,40,80 and 100ng/mL for perindopril and 0.2,0.5,1,2,5,10,16 and 20 ng/mL for perindoprilat.The low QC(LQC),medium QC(MQC)and high QC(HQC)were prepared with the same spiking procedure at concentrations of 2.5,25 and 200 ng/mL for indapamide,1,10 and 80 ng/mL for perindopril and 0.5,5,16ng/mL for perindoprilat.All standard solutions were stored at-20°C and plasma samples were stored at-80°C until analysis.
2.5.Sample preparation
2.5.1.Indapamide and perindopril
An aliquot of 100μL plasma or whole blood sample was spiked with 20μL of IS working solution.Then,200μL of acetonitrile was added to precipitate the protein by vortex mixing for 1 min.Following centrifugation(12,000rpm,10min at 4°C),20μL of the supernatant was injected into the LC-MS/MS system for the analysis.
2.5.2.Perindoprilat
An aliquot of 200μL plasma or whole blood sample was spiked with 40μL of IS working solution.Then,40μL of 14%perchloric acid was added to precipitate the protein by vortex mixing for 1 min.Following centrifugation(12,000 rpm,10 min at 4°C),20μL of the supernatant was injected into the LC-MS/MS system for the analysis.
2.6.Method validation
The proposed methods were validated according to the Guidelines for Bioanalytical Method Validation published by the European Medicines Agency(EMA)[17].
Specificity of the methods was investigated by comparing blank human plasma or whole blood samples obtained from six sources with those spiked with analytes and IS.The responses of endogenous interfering substances at retention time of the analytes are acceptable if there is less than 20%of the response of lower limit of quantitation(LLOQ).The responses of endogenous interfering substances at retention time of the internal standard are acceptable if there is less than 5%of the response of working internal standard.Carryover was assessed by immediately injecting blank plasma or whole blood samples after the injection of HQC samples.
Calibration curves for the methods were constructed by leastsquares linear regression analysis by plotting analyte-to-IS peak area ratio versus its nominal concentration,with 1/x2as the weighting factor.Sensitivity was determined by analyzing six replicates of plasma or whole blood spiked LLOQ samples.The backcalculated concentrations of each calibration standard have to be within±15%deviation(±20%for LLOQ)of the nominal values.
Intra-batch and inter-batch precision and accuracy were measured by analyzing QC samples at three concentration levels(LQC,MQC and HQC)with six determinations in three consecutive validation runs.The mean value should be within 15%of the actual value except at LLOQ,where it should not deviate by more than 20%.Inter-batch precision was assessed by One-way analysis of variance(ANOVA).Both intra-batch and inter-batch precision were expressed as relative standard deviation(RSD)and did not exceed 15%(20%for LLOQ).
Recovery of each analyte was determined by analyzing six replicates of QC samples at three concentration levels(LQC,MQC and HQC)and was calculated as the ratio of peak areas obtained from extracted spiked samples to that of non-extracted standard at corresponding concentrations.
Blank plasma or whole blood samples from six different single lots spiked with standard solutions at three concentration levels(LQC,MQC and HQC)were analyzed to assess the matrix effect.The matrix effect was expressed as the ratio of peak areas of extracted blank plasma or whole blood samples spiked with the pure authentic standard solutions to the peak areas of the pure authentic standard solutions at corresponding concentrations.The IS normalized matrix factors expressed as the ratio of the matrix factor of each analyte to the matrix factor of the IS were used to assess the effects of matrix on ionization.
The stability of each analyte in human plasma or whole blood was investigated at LQC and HQC(n=3)under the conditions of room temperature,three freeze-thaw(-80°C)cycles,long-term(-80 °C)storage,and autosampler(8 °C).
2.7.Pharmacokinetic study
The pharmacokinetics of perindopril tert-butylamine/indapamide compound tablets was conducted in Chinese healthy volunteers after oral administration.A total of 10 Chinese healthy volunteers were recruited after strict medical,biochemical and physical examinations and were given an informed consent approved by the Ethics Committee of National Medicine Clinical Trial Organization of Nanjing General Hospital(Nanjing,China)according to the principles of the Declaration of Helsinki.
