Taxonomic Clarification of A Well-Known Pathogenic Scuticociliate, Miamiensis avidus Thompson &Moewus, 1964 (Ciliophora, Scuticociliatia)
MA Mingzhen, LU Borong, , FAN Xinpeng, SHI Yuhong, ,and CHEN Xiangrui,
1) School of Marine Sciences, Ningbo University, Ningbo 315211, China
2) Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
3) School of Life Sciences, East China Normal University, Shanghai 200062, China
(Received October 19, 2017; revised November 21, 2017; accepted May 29, 2018)
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
Abstract Miamiensis avidus Thompson & Moewus, 1964, is a cosmopolitan and well-known marine pathogenic ciliated protist.However, the taxonomy of this species up to now has remained controversial, especially with respect to the validity of the morphologically similar species, Philasterides dicentrarchi, which was considered as a junior synonym of M. avidus. In this study, a population of M. avidus was collected from the skin of pharaoh cuttlefish (Sepia pharaonis) cultured near the East China Sea, Ningbo,China and its morphology and phylogeny were investigated in detail based on living characters, infraciliature, small subunit (SSU)rDNA and ITS1-5.8S-ITS2 region sequences. In addition, the morphometrics of a previously reported free-living population, collected from the Bohai Sea, were rechecked and analyzed. We compared the present two isolates with all historic populations of M.avidus and P. dicentrarchi, and found that their morphological characters were either highly similar or exactly identical, indicating that they are the same morphospecies. However, the phylogenetic analyses based on SSU rDNA or ITS1-5.8S-ITS2 region sequences revealed that most M. avidus and P. dicentrarchi populations formed one clade, and the two isolates of M. avidus from Weifang and American Type Culture Collection clustered in another clade, which indicated that there might be cryptic species in Miamiensis avidus.
Key words Miamiensis avidus; morphology; phylogeny; SSU rDNA; synonym; ITS1-5.8S-ITS2 region
1 Introduction
Over the past two decades, the number of reports on disease outbreaks in mariculture has increased significantly (Jung et al., 2007; Buchmann, 2015). One problem associated with those farming diseases is invasion by scuticociliates (Budino et al., 2011; Kim et al., 2004; Song et al., 2003). The subclass Scuticociliatia is a speciose assemblage of ciliates that are generally small in size,share a basic pattern of infraciliature, and show similar characters in vivo, as a result, their identifications remain difficult and confused despite of some interesting studies(Fan et al., 2010, 2011a, b; Pan et al., 2013; Song et al.,2009; Zhao et al., 2011). With the application of modern techniques in taxonomy, many poorly studied species need to be reinvestigated in detail after the original reports (Gao et al., 2010; Pan, 2016; Pan et al., 2013, 2016).Moreover, molecular phylogenetic analyses based on multi-gene as well as single genes have been increasingly used, which can give better understanding of the relationships among scuticociliate species (Gao et al., 2012a,b, 2014, 2017; Huang et al., 2016; Pan et al., 2017; Seo et al., 2013; Wang et al., 2017b; Xu et al., 2017; Yan et al.,2016).
Miamiensis avidus was first isolated by Thompson and Moewus (1964) from sea horses collected from Miami,Florida, USA, and re-described several times in the following years (Gomez-Saladin and Small, 1993a, b; Thompson and Moewus, 1964). Dragesco et al. (1995) isolated a similar organism from the Mediterranean Sea and named it Philasterides dicentrarchi. Based on the clear and detailed morphological characters, Song and Wilbert (2000)proposed P. dicentrarchi as a junior synonym of M.avidus (Dragesco et al., 1995; Song and Wilbert, 2000).This species was frequently studied worldwide as a fishpathogen in the past two decades (Budino et al., 2011;Jung et al., 2010, 2011; Rossteuscher et al., 2008; Seo et al., 2013; Smith et al., 2009). Tao et al. (2016) first isolated Miamiensis avidus from cephalopod mollusk,namely pharaoh cuttlefish, in China. Moreover, this species could also be found as a free-living form (Zhao et al.,2011). Most recently, Felipe et al. (2017) investigated four populations isolated from cultured fine flounder,turbot and a strain deposited in the American Type Culture Collection (ATCC? 50180TM). Based on the morphological and molecular data, they suggested that Miamiensis avidus and Philasterides dicentrarchi were different species.
We isolated Miamiensis avidus from the skin ulcers of pharaoh cuttlefish (Sepia pharaonis) as in Tao et al. (2016),and investigated the living morphology, infraciliature,small subunit (SSU) rDNA and ITS1-5.8S-ITS2 region sequences. In order to clarify the taxonomy of this species,we rechecked the taxonomic data of the problematic Weifang population, and compared all the historic information. These detailed works provided more useful evidences that Philasterides dicentrarchi is a junior synonym of Miamiensis avidus.
2 Materials and Methods
2.1 Sample Collection and Identification
Ningbo population of Miamiensis avidus was collected from the skin ulcers of pharaoh cuttlefish (Sepia pharaonis), which was reared in an aquaculture farm next to Xiangshan Bay, China (29?32′42′N, 121?45′09′E) (Fig.1).Water temperature was 21℃ ± 2℃ and salinity was 22-24.During parasitic inspection in December 2015, a larger number of ciliated organisms were discovered in the lesion tissues from all inspected animals. The ciliates were isolated using a pipette and transferred in Petri dishes with 0.22 μm filtered seawater. Weifang population was free living and collected from the Bohai Sea, China(36?52′42′′N, 119?25′08′′E). Water temperature was about 20℃ and salinity was 28. Samples were collected directly from the tidal pools using a syringe (200 mL).
The behavior of the organisms was studied in the Petri dishes under a dissecting microscope. The living morphology was investigated under a compound microscope equipped with a high-power oil immersion objective as well as differential interference contrast optics (Olympus BH-2, Olympus Optical Co., Ltd., Tokyo, Japan; Leica DM2500, Leica Microsystems, CMS GmbH, Wetzlar,Germany). The infraciliature was revealed with the protargol impregnation method (Wilbert, 1975). Drawings of stained specimens were made with the help of a camera lucida. Counts and measurements were performed at a magnification of ×1000. Terminology is according to Song and Wilbert (2000).

Fig.1 Sampling site, culturing pond and damaged skin of pharaoh cuttlefish. (A) The red dot indicates the location of pharaoh cuttlefish farm. (B) An indoor culturing pond. (C-E) A collected pharaoh cuttlefish with damaged skin or developed ulcer (arrows or red box) where ciliates were isolated.
