Unusual retention behavior of omeprazole and its chiral impurities B and E on the amylose tris(3-chloro-5-methylphenylcarbamate)chiral stationary phase in polar organic mode
Rosell Ferretti,Leo Znitti,Adrino Csulli,Roberto Cirilli,*
aCentro nazionale per il controllo e la valutazione dei farmaci,Istituto Superiore di Sanità,Viale Regina Elena 299,00161 Rome,Italy
bEuropean Union Reference Laboratory for the Parasites,Istituto Superiore di Sanità,Viale Regina Elena 299,00161 Rome,Italy
cWorld Health Organization Collaborating Centre for the Epidemiology,Detection and Control of Cystic and Alveolar Echinococcosis(In Animals and Humans),Istituto Superiore di Sanità,Viale Regina Elena 299,00161 Rome,Italy
Keywords:Chiralpak?IG-3 Retention Enantioselective HPLC Omeprazole Column temperature Basic/acid additives
ABSTRACT Recent reports have demonstrated that the new commercially available immobilized-type chiral stationary phases(CSPs)containing amylose tris(3-chloro-5-methylphenylcarbamate)(ACMPC)as a selector exhibit not only an exceptionally high enantioselectivity in high-performance liquid chromatography(HPLC)but they are also applicable to a wide range of chiral analytes.Herein,we report the results obtained in the HPLC analysis of omeprazole and its impurities B and E on the ACMPC-based Chiralpak IG-3 CSP(CSP)under polar organic conditions.A systematic evaluation of the retention characteristics of the selected benzimidazole chiral probes was carried out by changing the composition of the mobile phase and the column temperature.It is worth emphasizing that the high affinity of both enantiomers of all analytes recorded in pure methanol mode dramatically decreased incorporating small volumes of either basic or acid additives in the mobile phase.Unspecified sites of the IG-3 CSP presumably involved in strong and non-stereoselective H-bonding contacts with chiral analytes are assumed responsible for the unproductive retention process.
1.Introduction
Amylose tris(3-chloro-5-methylphenylcarbamate)(ACMPC)is a new meta-substituted polysaccharide-based selectorforenantioselective HPLC.Three versions of chiral stationary phases(CSPs)have been prepared by immobilizing the chlorinated amylose phenylcarbamate derivative onto particles of silica of different diameters(typically 1.6,3.0 and 5.0 μm,from which the trade names Chiralpak?IG-U,Chiralpak?IG-3 and Chiralpak IG?,respectively,came).Although only since 2016 it has become commercially available,the Chiralpak?IG CSP seems to have suitable characteristics for the separation of enantiomers on an analytical and semipreparative scale.In fact,as demonstrated in an accurate study carried out by Ghanem et al.[1],it combines a broad chiral resolving ability,which is typical of other more popular polysaccharide selectors such as tris-(3,5-dimethylphenylcarbamate)of amylose(ADMPC)and cellulose,with a universal solvent compatibility resulting from the immobilization process.In the same report it was highlighted that the replacing of a methyl group on the phenyl moiety of the parent ADMPC by a chlorine atom gives rise to a substantial enlarging of the enantioselectivity spectrum toward chiral analytes of pharmaceutical interest.
Recently,our research group has showed that the ACMPC-based CSP can be successfully applied to develop efficient analytical and semipreparative protocols for the enantiomer separation of the anthelmintic drug albendazole sulfoxide[2],known also as ricobendazole,and a series of novel secondary alcohols,endowed with rhinovirus inhibitory activity[3].
However,exhaustive and clear chromatographic and spectroscopic data to understand its retentive and chiral recognition mechanism are still missing.
In the present article,three biologically active chiral sulfoxides,omeprazole(OME)and its impurities B and E(IMP-B and IMP-E),have been selected as molecular probes to investigate the chromatographic behavior of the Chiralpak IG-3 column under polar organic mode.OME is currently marketed as racemic mixture and single(S)-enantiomer in treatment of gastric-acid related diseases[4,5].As shown in Fig.1,the structures of IMP-B and IMP-E are strictly related to OME lacking in the former the methoxy group at 4-position of the pyridine moiety and the latter resulting from the oxidation of the pyridine nitrogen.