After supervised overnight fasting of at least 10 h,all volunteers received study drug with 240mL of warm water according to the randomization schedule.Blood samples were collected following oral administration of perindopril tert-butylamine/indapamide compound tablets(4 mg/1.25 mg)at pre-dose and 0.25,0.5,0.75,1,1.25,1.5,2,2.5,3,4,6,8,10,12,24 and 48h.The blood samples were separated in two separate K2EDTA vacutainer collection tubes.One of the tubes was centrifuged at 3000 rpm for 10 min and the plasma was collected.The collected plasma and whole blood samples were stored at-80°C till use.Pharmacokinetic analysis was performed by Phoenix WinNonlin 6.0.
3.Results and discussion
3.1.Method development
3.1.1.Chromatography
Indapamide,perindopril and perindoprilat have different physicochemical properties,which leads to the difficulty in setting chromatographic conditions that produce symmetrical peak shape and adequate response for all three compounds simultaneously.Hence,two different chromatographic conditions were established according to their physicochemical properties.The composition of mobile phase and column types were mainly optimized to obtain good peak shape and chromatograph separation.The mobile phases consisting of methanol-water-formic acid were chosen to enhance the response and 0.05%ammonium acetate was added to resolve the peaks free from tailing.The use of Thermo BDS Hypersil C18(4.6 mm × 100 mm,2.4 μm)column provided good resolution and peak shapes for simultaneously indapamide and perindopril determination.In order to avoid carryover peaks after injections of high concentration samples,the proportion of organic phase was increased to 95%when indapamide and perindopril were eluted.Different types of columns like C8,C18and PFP were studied for the analysis of perindoprilat and the best conditions were achieved with gradient elution using a Phenomenex PFP column(4.6 mm × 150 mm,5 μm).
3.1.2.Mass spectrometry
Mass parameters were optimized in both positive and negative ionization modes by infusion of the standard solutions into the mass spectrometer using electrospray ionization source and the optimal parameters were obtained by automatic tuning.All three compounds showed better and more stable response when detected with positive ionization.The most sensitive mass transition was monitored fromm/z366.4–132.1 for indapamide,fromm/z369.1–135.0 for indapamide-d3,fromm/z369.3–172.1 for perindopril,fromm/z373.3–176.1 for perindopril-d4,fromm/z341.2–170.1 for perindoprilat,and fromm/z345.2–170.1 for perindoprilat-d4.
3.1.3.Sample preparation
Sample preparation is a crucial factor for method development in bio-analysis to reach the maximum extraction recovery,minimum matrix effects and also minimize the sample preparation procedure.Simple protein precipitation(PP)procedure was recommended for this work since complicated liquid-liquid extraction(LLE)may compromise the stability of perindopril and influence the quantitation of perindoprilat.Protein precipitants such as acetonitrile,methanol and ethyl alcohol were investigated for the extraction of indapamide and perindopril.14%perchloric acid,7%perchloric acid and 10%trifluoroacetic acid solution were tested for the extraction of highly polar perindoprilat.As a result,acetonitrile and 14%perchloric acid solution were respectively found to reach the best recovery,lowest diluting ratio and highest method sensitivity.The use of stable isotope labeled IS helped the improving of the determination accuracy of the analytes.The extraction methods were appropriate for both plasma and whole blood.