2.2 DNA Extraction, PCR and Sequencing
Total genomic DNA was extracted from cells using the Dneasy Blood and Tissue Kit (Qiagen, Hilden, Germany)following the manufacturer’s instructions. The PCR amplification of the SSU rRNA gene sequence was performed using Q5?Hot Start High-Fidelity DNA Ploymerase (NEB Co., Ltd., M0493, Beijing) with the universal eukaryotic primers 18S-F (5’-AAC CTG GTT GAT CCT GCC AGT-3’) and 18S-R (5’-TGA TCC TTC TGC AGG TTC ACC TAC-3’) (Medlin et al., 1988). A fragment of approximately 500 bp containing the ITS1,5.8S ribosomal RNA gene and ITS2 was amplified using primers ITS-F (5’-GTA GGT GAA CCT GCG GAA GGA TCA TTA-3’) and ITS-R (5’-TAC TGA TAT GCT TAA GTT CAG CGG-3’) (Gao et al., 2012a; Wang et al., 2017a).PCR products were purified using an E.Z.N.A.TMQuik Gel Extraction Kit (OMEGA Bio-Tek, D2500-01, Guangzhou), then cloned using a pEASY?-Blunt Cloning Kit(TransGen, CB101, Beijing). Sequencing was performed bidirectionally (BGI Co., Ltd., Shanghai, China).
2.3 Phylogenetic Analysis
Newly-characterized sequences were combined with relevant sequences obtained from NCBI GenBank database. Sequences were aligned using Clustal W implemented in Bioedit v7.1.3.0. with default parameters (Hall,1999). The resulting alignments were manually refined by trimming both ends. The numbers of unmatched sites and sequence similarities were calculated using Bioedit v7.1.3.0. Bayesian inference (BI) and maximum likelihood (ML) analyses were carried out online on the CIPRES Science Gateway v3.3 (http://www.phylo.org/portal2). Bayesian analysis was performed with MrBayes on XSEDE v3.2.6 (Ronquist and Huelsenbeck, 2003)using the GTR + I + G model as selected by MrModeltest v2.2 (Nylander, 2004). The chain length was 1000000 generations and sampled every 100 generations. The first 25% were discarded as burn-in. ML tree was constructed with RAxML-HPC2 on XSEDE v8.2.4 (Stamatakis et al.,2008) using the GTR + I + G model as selected according to the AIC criterion by Modeltest v3.4 (Posada and Crandall, 1998). The reliability of ML internal branches was assessed using a nonparametric bootstrap method with 1000 replicates. MEGA v5.0 (Tamura et al., 2011)was used to visualize tree topologies. Systematic classification mainly follows Gao et al. (2016) and Lynn (2008).
3 Results
In the past half century, Miamiensis avidus was re- described several times and some more detailed features were presented. Thus, an improved diagnosis based on data of historic and Chinese populations is supplied here.
3.1 Improved Diagnosis
Body shape ovoid, cells about 21-58 μm × 11-38 μm in vivo; buccal field about 3/10-1/2 of body length; on average 9-15 somatic kineties, single caudal cilium; membranelles 1-3 (M1-3) with 2 or 3, 3-5, 2 or 3 longitudinal rows of basal bodies, respectively; paroral membrane(PM) formed by two distinct parts, anterior portion composed of monokinetids with sparsely arranged kinetosomes, posterior part with kinetosomes positioned in zigzag pattern.
3.2 Voucher Slide
Two voucher slides with protargol-impregnated specimens of Ningbo population (registration number: LU-20151209-01); two slides with protargol-impregnated specimens and three slides with silver nitrate-stained specimens of Weifang population (registration number:FXP-090506-02) were deposited in the Laboratory of Protozoology, Ocean University of China (OUC).
3.3 Morphological Description of the Ningbo Population (Figs.2, 3; Table 1)
Living cells about 35-45 μm × 15-20 μm in size, body shape thick ovoid with rounded posterior and pointed anterior end, ratio of length to width about 2:1, asymmetrical in outline when viewed from ventral side with anterior end slightly curved sideways (Figs.2A; 3A-D).Cross section often slightly bilaterally flattened with distinct depression at buccal field. Pellicle slightly indented at bases of cilia. Densely arranged somatic cilia about 8-12 μm long and single caudal cilium about 15-20 μm in length. Cytoplasm generally hyaline and colorless, always filled with many small granules in newly isolated individuals from skin ulcers (Figs.3A-D). After two days’starvation, cells usually more slender and no such granules recognizable. One spherical to ovoid macronucleus located in central of body, about 8-13 μm in diameter,one micronucleus often positioned anterior to macronucleus with a diameter of about 2 μm (Figs.2D; 3I). One contractile vacuole about 8-10 μm in across, caudally positioned (Figs.2A; 3A, B). Inactive when in ulcer tissues, constantly moving forward without pause in filtered sea water.
Buccal field conspicuous and about 35% of cell length.Oral apparatus characteristically as shown in Fig.2B,consisting of paroral membrane and three small linearly arranged membranelles. Membranelle 1 (M1) small and obviously separated from others, consisting of 2 or 3 longitudinal rows of kinetosomes; membranelle 2 (M2) distinctly larger than M1, with 3-5 longitudinal rows, each possessing about 6 or 7 basal bodies; membranelle 3 (M3)close to M2, comprising about 2 or 3 longitudinal rows of basal bodies. Paroral membrane on right border of buccal cavity, indented near M3 and bipartite, anterior part composed of sparsely arranged monokinetids, posterior part with dikinetids that arranged in zig-zag pattern (Figs.2B;3F).
On average twelve somatic kineties, and most of them commencing at bald plate in the apical region and ending in posterior pole area. Cytopyge between kineties1 and n,slightly curved (Figs.2C; 3F, H).
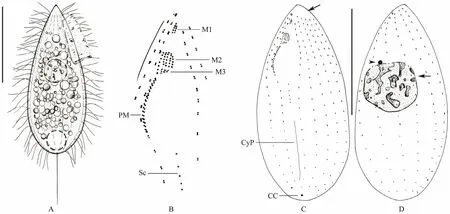
Fig.2 Morphology and infraciliature of Ningbo population of Miamiensis avidus. (A) Lateral view of a representative individual. (B) Fine structure of buccal apparatus. (C, D) Infraciliature on ventral (C) and dorsal (D) sides, arrow in (C) indicates the bald area, in (D) denotes the macronucleus, arrowhead marks the micronucleus. Abbreviations: CC = caudal cilium; CyP = cytopyge; M1, 2, 3 = membranelles 1, 2 and 3; PM = paroral membrane; Sc = scutica; Scale bars = 20 μm.