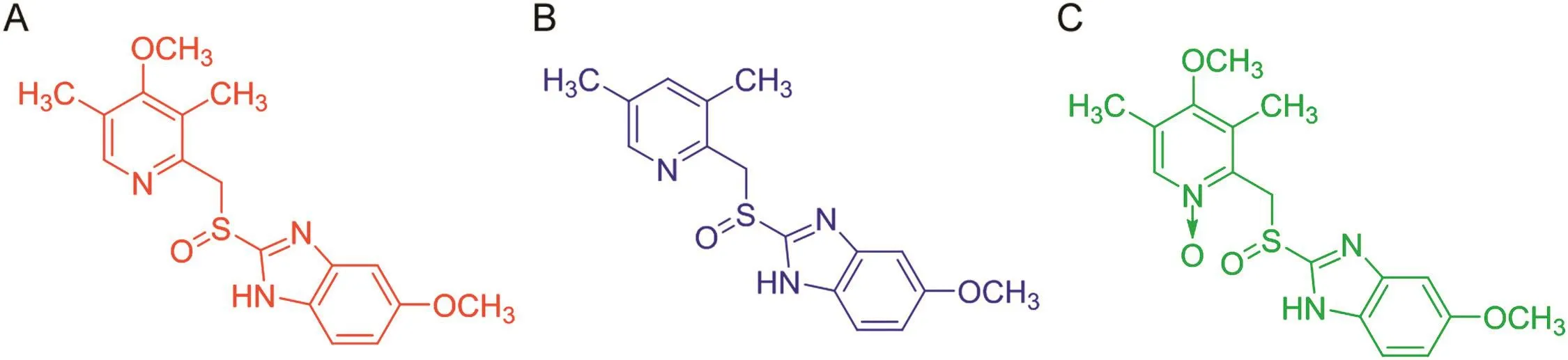
Fig.1.Structure of(S)-OME(A)and its chiral impurities B(B)and E(C).
A particular attention was devoted to the study of the in fluence of mobile phase composition and column temperature on the retentive comportment of the IG-3 CSP.
The knowledge acquired on an analytical scale was finally applied to set up productive multimilligram chiral separations of OME,IMP-B and IMP-E.
2.Experimental
2.1.Chemicals and reagents
OME and the impurities shown in Fig.1 were purchased from the European Directorate for the Quality of Medicines&Healthcare(EDQM)(France)and United States Pharmacopoeial Convention,Rockville(MD).HPLC-grade solvents were used as supplied by Aldrich(Milan,Italy).HPLC enantioseparations were performed by using stainless-steel Chiralpak?IG-3(250 mm × 4.6 mm,3μm)and Chiralpak?IG(250 mm × 10mm,5μm)columns(Chiral Technologies Europe,Illkirch,France).
2.2.Instruments and chromatographic conditions
The HPLC apparatus consisted of a Dionex P580 LPG pump,an ASI-100 T autosampler,an STH 585 column oven,a PDA-100 UV detector or a Jasco(Tokyo,Japan)Model CD 2095 Plus UV/CD detector;data were acquired and processed by a Chromeleon Datasystem (DionexCorporation,Sunnyvale,CA).Forsemipreparative separation,a Perkin-Elmer(Norwalk,CT,USA)200 LC pump equipped with a Rheodyne(Cotati,CA,USA)injector,a 5000μL sample loop,a Perkin-Elmer LC 101 oven and Waters 484 detector(Waters Corporation,Milford,MA,USA)was used.The signal was acquired and processed by Clarity software(DataApex,Prague,The Czech Republic).
In analytical separations,fresh standard solution of OME and single impurities were prepared shortly before using by dissolving 1–3 mg of each analyte in the mobile phase.
In order to ensure reproducible chromatographic performance,after the incorporation of acid or basic additives into the mobile phase,the IG-3 column was washed with the following mobile phases:ethanol at 0.5 mL/min for 30 min,followed by tetrahydrofuran at 0.5 mL/min for 120 min and, finally,ethanol at 0.05 mL/min for 120 min.
2.3.Enantiomer elution order
Theenantiomerelution orderoftheinvestigated chiral compounds was unambiguously established by evaluating the on-line CD signal monitored at 280 nm during the enantioselective analysis.As demonstrated previously[6],at the diagnostic wavelength of 280 nm there is a univocal correlation between CD properties and absolute configuration of the eluting enantiomer.In all elution modes investigated in this work,the first eluting enantiomer of OME and IMP-E on the IG-3 CSP exhibited negative CD signal at 280 nm which was related to the(S)-configuration.On the contrary,the first eluting (R)-enantiomer of IMP-B on the IG-3 CSP exhibited positive CD signal at the same wavelength.
3.Results and discussion
3.1.Analytical HPLC enantioseparation in polar organic conditions
Initially,pure methanol and ethanol were used as mobile phases for the enantioseparation of OME,IMP-B and IMP-E on the Chiralpak IG-3 CSP.
Chromatographic data collected from the measurements of retention,enantioselectivity and resolution at the column temperature of 25°C and flow rate of 1mL/min are summarized in Table 1.
Comparing the data obtained with two alcoholic mobile phases it can be noted that methanol provided superior chiral resolving ability than ethanol for all the tested chiral analytes.