3.1.4.Comparison between the developed methods and the existing methods
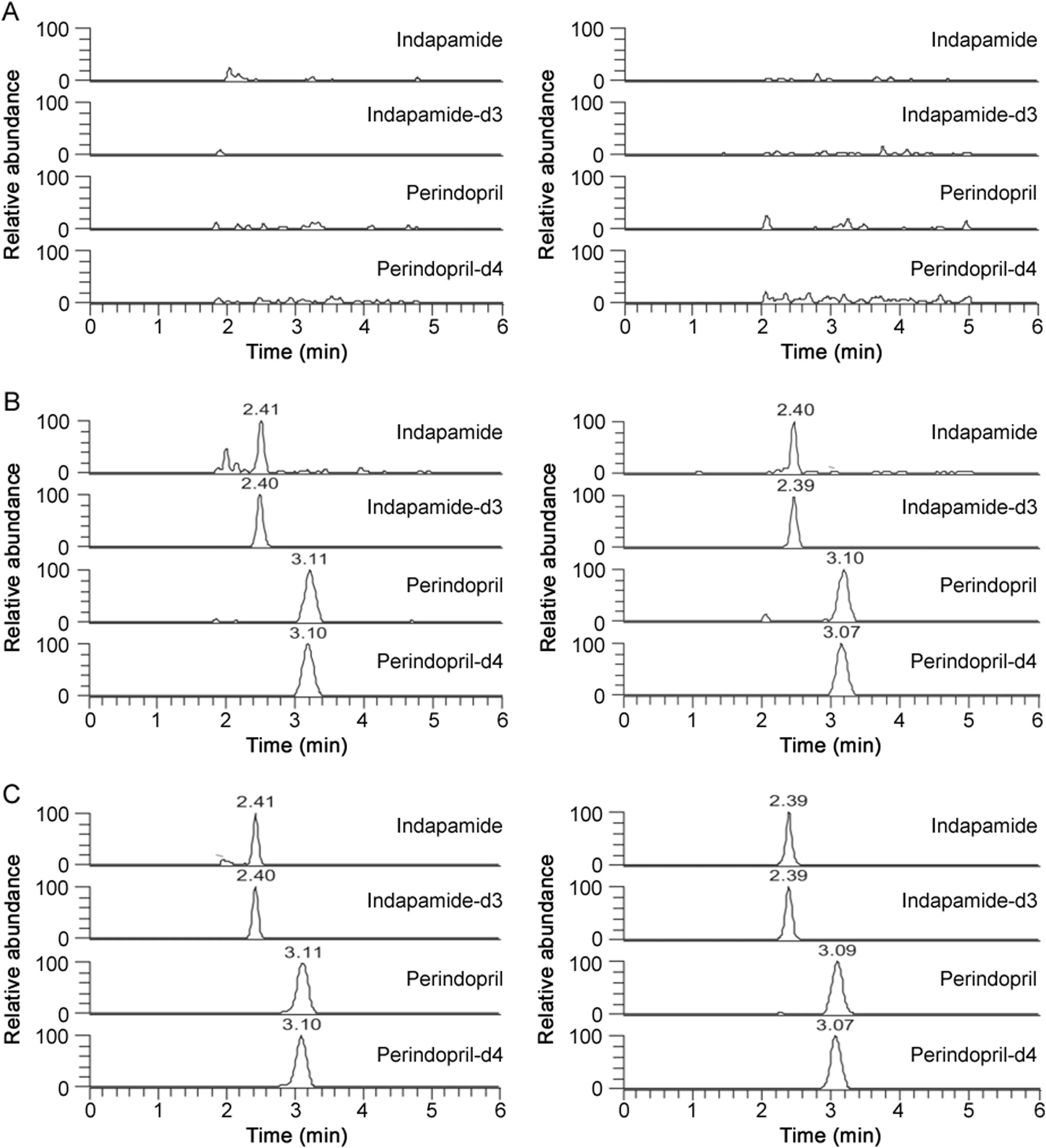
Fig.2.Typical MRM chromatograms of indapamide and perindopril in(A)blank plasma(left panel)and whole blood(right panel);(B)LLOQ sample plasma(1.00 ng/mL for indapamide and 0.400 ng/mL for perindopril)and whole blood(1.00 ng/mL for indapamide and 0.400 ng/mL for perindopril);(C)subject sample plasma(5.32 ng/mL for indapamide and 11.1 ng/mL for perindopril)and whole blood(14.9 ng/mL for indapamide and 7.35ng/mL for perindopril)at 2 h.