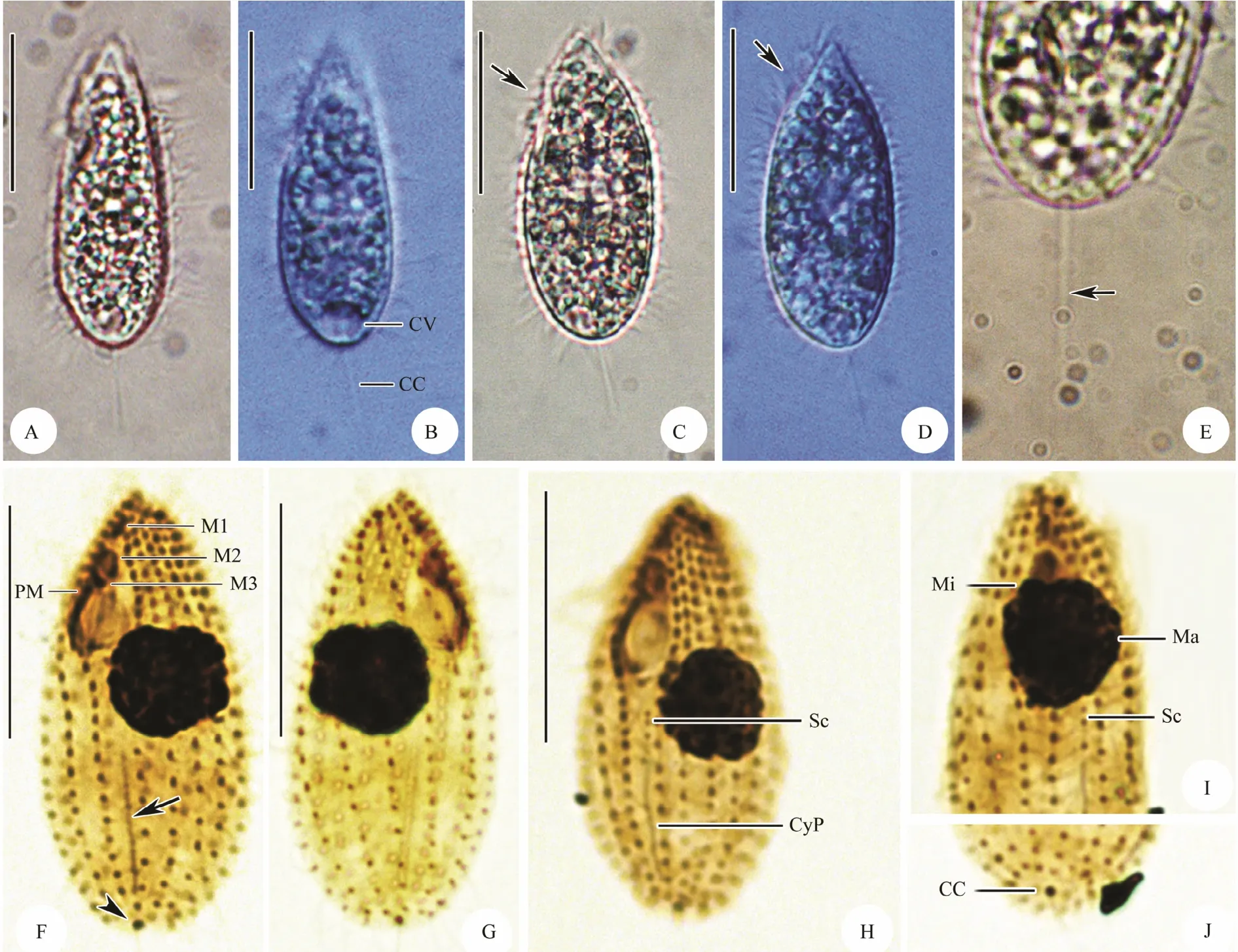
Fig.3 Photomicrographs of Ningbo population of Miamiensis avidus from life (A-E) and after protargol staining (F-J).(A-D) Lateral view of different individuals in life, arrows mark the oral area. (E) The posterior end to show the caudal cilium (arrow). (F, H) Ventral view of the infraciliature, arrow in (F) marks the cytopyge, arrowhead in (F) denotes the kinetosomes of the caudal cilium. (G) Dorsal view of the infraciliature. (I) The anterior portion of a stained individual to show the macronucleus and micronucleus as well scutica. (J) Caudal view of the infraciliature. Abbreviations: CC = caudal cilium; CV = contractile vacuole; CyP = cytopyge; Ma = macronucleus; Mi = micronucleus; M1, 2, and 3 = membranelle 1, 2, 3; PM = paroral membrane; Sc = scutica. Scale bars = 20 μm.