Undermethanolmode,the highestvalue ofthe enantioseparation factor was observed for IMP-B(α=8.83)whereas the lowest degree of discrimination was observed for the IMP-E enantiomers(α=1.54).Even in the case of OME,a rather high value of enantioselectivity factor(α=2.35)was recorded.
As appears in Table 1,the(S)-enantiomers of OME and IMP-E were found to be eluted before than(R)-counterparts.On the contrary,for IMP-B the(S)-configuration was assigned to the more retained enantiomer.The reversal of the enantiomer elution order suggests that the methoxy group of OME and IMP-E,which is lacking in the IMP-B,takes a significant part in the enantioseparation process on IG-3 CSP,probably participating in H-bonding interactions with the hydrogen atom of the carbamate group of the stationary phase.
Another interesting aspect of the enantioselective HPLC analysis on the IG-3 CSP is the retention behavior of the three chiral compounds.Based on the results reported in Table 1,the retention was unusually high in methanol and in the cases of OME and IMPB significantly higher than that recorded in ethanol.In particular,the retention factor values of the more retained enantiomers were higher than 7.72.The reasons for such uncommon retentive behavior under methanol mode are unclear.It has already been reported that methanol can produce a stronger retention than ethanol due to its capability to promote solvophobic interactions between the CSP and chiral analytes[7]and/or induce conformational changes in the helical structure of the polysaccharide selectors[8],which can result in a higher affinity for selectands.
The chromatographic behavior of the IG-3 CSP was even more surprising when aprotic polar organic such as acetonitrile and acetone were used as mobile phases.In fact,in aprotic polar organic mode, fluxing the mobile phase at 1 mL/min,the first enantiomers of chiral compounds were not eluted from the 250 mm×4.6 mm IG-3 column after 40 min;thus they were much more retained than in polar alcoholic conditions.
On the basis of these findings,other factors potentially in fluencing the retention such as the column temperature and the presence of basic/acid additives in the mobile phase were evaluated.Under all conditions investigated methanol was the unique solvent component.
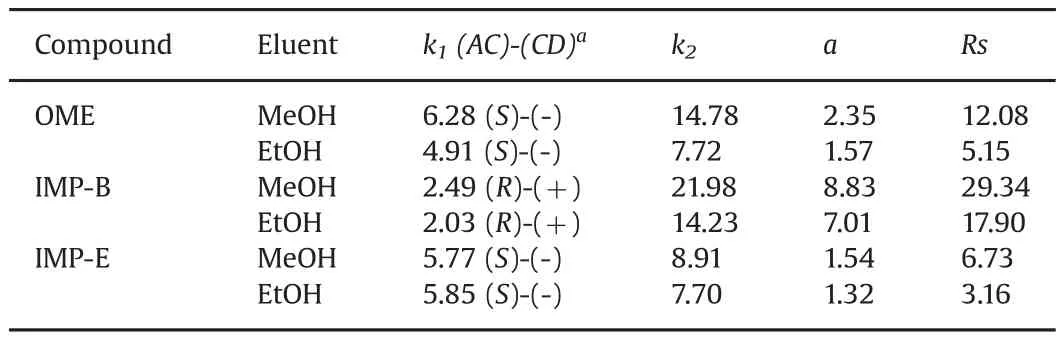
Table 1 Chromatographic results in polar organic conditions.
3.2.Effect of temperature on retention and enantioselectivity
In order to evaluate the impact of temperature on retention of OME,IMP-B and IMP-E enantiomers and calculate thermodynamic parameters,column temperature was changed in the 25–45 °C range at progressive intervals of 5°C.
As established by van’t Hoff analysis[9],the ln values of the resultant retention factors were related to the inverse of the temperature and the enthalpy and entropy of adsorption onto stationary phase(ΔH°and ΔS°)were calculated.The linear van’t Hoff plots shown in Fig.2 reveal that for all chiral compounds investigated the retention progressively decreased with increasing temperature.
Differently,as can be seen in Fig.2,column temperature affected the enantioseparation of the chiral analytes through two different ways.The enantioselectivity of OME and IMP-E increased significantly when the temperature changed from 25 °C to 45 °C.On the basis ofΔΔH°andΔΔS°values,it was possible to calculate the correspondent isoenantioselective temperatures,TISO(i.e.temperature at whichα=1)[10]and to establish that the two chiral resolution processes were entropically driven(i.e.TISOwas always lower than column temperature).
Instead,the enantioseparation of IMP-B was enthalpically driven(TISO=1020 K)andαlowered when temperature increased.
3.3.Effect of basic and acid additives on retention
During optimization of enantioselective analysis of chiral basic and acidic analytes it is a common practice to incorporate low concentrations of basic and acidic additives,respectively,into the eluent[11].Numerous studies highlight that this alteration of the mobile phase composition improves peak efficiency and symmetry,and consequently favors the resolution through the competitive interactions of the additives with the underivatized silanol groups of the CSP.Usually,the additive addition only slightly affects the retention[12].However,in the event that the additive produces an alteration of the complex network of stereoselective interactions between enantiomers of chiral analyte and selector,a change in enantioseparation can be observed[13].