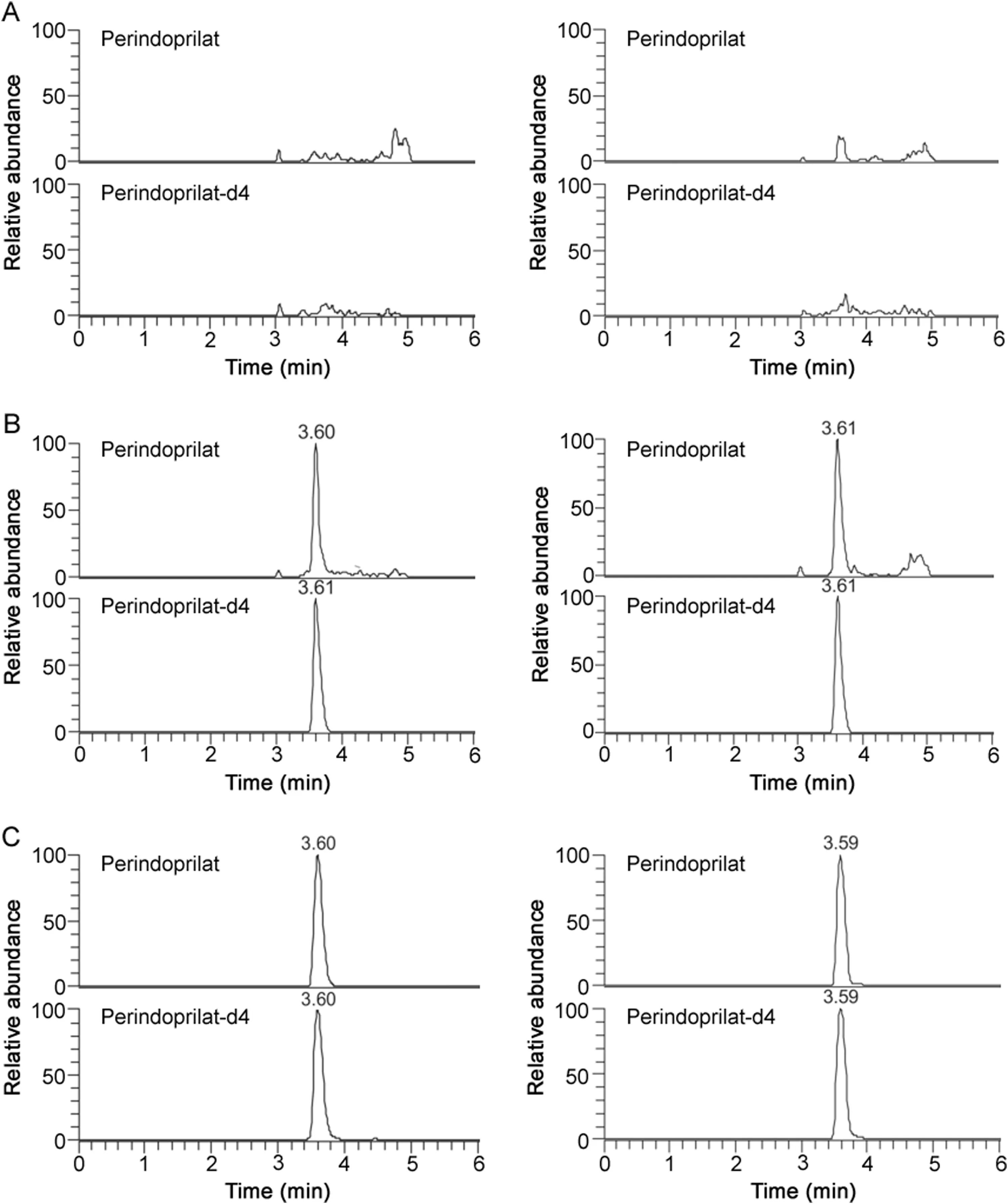
Fig.3.Typical MRM chromatograms of perindoprilat in(A)blank plasma(left panel)and whole blood(right panel);(B)LLOQ sample plasma(0.200 ng/mL)and whole blood(0.200 ng/mL);(C)subject sample plasma(2.49 ng/mL)and whole blood(1.31 ng/mL)at 2 h.