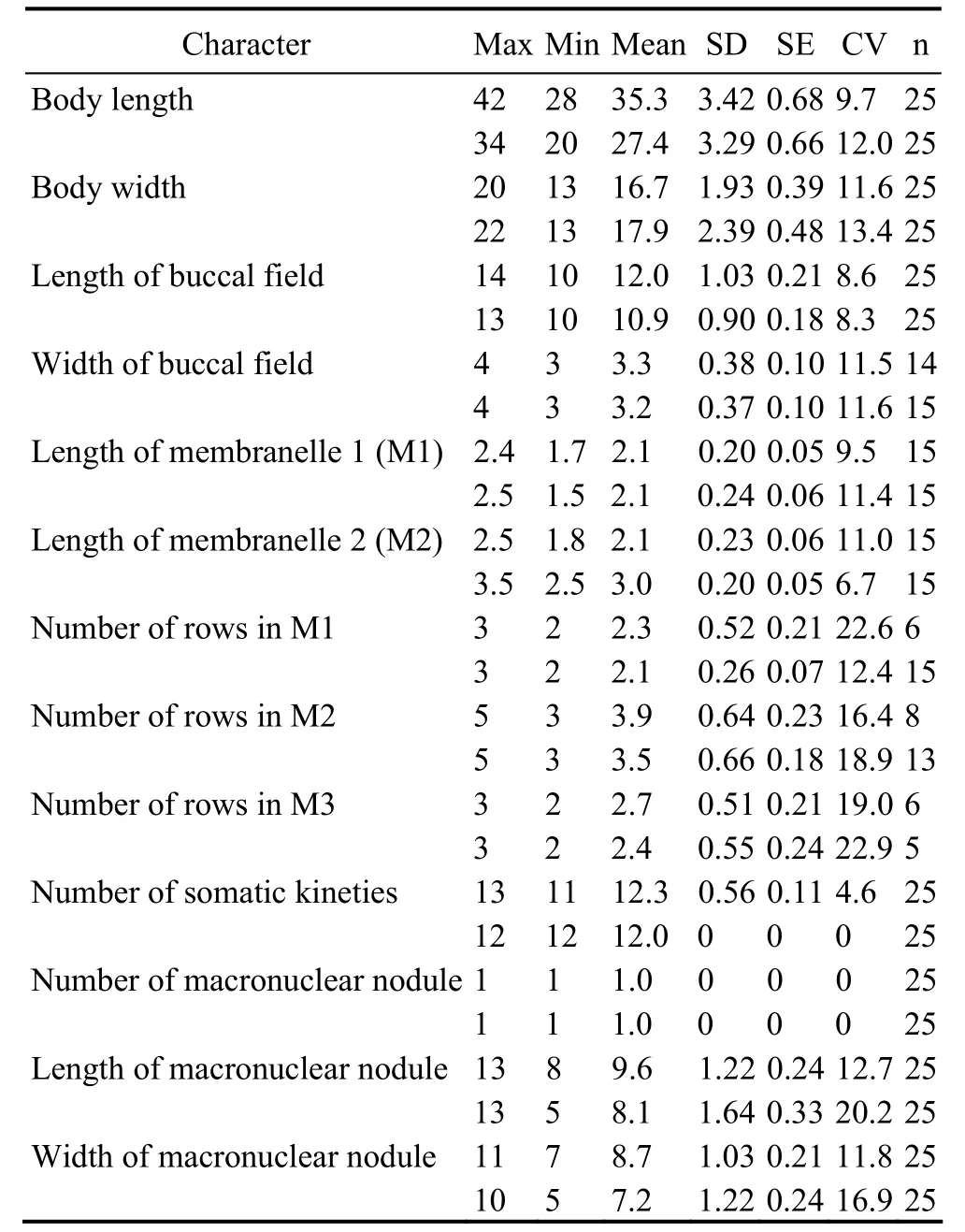
Table 1 Morphometric data of Miamiensis avidus from protargol stained specimens (first line, Ningbo population; second line, Weifang population)
3.4 Additional Description of the Weifang Population (Fig.4; Table 1)
The Weifang population was recently described by Zhao et al. (2011). Therefore, a full re-description is unnecessary. However, some structures, i.e., the caudal cilium, the paroral membrane, are important characters for taxonomic description of Miamiensis avidus. Having carefully examined the original data and permanent slides,we provide some additional description of these characters:1) the caudal cilium about 20 μm long which is obviously longer than other body cilia (Fig.4A); 2) the micronucleus attached to the macronucleus and always located in the anterior cell half; 3) the paroral membrane contained two distinct parts, anterior part composed of monokinetids with sparsely arranged kinetosomes, posterior part with dikinetids arranged in zig-zag pattern (Figs.4B-D); in silver nitrate-stained specimens, these two parts are usually joined together but sometimes slightly separated(Figs.4E-G).
3.5 Sequence Information and Phylogenetic Analysis
The SSU rDNA sequence and ITS1-5.8S-ITS2 region sequence of Miamiensis avidus were deposited in Gen-Bank database with the accession numbers KY082893 and KY082894. The length and G + C content of the SSU rRNA gene sequence are 1712 bp and 44.45%, while these of the ITS1-5.8S-ITS2 region sequence are 506 bp and 36.96%.
Fifty-five sequences were included in the present phylogenetic analysis based on SSU rDNA sequences, including all species of Philasterida for which SSU rDNA sequence data are available, and five species of Pleuronematida as the outgroup. (Fig.5). In the order Philasterida, there are 50 sequences that represent 10 families(Orchitophryidae, Uronematidae, Schizocaryidae, Philasteridae, Cryptochilidae, Entorhipidiidae, Pseudocoh nilembidae, Cohnilembidae, Entodiscidae, Thyrophylacidae).The topologies of the ML and BI trees were basically congruent and, therefore, a single topology was presented based on the ML tree with support values from both algorithms indicated on branches. In the phylogenetic trees,all the Miamiensis avidus and Philasterides dicentrarchi grouped into one clade (100% ML, 1.00 BI), except two populations of M. avidus (Weifang population, JN885091 and the strain Ma/2, ATCC?50180TM, KX357144), which grouped with Anophyroides haemophila (U51554, 84%ML, 1.00 BI). In GenBank database, there are 34 SSU rDNA sequences of Miamiensis avidus (including Philasterides dicentrarchi, which was considered a synonym of M. avidus). Sequence comparison showed that the newly characterized sequence (Ningbo population, KY083893)is identical to the other 20 sequences of M. avidus and P.dicentrarchi from GenBank (Accession no. AY550080,EU831204, JN689230, EU831201, EU831207, EU831208,EU831200, EU831197, EU831202, EU831203, EU831212,EU831210, EU831209, EU831211, EU831206, EU831205,JX914665, GU572375, KU992658, KX259260), while they one nucleotide differ from another six sequences (EU-831192, EU8311193, EU831194, EU831195, EU831196,KU720304), two nucleotides from another three sequences (AY642280, EU831198, JN689229), and three or four nucleotides from another two sequences respectively (EU831199, FJ936000). The Weifang population(JN885091) and the strain Ma/2, ATCC?50180TM(KX-357144), 67 and 83 nucleotides, respectively, differ from most sequences of M. avidus and P. dicentrarchi (Table 2).
Thirty-four populations were included in the phylogenetic analysis based on ITS1-5.8S-ITS2 region, with Pleuronema coronatum, a species of the order Pleuronematida, as the out-group (Fig.6). All the Miamiensis species and Philasterides dicentrarchi that are available for which ITS1-5.8S-ITS2 sequences data were included. The newly characterized sequence (Ningbo population, KY08 2894) formed a fully supported clade with M. avidus(HM768743 Jung et al., 2011 and KU720303 Tao et al.,2016), which then grouped with M. avidus JN885095(Weifang population) in the ML analyses with low support (41% ML). The results of the sequence comparison showed that M. avidus KY083894 is identical to M.avidus HM720303 and differs from M. avidus HM768743 and M. avidus JN885095 in one and 124 nucleotides, respectively (Table 3).