In this work,variable concentrations of DEA in the range of 0.01%–0.1%were added to methanol in the resolution of OME,IMPB and IMP-E on the Chiralpak IG-3.Fig.3 shows the in fluence of concentration of amine additive on retention and enantioseparation parameters for one selected compound.As can be seen,the presence of growing levels of DEA in the methanol eluent system reduced deeply and progressively the elution times of both enantiomers of OME,leaving the enantioseparation value almost on the same value.Examination of the chromatographic data illustrated in Fig.4 reveals that the retention factors of the more retained enantiomer(R)-OME recorded in absence and at 0.08%DEA level were 11.82 and 3.48,respectively,while the enantioseparation factors changed from 2.35 to 2.42.
The effect of DEA in significantly shortening retention was evident also for enantiomers of other two related chiral benzimidazoles(data not shown).However,in the case of IMP-B the lowering in retention was much more pronounced for the first eluting enantiomer.Therefore,the presence of DEA substantially improved enantioselectivity.Changesin retention and enantioseparation values from methanol to methanol-DEA(0.1%)are presented in Fig.4.
Dramatic shifts in retention were also observed when TFA was used as an acid additive.As shown in Fig.5,the addition of only 0.01%TFA to methanol was sufficient to reduce the elution times for the second eluting enantiomer of OME and IMP-B by over 70%without greatly altering enantioselectivity.
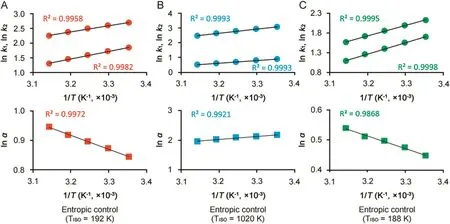
Fig.2.Plots of ln k vs.1/T × 103and ln α vs.1/T × 103for OME(A),IMP-B(B)and IMP-E(C).Column:Chiralpak IG-3(250 mm × 4.6 mm I.D.);detection:UV at 280 nm; flow rate:1.0 mL/min;column temperature:25–45 °C.