As it was mentioned in Introduction,several methods have been reported for the determination of perindopril and perindoprilat or indapamide in biological samples[9–16].However,most of the reported methods involved only one or two analytes in one bio fluid.In this study,simple and sensitive PP methods were established for all three analytes both in plasma and whole blood.And compared to the LLE and solid phase extraction(SPE)methods in literature,the developed methods were simpler and faster when applied to high-throughput analysis.
3.2.Method validation
Fig.2 shows the typical chromatograms of indapamide and perindopril in both the blank plasma and whole blood and the samples after oral administration of perindopril tert-butylamine/indapamide compound tablets.Fig.3 shows the typical chromatograms of perindoprilat in both the blank plasma and whole blood and the samples accordingly.No significant interfering peaks were observed at the retention time of analytes and ISs.No significant carry-over peaks were observed for both analytes and ISs(Tables S1 and S2).
The calibration curves(n=3)were linear covering the ranges of 1–250,0.4–100 and 0.2–20 ng/mL for indapamide,perindopril and perindoprilat,respectively,with correlation coefficient(r)>0.99(Tables S3–S8).
The validation results for both plasma and whole blood quality control samples are summarized in Table 1 and Table 2.
The established methods showed very good intra-batch and inter-batch precision and accuracy.The extraction was proved successful and no significant matrix effects were observed both inplasma and whole blood samples for indapamide and perindopril.Though some matrix effects about 180%were observed both in plasma and whole blood samples for perindoprilat,they were stable(RSD%less than 15%).And the ion enhancement was corrected with stable isotope labeled IS.

Table 1 Validation results for the determination of indapamide,perindopril and perindoprilat in human plasma.
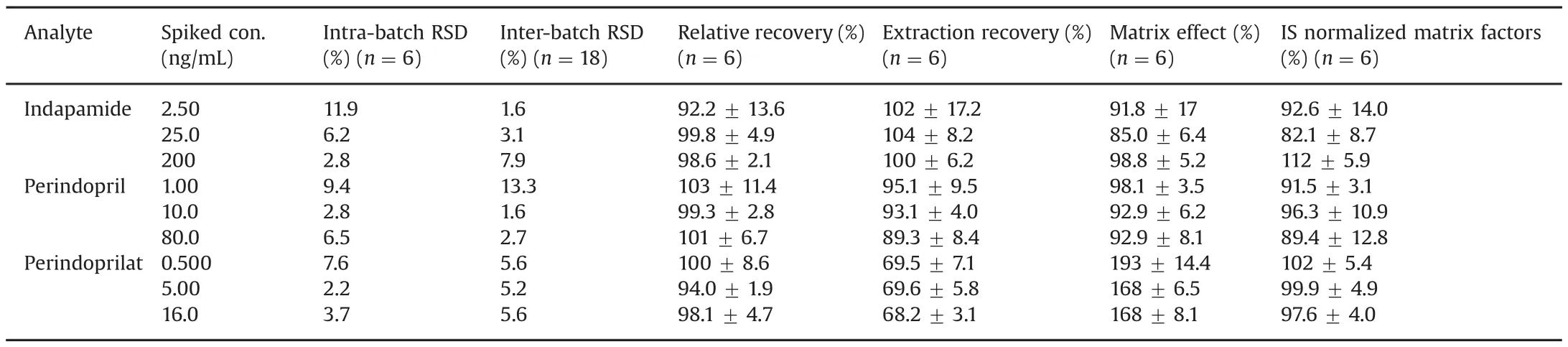
Table 2 Validation results for the determination of indapamide,perindopril and perindoprilat in human whole blood.
Three freeze-thaw cycles,long-term storage at-80°C for one month and short-term stability of the biological samples did not result in obvious changes in the drug concentration,as earlier publications have reported[9–14,18].And the stability results of analytes after 30 h in autosampler at 8 °C were within 85%–115%of the actual concentration(Tables S9 and S10).