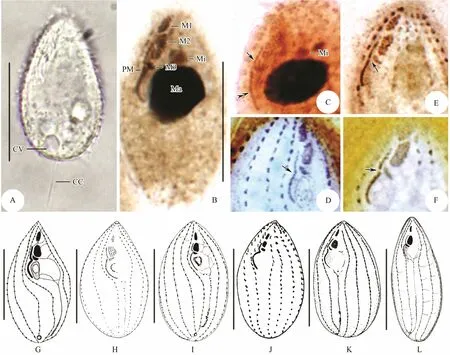
Fig.4 Photomicrographs of Weifang population of Miamiensis avidus from life (A), after protargol staining (B-C) and silver nitrate impregnation (D-F), and infraciliature of historic populations (G-L). (A) Lateral view of one individual in life, showing the contractile vacuole position and the long caudal cilium. (B) Ventral view of the infraciliature, showing the buccal apparatus and micronucleus position. (C) Detailed structure of paroral membrane, arrow indicates the anterior part composed of monokinetids with sparsely arranged kinetosomes, double-arrowhead denote the posterior part with dikinetids arranged in zig-zag pattern. (D-F) Three types of paroral membrane, arrow in (D) denotes the continuous paroral membrane, arrow in (E) marks an indistinct gap between the anterior and posterior parts of paroral membrane,while in (F) indicates a clear gap within paroral membrane. (G-L) Ventral views of infraciliature (G from Thompson and Moewus (1964); H, I from Song and Wilbert (2000); J from Jung et al. (2007); K and L identified as Philasterides dicentrarchi from Iglesias et al. (2001) and Dragesco et al. (1995), respectively). Abbreviations: CC = caudal cilium; CV = contractile vacuole; Ma = macronucleus; Mi = micronucleus; M1, 2, 3 = membranelle 1, 2 and 3; PM = paroral membrane.Scale bars = 20 μm.
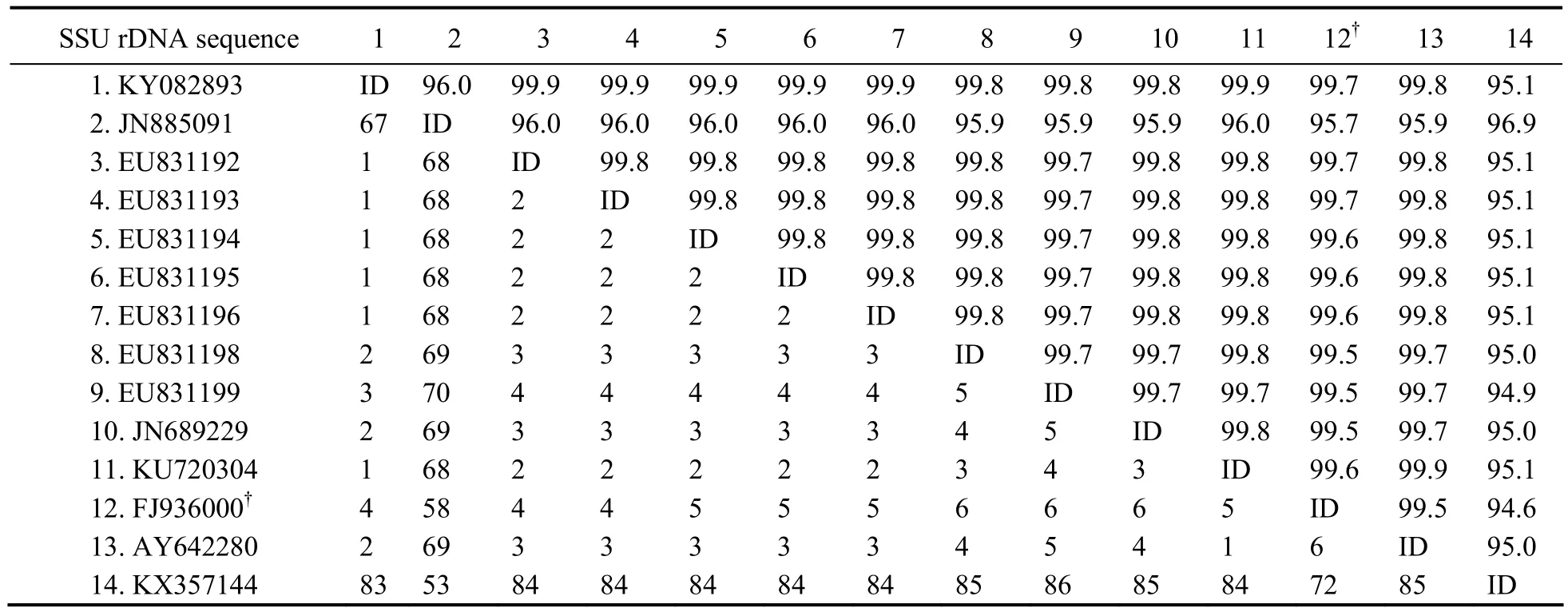
Table 2 Sequence comparisons of the small subunit ribosomal DNA sequences in Miamiensis avidus determined by Bioedit v7.1.3.0
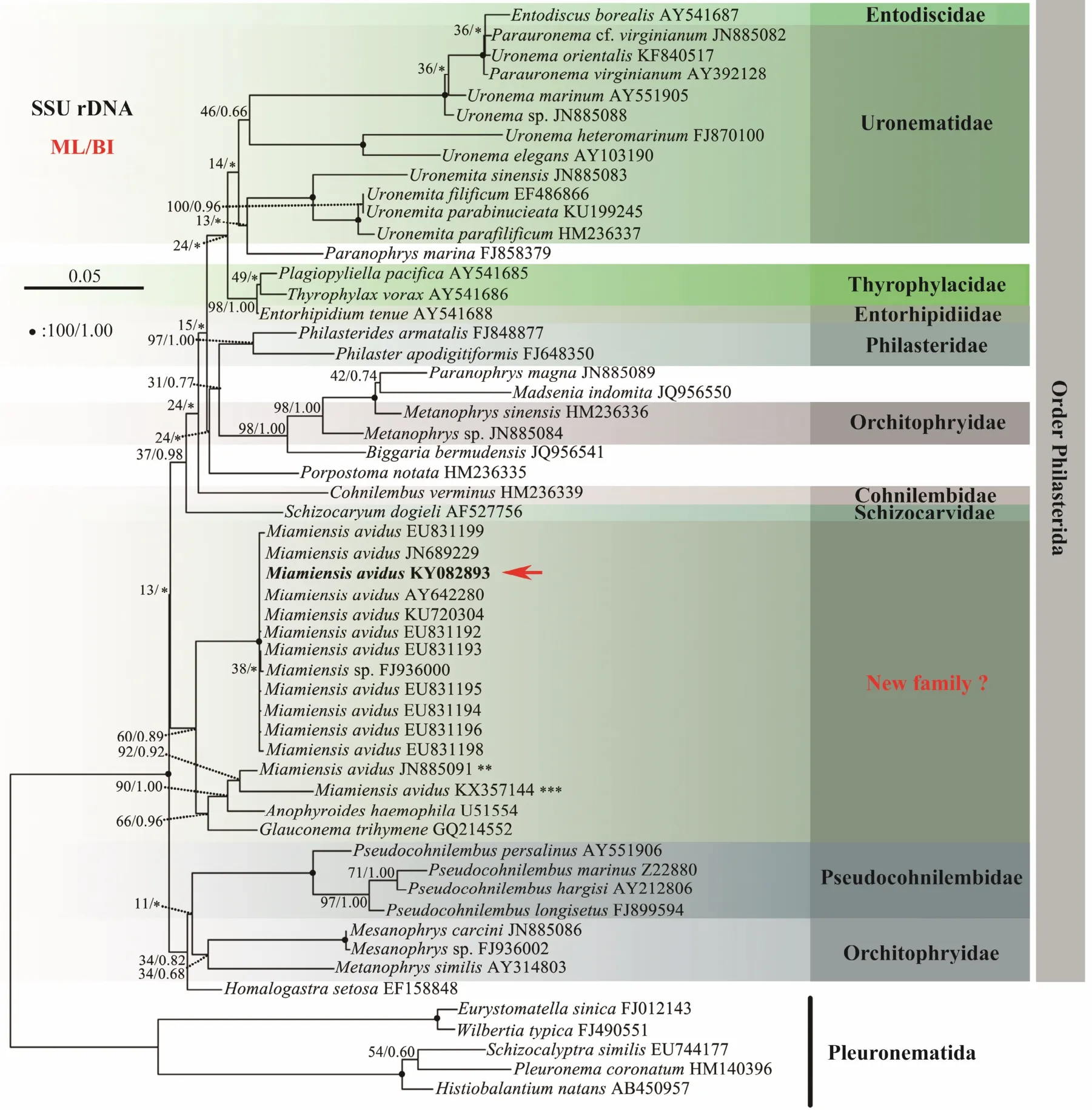
Fig.5 Maximum likelihood (ML) tree inferred from SSU rDNA sequences of representative taxa (55 taxa). Numbers at nodes represent the bootstrap values of ML and the posterior probabilities of Bayesian analysis (BI), respectively. * indicates the disagreement between BI tree and the reference ML tree. Fully supported (100%/1.00) branches are marked with solid circle. **, JN885091 (Weifang population). ***, KX357144: should be a misidentified material (the strain Ma/2,ATCC? 50180TM). Red arrow indicates that 21 SSU rRNA gene sequences are identical and here only exhibit KY082893(Ningbo population). The scale bar corresponds to five substitutions per 100 nucleotide positions. Newly sequenced species in this work are in bold.
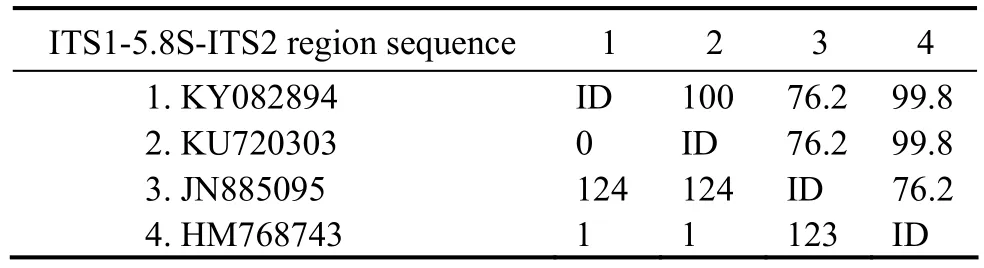
Table 3 Sequence comparisons of the ITS1-5.8S-ITS2 region sequences in Miamiensis avidus determined by Bioedit v7.1.3.0
4 Discussion
4.1 Morphological Comparison of the Previous and Present Populations of Miamiensis avidus