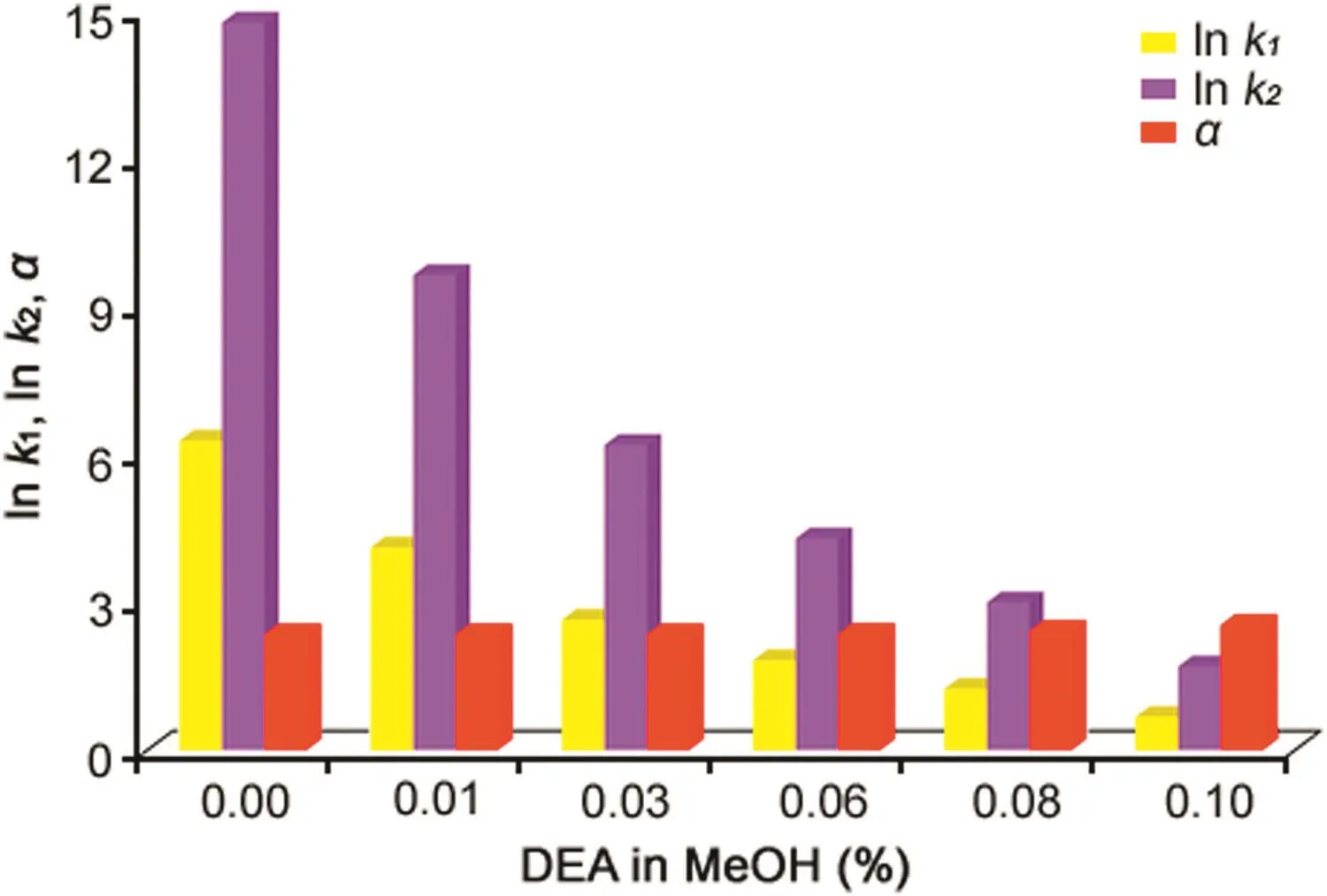
Fig.3.Effect of DEA content on k1,k2and α of selected OME and IMP-E.Column:Chiralpak IG-3(250 mm×4.6 mm I.D.);detection:UV at 280 nm; flow rate:1.0 mL/min;column temperature:25°C.
The exact mechanism by which the basic/acid additives produced the retention changes is not clear.However,since the interactions responsible for enantioseparation are slightly affected bytheaction ofacid orbasicmodifier,itispresumable thatnon-stereoselectivestrongH-bondingcontactsbetween chiral analytes and binding sites of the polysaccharide-based CSP may be involved in determining the unusual increment in affinity with CSP.
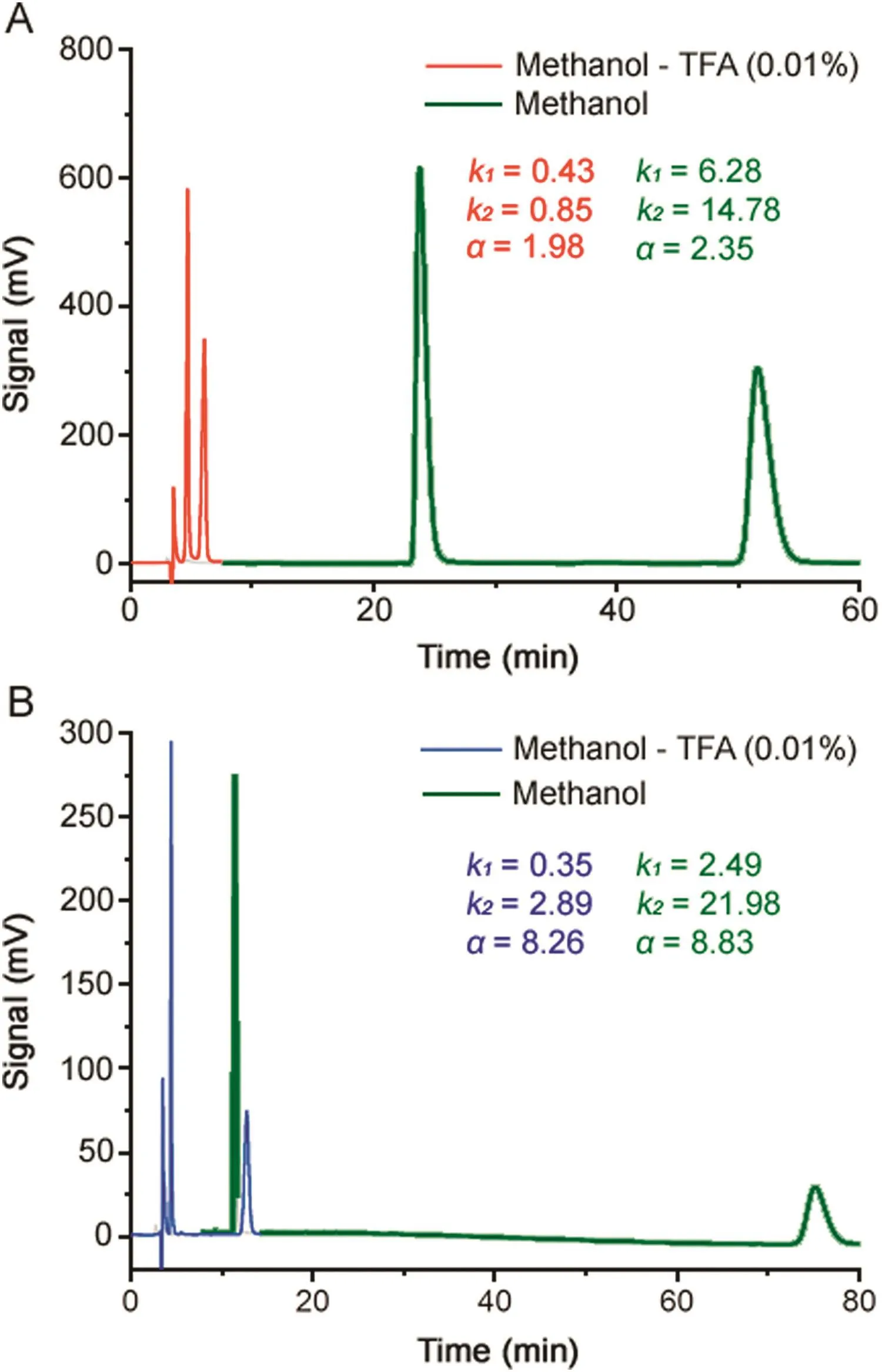
Fig.5.Typical HPLC chromatograms illustrating the separation of the enantiomers of OME(A)and IMP-B(B)in absence and in presence of TFA(0.01%)in methanol.Column:Chiralpak IG-3(250mm×4.6 mm I.D.);detection:UV at 280 nm; flow rate:1.0 mL/min;column temperature:25°C.
3.4.Semipreparative HPLC enantioseparation
The availability of chiral impurities of enantiopure APIs is a challenge in the development of effective analytical methods with strict quality requirements established by regulatory agencies.Chiral impurities include all the chiral related substances formed as undesired reaction or degradation products which have the same absolute configuration of the API as well as its enantiomeric form[14].
The mg-scale production of enantiomerically pure impurities of(S)-OME by enantioselective HPLC on the immobilized ADMPC-based Chiralpak IA CSP under normal phase mode was reported in a previous paper by our research group[15].