3.3.Pharmacokinetic study
The validated LC-MS/MS bioanalytical method was successfully applied to determine indapamide,perindopril and its active metabolite perindoprilat in human plasma or whole blood for pharmacokinetic study in ten Chinese healthy volunteers,after oral administration of a tablet containing 4/1.25 mg perindopril tertbutylamine/indapamide in a fasting condition.
Table 3 shows the main pharmacokinetic parameters of indapamide,perindopril and perindoprilat in plasma and whole blood,respectively.The comparison between mean plasma and whole blood concentrations versus time curves of indapamide,perindopril and perindoprilat is shown in Fig.4.Cmaxand AUC0-tshowed significant differences between plasma and whole blood for all three analytes.For indapamide,due to the high binding to the red blood cell[18,19]the Cmaxand AUC0-tin whole blood were higher than those in plasma while it presented an opposite result for perindopril and perindoprilat.As per the literature[20],perindopril and perindoprilat bind mainly to plasma protein in blood,which resulted in the lower concentration levels in whole blood.
Table 4 shows comparison of main pharmacokinetic parameters of indapamide in whole blood,perindopril and perindoprilat in plasma between this study and reported methods.The results were in close agreement with the earlier reported values for pharmacokinetic study of two fixed dose combination formulations[16].The Tmaxand AUC0-tof indapamide in whole blood showed disparity after oral administration of indapamide tablets(1.5 mg)individually[11,21],which may be attributed to thedifferent dosage forms as it has been reported that the co-administration of perindopril and indapamide does not change their pharmacokinetic properties[5].In regard to pharmacokinetic results of perindopril and perindoprilat in plasma,they were in close proximity when compared to the others found in the literature[22,23].However,the AUC0-tvalues in literature[24]showed some differences,which may relate to the insensitive method of determination as the literature was published more than 20 years ago.
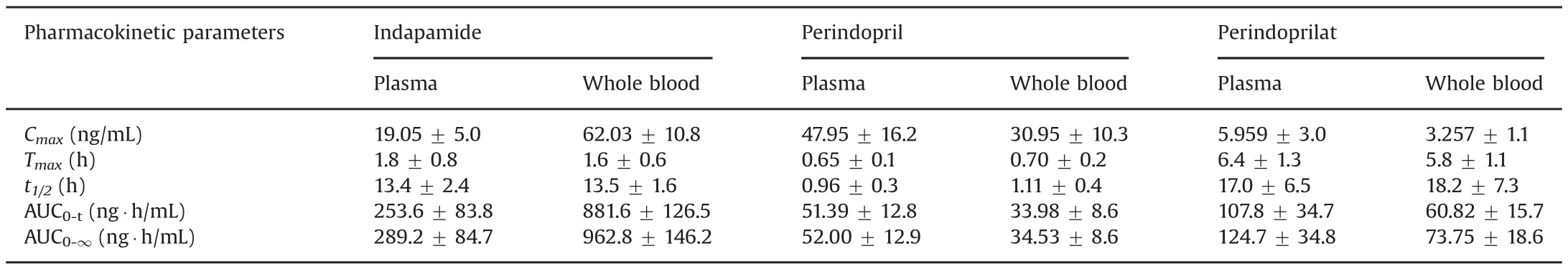
Table 3 The pharmacokinetic parameters of indapamide,perindopril and perindoprilat(mean±SD)in Chinese healthy volunteers after oral administration of perindopril tertbutylamine/indapamide compound tablets.
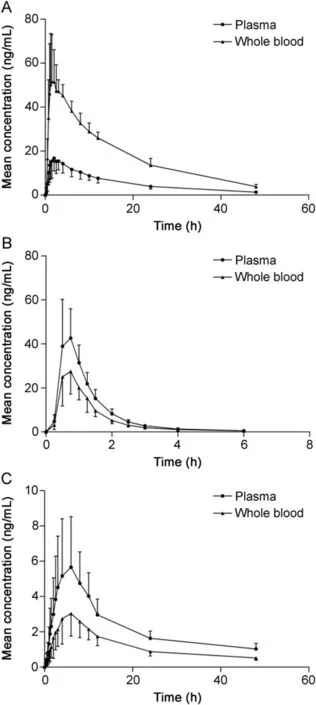
Fig.4.The mean plasma and whole blood concentrations versus time curves of(A)indapamide,(B)perindopril and(C)perindoprilat,respectively.