Miamiensis avidus, which is commonly found as an ectoparasite, was originally isolated from sea horse in coastal waters near Miami, USA (Thompson and Moewus,1964). During the past half century, numerous ‘populations’ of this taxon have been reported worldwide collected from various hosts, most of them were teleost, such as flounder (Jung et al., 2007; Song and Wilbert, 2000),and turbot (Budino et al., 2011). Zhao et al. (2011) described one free-living popu lation collected from Bohai Sea, Weifang, China. The present population, named Ningbo population, isolated from the mantle skin of reared pharaoh cuttlefish (Sepia pharaonis) is similar to the original and historic descriptions in the fixed cell size,number of somatic kineties, general structure of buccal apparatus, position of contractile vacuole, and habitat.

Fig.6 Maximum likelihood (ML) tree inferred from ITS1-5.8S-ITS2 sequences of representative taxa (34 taxa). Numbers at nodes represent the bootstrap values of ML and the posterior probabilities of Bayesian analysis (BI), respectively. * indicates the disagreement between BI tree and the reference ML tree. Fully supported (100%/1.00) branches are marked with solid circle. The scale bar corresponds to five substitutions per 100 nucleotide positions. Newly sequenced species in this work are in bold. JN885095 (Weifang population).
Thompson and Moewus (1964) described Miamiensis avidus only based on silver nitrate impregnated specimens,therefore, the structure of paroral membrane was not provided exactly because that paroral membrane kinetosomes were heavily stained and connected to each other.Even so, there was an important note about this structure in the original report, that is, ‘the infraciliature of the undulating membrane is indented near membranelle three(M3), the anterior portion is relatively straight and the posterior portion curved; sometimes a narrow gap appears between the anterior and posterior portion’ (Fig.4H; Table 4a). Song and Wilbert (2000) firstly provided the detailed oral structure of M. avidus based on the protargol-impregnated specimens, namely paroral membrane indented near M3 and formed by two distinct parts, which are generally joined together, sometimes might be slightly separated, the anterior portion composed of monokinetid, the posterior part with kinetosomes arranged in zig-zag pattern (Figs.4I, J; Table 4a). A free- living population of M.avidus was collected from Bohai Sea, Weifang, whose morphology and molecular data were provided by Zhao et al. (2011) and Gao et al. (2012a), respectively. We rechecked the permanent slides and found that the paroral membrane is composed of two different parts, which were joined together or slightly separated (Figs.4B, D-G; Table 4a). Combining the historic and present data, we can conclude that the paroral membrane of M. avidus is bipartite.Also, these two different parts are joined together or slightly separated.
Philasterides dicentrarchi Dragesco et al., 1995, found as a histophagous parasite of sea bass (Dicentrarchus labrax) in the Mediterranean Sea, was differed from Miamiensis avidus mainly by the separated paroral membrane (Fig.4M; Table 4a) (Dragesco et al., 1995). Subsequently, Iglesias et al. (2001) and Kim et al. (2004) also isolated this organism from turbot and olive flounder(Paralichthys olivaceus), respectively (Figs.4K, L; Table 4b). Based on the identical morphological characters,especially the similar structure of paroral membrane,Song and Wilbert (2000) proposed it as a junior synonym of M. avidus. About ten years later, Budino et al. (2011)provided further support for this proposal on the basis of morphological and molecular data with seven isolates
from Spain and Portugal. However, Felipe et al. (2017)rejected the synonymy/conspecificity of M. avidus and P.dicentrarchi. They stained three populations of P. dicentrarchi and one population of M. avidus (the strain Ma/2,ATCC? 50180TM) using silver carbonate method, and found that the former two had two clearly separated paroral membranes, while the latter had invariably continuous paroral membrane. Unfortunately, the staining result was not clear enough to illustrate the detailed structure of paroral membrane.
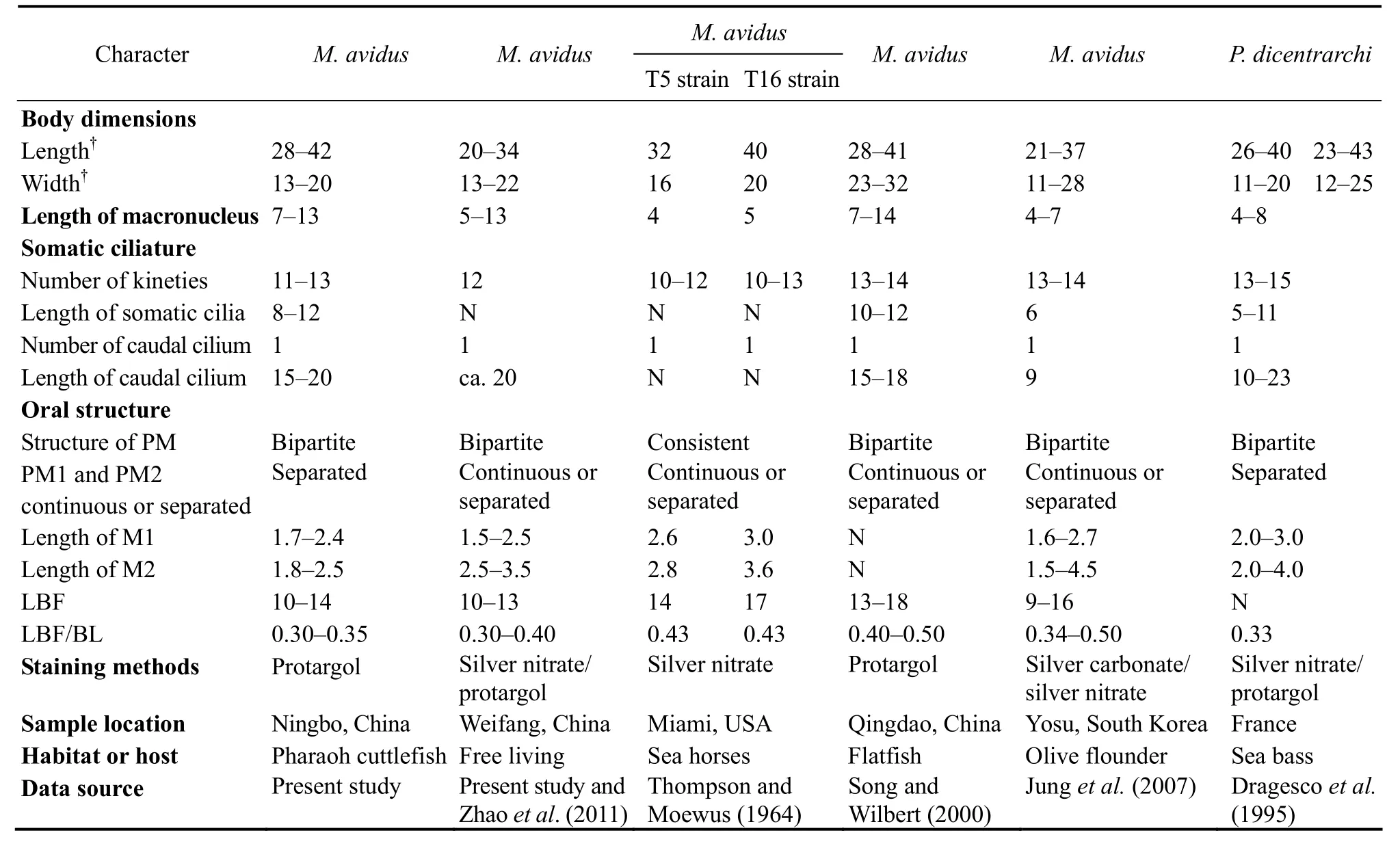
Table 4 a Morphometric comparison of Miamiensis avidus population
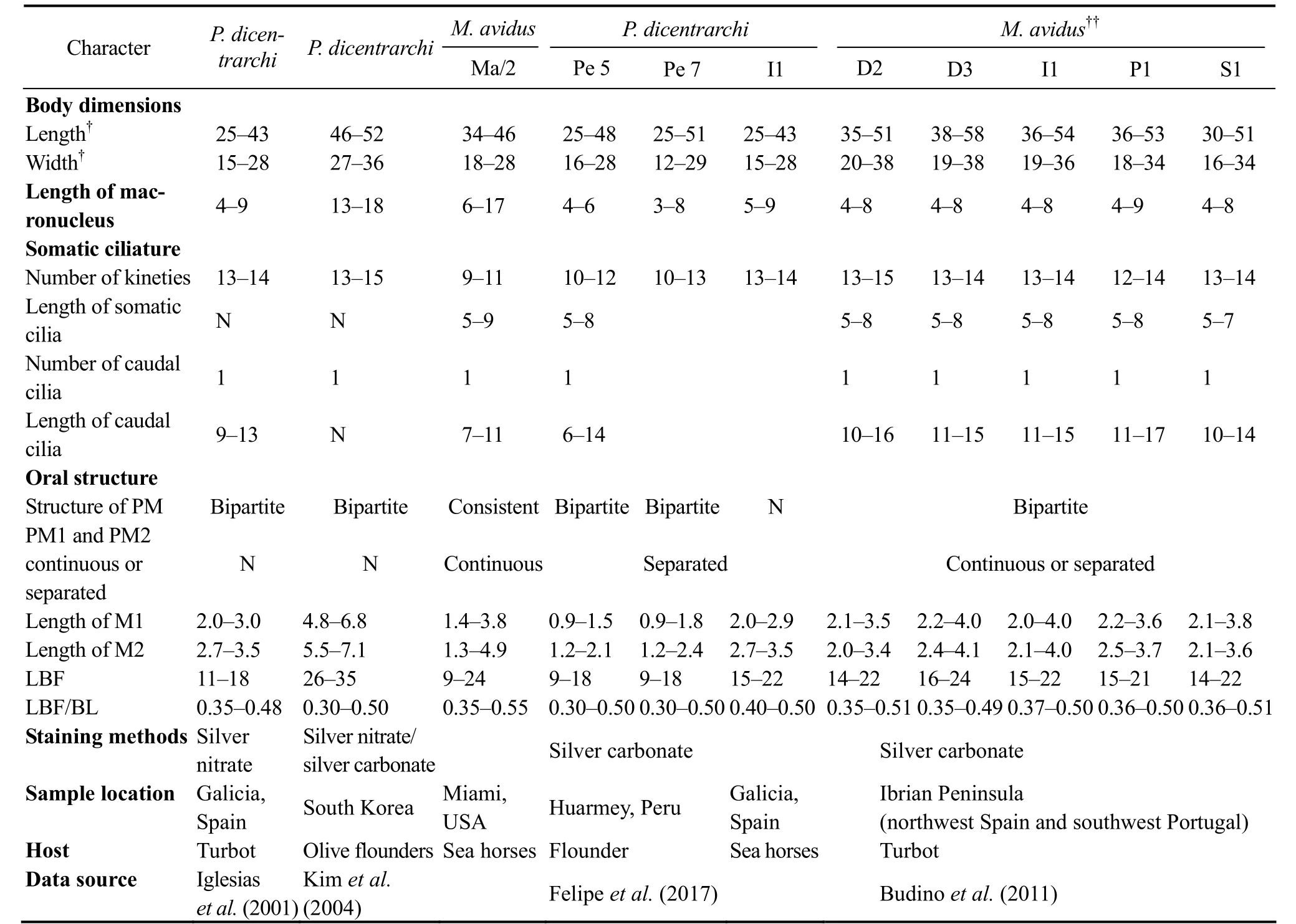
Table 4 b Morphometric comparison of Miamiensis avidus population
Jung et al. (2007) found that Miamiensis avidus has one or two paroral membranes and two or three oral membranes, so the authors concluded that the morphology of buccal structures ‘cannot be used as a consistent key for identification of the species’. After reviewing most of the historic morphological data of M. avidus and Philasterides dicentrarchi (as shown in Tables 4a and b), we agree with Jung et al. (2007) that the continuous or separated paroral membrane is not a reliable criterion for species separation. Consequently we agree with previous studies that P. dicentrarchi is a junior synonym of M.avidus (Budino et al., 2011; Jung et al., 2007; Song and Wilbert, 2000).
4.2 Molecular Analysis and Phylogeny of Miamiensis
Miamiensis avidus has been re-described several times since the original report, and some are misidentified as M.blitzae or Philasterides dicentrarchi (Dragesco et al., 1995;Small and Lynn, 1985; Song and Wilbert, 2000; Zhao et al.,2011). Till now, more than 30 SSU rRNA gene sequences and four ITS1-5.8S-ITS2 sequences of M. avidus have been submitted in GenBank, although most are not accompanied by detailed morphological data (Felipe et al.,2017; Jung et al., 2010). All the SSU rRNA gene sequences of M. avidus are very similar to each other with 1-3 nucleotides difference, except that from the two populations isolated from Weifang, Bohai Sea, (Accession number JN885091) and the strain Ma/2 (Accession number KX357144), which differ in ca. 70 bp and 80 bp respectively from other sequences. For the four ITS1-5.8S-ITS2 sequences of M. avidus in GenBank (KU72-0303, KY082894, HM768743 and JN885095), the sequence of the population isolated from Weifang, Bohai Sea (Accession number JN885095) differs in about 120 bp from that of other populations. However, the Weifang population and the strain Ma/2 cannot be morphologically distinguished from our population, indicating that there might be cryptic species in M. avidus, which need further information to distinguish and define the species.
The genus Miamiensis was first proposed based on the marine facultative parasite M. avidus (Thompson and Moewus, 1964). Small and Lynn (1985) described another Miamiensis species, M. blitzae, which was considered as a junior synonym of M. avidus (Song and Wilbert, 2000).Therefore, Miamiensis only contains one species M.avidus. However, the family assignment of Miamiensis is still unsolved. According to Corliss (1979), Miamiensis is assigned in the family Philasteridae, while it was assigned in the family Uronematidae by Song and Wilbert (2000),or Parauronematidae according to Lynn (2008). None of these assignments, however, are supported by the phylogenetic analyses based on SSU rRNA gene or multigenes (Gao et al., 2012a), which reveal that Miamiensis does not group in Philasteridae or Uronematidae, but forms a clade with Glauconema trihymene and Anophryoides haemophila (Fig.5). M. avidus, A. haemophila and G. trihymene are morphologically very similar in buccal apparatus, body size, and body shape, which indicate that this clade might represent a new family as suggested in Gao et al. (2012a).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31572230), the Open Fund of Zhejiang Provincial Top Key Discipline of Aquaculture in Ningbo University (No. xkzsc1417), the K. C.Wong Magna Fund in Ningbo University, and the Scientific Research Foundation of Graduate School of Ningbo University. We are very grateful to Dr. Jie Huang (Institute of Hydrobiology, Chinese Academy of Sciences) for her help with the phylogenetic analysis.
 Journal of Ocean University of China2018年5期
Journal of Ocean University of China2018年5期
- Journal of Ocean University of China的其它文章
- Effect of Different Dietary Protein and Lipid Levels on the Growth, Body Composition, and Intestinal Digestive Enzyme Activities of Juvenile Yellow Drum Nibea albiflora (Richardson)
- Establishing Gene Delivery Systems Based on Small-Sized Chitosan Nanoparticles
- Methylation Status of the Follistatin Gene at Different Development Stages of Japanese Flounder(Paralichthys olivaceus)
- Structural Variation Analysis of Mutated Nannochloropsis oceanica Caused by Zeocin Through Genome Re-Sequencing
- A Comparative Study on Hydrodynamic Performance of Double Deflector Rectangular Cambered Otter Board
- Characterization of Polysaccharides Extracted from a Cultivated Brown Alga Costaria costata During the Harvest Period