Here,as high retention of racemic sample is generally recognized as a limiting factor when approaching scale-up of enantioseparation conditions,for each chiral benzimidazole derivative it was searched the best analytical compromise between enantioselectivity,time and solvent consumption.Method development and optimization on the IG-3 CSP was carried out according to the findings of the above described study of the in fluence of mobile phase composition and column temperature on retention and enantioseparation.Fig.6 shows the advantages obtained in the enantioselective HPLC of OME by substituting in the mobile phase 60%of methanol with ethanol and increasing the column temperature from 25 to 45°C.As a result,the elution time was reduced by more than half and the factor of resolution appreciably improved from 12.08 to 14.47.
As illustrated again in Fig.6,in the case of IMP-B the total replacement of methanol with the green solvent ethanol and the use of a column temperature of 45°C,dramatically reduced the retention time of the second elution enantiomer maintaining the resolution factor value at a rather high level(i.e.Rs=20.91).
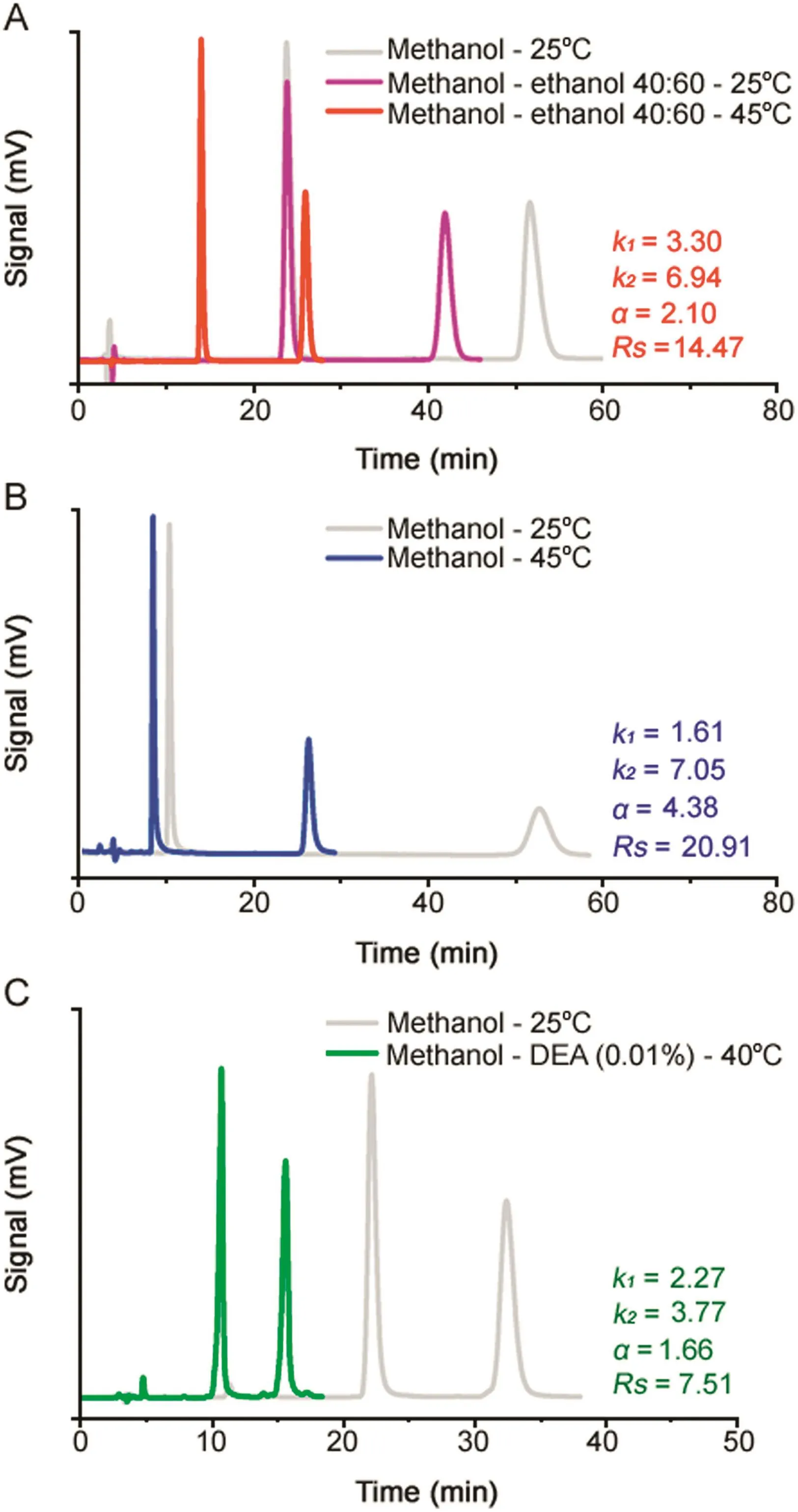
Fig.6.Optimization of the analytical HPLC enantioseparation of OME(A),IMP-B(B)and IMP-E(C).Column:Chiralpak IG-3(250 mm×4.6 mm I.D.);detection:UV at 280 nm; flow rate:1.0 mL/min.
Finally,the optimized HPLC enantioseparation of IMP-E on IG-3 CSP was carried out at 40°C and using the mixture methanol-DEA(0.01%)as a mobile phase(Fig.6).
Fig.7 shows the typical chromatograms pertinent to the resolution of 40mg of OME,70mg of IMP-B and 9mg of IMP-E by a 1-cm i.d.Chiralpak IG column in a single chromatographic run.The high efficiency and loading capacity of the column as well as the good degree of enantioselectivity achieved in polar alcoholic conditions allowed to obtain,in all cases,two enantiomerically pure fractions(e.e.>99%).
4.Conclusions
The immobilized ACMPC-based IG-3 CSP has been tested in HPLC enantioseparation of OME and its chiral impurities B and E under alcoholic organic mode.
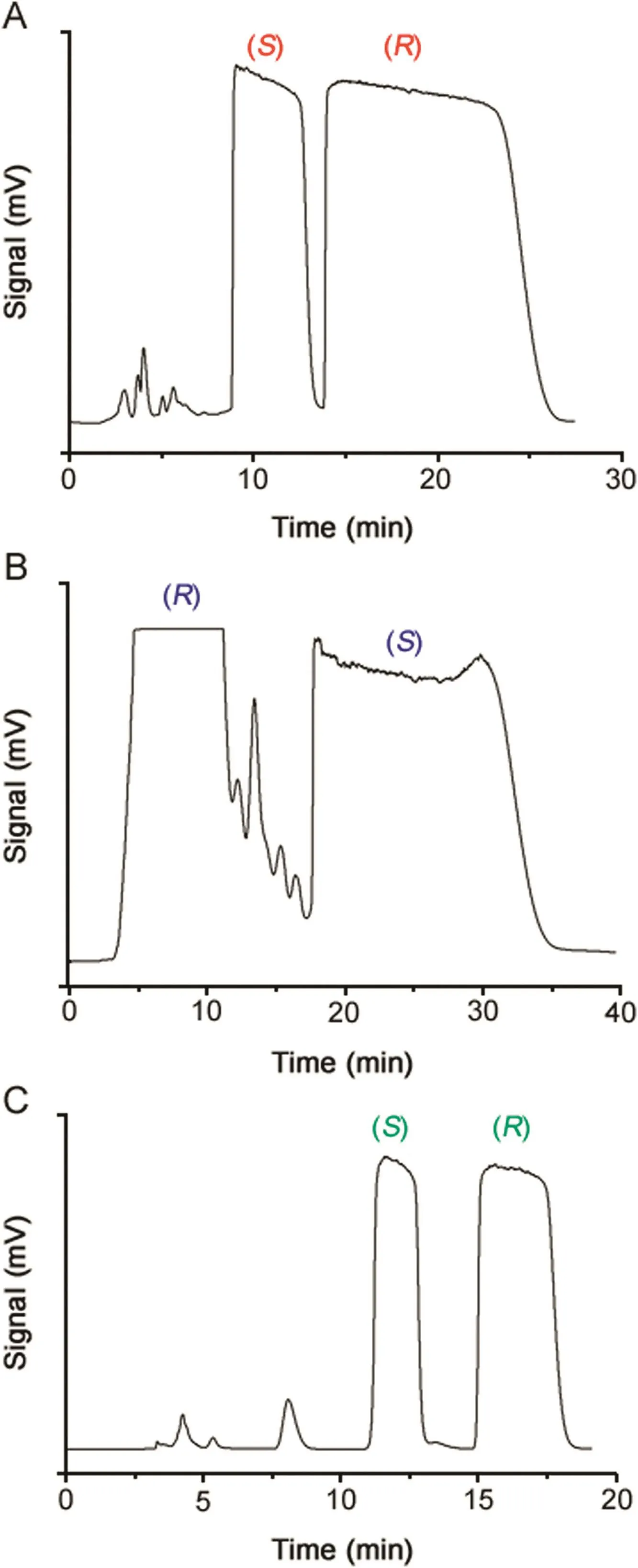
Fig.7.Typical chromatograms illustrating the resolution of 40 mg(in 3 mL of ethanol)of OME(A),70mg(in 5 mL of ethanol)of IMP-B(B)and 9 mg(in 3.5 mL of ethanol)of IMP-E(C)on the Chiralpak IG column.Column:Chiralpak IG(250 mm×10 mm I.D.);eluent:ethanol-methanol 60:40(v/v)(OME),ethanol(IMP-B)and methanol-DEA(0.01%)(IMP-E); flow rate:4.7(OME),3.7(IMP-B)and 5.0(IMPE)mL/min;column temperature:45 °C(OME and IMP-B)and 40 °C(IMP-E);detection:UV at 310 nm.
The enantioselective HPLC analysis reveals that:i)the retentive behavior of the IG-3 CSP towards benzimidazoles is strongly affected by the type of solvent used as mobile phase and it decreases in the following order:acetone/acetonitrile>>methanol>ethanol;ii)under methanol conditions the enantiomers of the chiral analytes are discriminated at the best level although their elution times are excessively high;iii)the high retention recorded in methanol can be reduced by the addition of basic/acid additives or increasing the column temperature;iv)temperature exerts a significant in fluence on the chromatographic performance of the IG-3 CSP;in particular,the enantioseparations of OME and IMP-E are entropically-driven whereas the resolution of IMP-B is enthalpically driven.
The manipulation and control of the chromatographic parameters affecting retention allowed minimizing the unproductive CSP/selectands interactions responsible for the undesirable increment in retention and setting up productive separations of the enantiomers of chiral analytes on a semipreparative scale.
Therefore,a simple screening with a reduced number of mobile phase compositions and fluctuations in temperature has demonstrated the effectiveness of the immobilized ACMPC-based IG-3 CSP for enantiomer resolution of the chiral benzimidazole derivatives structurally related to OME.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are grateful to Ms.A.Mosca for her technical assistance.
The research that has led to these results has received funding from the European Community's Seventh Framework Programme under the grant agreement 602051(Project HERACLES:Humancystic Echinococcosis ReseArch in CentraL and Eastern Societies;http://www.Heracles-fp7.eu/).The funding body had no involvement in the preparation,ideas,writing,interpretation,or the decision to submit this article.
 Journal of Pharmaceutical Analysis2018年4期
Journal of Pharmaceutical Analysis2018年4期
- Journal of Pharmaceutical Analysis的其它文章
- Quantitation of tadala fil in human plasma using a sensitive and rapid LC-MS/MS method for a bioequivalence study
- Duplex microRNAs assay based on target-triggered universal reporter hybridization
- Extracellular synthesis of silver nanoparticles by Pseudomonas sp.THG-LS1.4 and their antimicrobial application
- Simultaneous determination of steroid drugs in the ointment via magnetic solid phase extraction followed by HPLC-UV
- Screening potential mitochondria-targeting compounds from traditional Chinese medicines using a mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry method
- Recent advances in screening of enzymes inhibitors based on capillary electrophoresis