4.Conclusions
Simple and sensitive UPLC-MS/MS methods for the determination of indapamide,perindopril and perindoprilat in humanplasma or whole blood were developed and validated.The methods were successfully applied to pharmacokinetic studies in humans.This is the first report on the pharmacokinetics of perindopril tert-butylamine/indapamide compound tablets in Chinese healthy volunteers and the pharmacokinetic results of indapamide,perindopril and perindoprilat in different biological matrices would be helpful in providing some reference for clinical application and clinical medication safety.
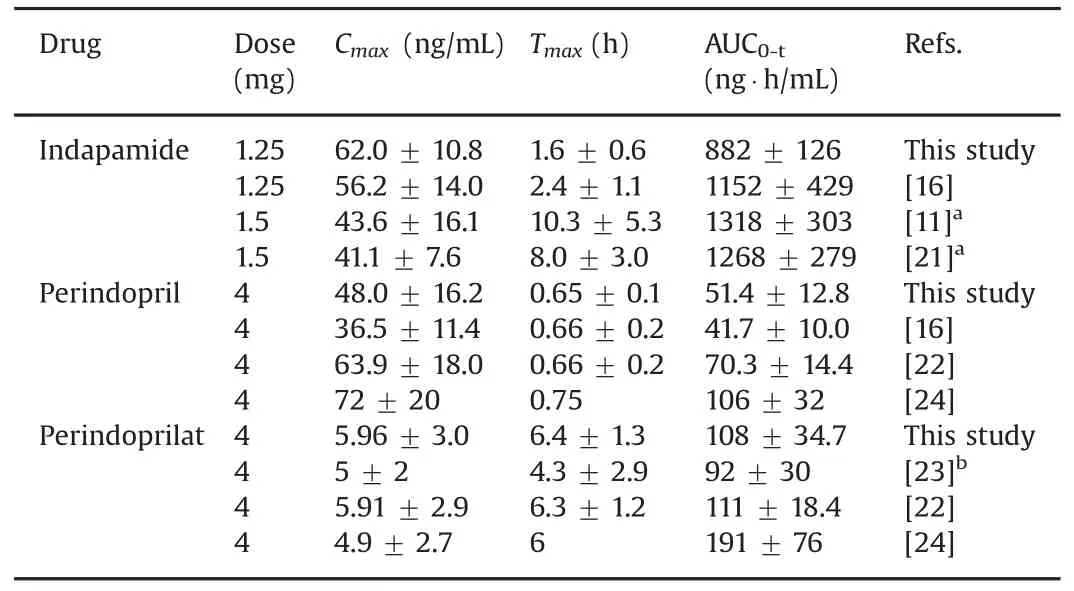
Table 4 Comparison of main pharmacokinetic parameters of indapamide in whole blood,perindopril and perindoprilat in plasma between this study and reported methods.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge Jiangsu Jiayi Pharmaceutical Co.,Ltd.(Nanjing,China)for graciously providing help during the research and Nanjing General Hospital(Nanjing,China)for providing necessary facilities to carry out this work.
This project was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Policy Directive Program of Jiangsu Province(BY2015072-03).
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jpha.2018.05.004.
 Journal of Pharmaceutical Analysis2018年5期
Journal of Pharmaceutical Analysis2018年5期
- Journal of Pharmaceutical Analysis的其它文章
- JPA Prize in 2016
- Determination of asenapine in presence of its inactive metabolites in human plasma by LC-MS/MS
- Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases
- Molecular docking studies of human MCT8 protein with soy isoflavones in Allan-Herndon-Dudley syndrome(AHDS)
- Discoursing on Soxhlet extraction of ginseng using association analysis and scanning electron microscopy
- Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells
