Sexual characteristics of male guppies Poecilia reticulata serve as effect biomarkers of estrogens*
TIAN Hua (田華), LI Yun (李赟), WANG Wei (王蔚), ZHAO Fei (趙飛), GAO Su (高素),RU Shaoguo (汝少國)
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
Abstract Guppies ( Poecilia reticulata) are considered a candidate model species for the identi fication and testing of endocrine-disrupting chemicals. Male guppies may be used to address the challenge of making potential linkages between alterations of biomarkers, both at the cellular and organ level, and adverse outcomes. In the present study, a predictive relationship between sex characteristics and reproductive output was observed in male guppies that underwent a long-term toxicity test with 0.5 μg/L 17 β-estradiol administered during the juvenile period. Radioimmunoassay and western blot analyses demonstrated that 17 β-estradiol exposure caused a signi ficant increase in testicular 17 β-estradiol levels as well as the induction of exposure biomarkers, namely hepatic vitellogenin. Exposure to 17 β-estradiol also caused a signi ficant decrease in testosterone levels, which consequently reduced the gonadosomatic index, sperm counts, and the coloration index. These changes of male sexual characteristics further translated into adverse in fluences on reproduction, as measured by a decrease in offspring production and survival rate. Our results suggest that the above-mentioned sexual characteristics of male guppies may be considered potential in vivo biomarkers of estrogen effects on reproduction.
Keyword: effect biomarker; endocrine-disrupting chemical; 17 β-estradiol; Poecilia reticulata; reproduction;sexual characteristic
1 INTRODUCTION
Endocrine-disrupting chemicals (EDCs), which enter into the aquatic environment by avenues such as sewage effluence, may affect the reproductive potential of aquatic animals by exerting estrogenic,anti-estrogenic, androgenic, or/and anti-androgenic activities. 17 β-Estradiol (E2) was detected in industrial and municipal sewage treatment works effluents, and these discharges represent one of the main sources of EDCs in the aquatic environment (Desbrow et al.,1998). The concentration of natural estrogens in effluents and surface waters ranged from the low nanograms per liter up to hundreds of nanograms per liter (Desbrow et al., 1998; Ternes et al., 1999; Baronti et al., 2000; Rodgers-Gray et al., 2000).
Various screening and testing systems for EDCs have been established by the Organization for Economic Cooperation and Development (OECD)and the U.S. Environmental Protection Agency (EPA).In most teleosts, lipophilic compound uptake from solution occurs via permeable surfaces of the body,especially across the gills (Randall et al., 1998; Van Der Kraak et al., 2001). Thus, in terms of the efficiency of chemical delivery/exposure, fish offer a signi ficant advantage over mammalian systems for certain types of toxicological studies. Small fish species, principally the fathead minnow ( Pimephales promelas), zebra fish( Danio rerio), and Japanese medaka ( Oryzias latipes),have been used as model organisms for several of these EDCs testing programs (Ankley and Johnson,2004). However, the importance of including other fish species in EDC research should not be overlooked(Hutchinson et al., 2006a). Among these candidate model organisms, the male guppy ( Poecilia reticulata)has been suggested to be a top priority due to the strong visibility and biological relevance of its secondary sex characteristics (gonopodium and pigmentation) (Baatrup and Junge, 2001; Bayley et al.,2002; Cardinali et al., 2004). Guppies are ovoviviparous fish, of which the differentiation of gonads and the development of sexual characteristics last for approximately 3 months (Houde, 1997). During the juvenile period of male guppies, pigments (such as carotenoids) are continually deposited in the skin to produce the sexually attractive nuptial colors (yellow,orange, and red) (Hudon, 1994), and the anal fin undergo a series of hormone-dependent changes that result in the development of gonopodium. Gonopodium in the adult is insensitive to environmental chemicals because the morphogenesis of the skeletal elements has already been completed by adulthood. Hence,juvenile male guppies are suitable for studying the effects of EDCs on reproductive system.
Teleost assays developed for EDC testing are comprised of a set of endpoints at multiple levels of biological organization, including exposure biomarkers, effect biomarkers, and adverse effect endpoints (Ankley and Johnson, 2004; Hutchinson et al., 2006a). These endpoints are indicative of possible adverse outcomes of exogenous chemicals on reproduction as well as their modes of action (MOA).For example, biomarkers of exposure, such as vitellogenin (VTG) and zona radiata protein (ZRP)induction in male fish, present useful features such as the speci ficity for estrogens, sensitivity, and the magnitude of the response (Emmersen et al., 1979;Sumpter and Jobling, 1995; Marin and Matozzo,2004; Nilsen et al., 2004; Saaristo et al., 2010; Humble et al., 2013; Adeogun et al., 2016). Effect biomarkers(including secondary sexual characteristics,gonadosomatic index (GSI), plasma steroids, and gonad histology) are assumed to be more directly and mechanistically related to reproductive capability to assess the potential threat posed by the presence of EDCs in the environment and to lend insight to causative MOA. There may be scope to increasingly use biomarker data in EDC ecological risk assessments if plausible linkages can be made across levels of organization so that adverse outcomes (i.e., survival,growth, development, and reproduction) might be envisaged relative to biomarker responses (Hutchinson et al., 2006a).
In this present study, male guppies were exposed to a nominal concentration of 0.5 μg/L E2during the entire juvenile period of gonad development. First,the testicular E2levels and the presence of hepatic VTG were determined to examine the bioavailability and estrogenic activity of E2in male guppies. Second,testicular testosterone levels were quanti fied to con firm the inhibition of E2on testosterone synthesis,and a series of biomarkers was selected to evaluate the in fluences on male sexual characteristics,including the number of ejaculated sperm cells, GSI,gonopodial index (GPI), and coloration index (CI).Finally, the phenotypic sex ratio, offspring production,and survival rate were analyzed to determine if the above mentioned changes to the sexual characteristics propagated into a disturbance of the sex differentiation and/or reproductive output. The main purpose of this study is to provide an example of how changes in biomarkers at the cellular and organ levels can be translated into adverse effects at the individual level and thus evaluate the potential use of sexual characteristics in male guppies, primarily secondary sex characteristics such as the bright coloration, as effect biomarkers for EDC assessment.
2 MATERIAL AND METHOD
2.1 Fish exposure and sample protocol
Adult Red Albino guppies ( Poecilia reticulata)were obtained from a local dealer in Qingdao, China and used as a broodstock. Within 3 days, 96 newborn guppies of two sexes was collected and distributed to eight 10-L aquaria (12 fish/tank, 4 tanks/treatment).The aquaria contained 6 L of dechlorinated tap water(a control) or dechlorinated tap water containing the appropriate E2dose (nominal concentration of 0.5 μg/L) using a semi-static toxicity test (3 L of water was renewed daily to maintain a constant E2concentration). Stock solutions of E2(Sigma, St.Louis, MO, USA) were prepared in double-distilled water containing dimethylsulfoxide (DMSO) as a solvent vehicle (terminal concentration 5 μL/L).Previous experiments demonstrated that no solventderived effects of DMSO existed at this concentration,which was 20 times lower than the OECD recommended maximum (100 μL/L) (Seki et al.,2003; Pawlowski et al., 2004a, b; Hutchinson et al,2006b). The water temperature was maintained at 25±2°C. The dissolved oxygen content was 7.0±0.1 mg/L, and the pH was 7.6±0.2. The photoperiod was 14 h light and 10 h dark. The guppies were fed with freshly hatched Artemia nauplii and commercial fish feed. Feces and remaining food were removed daily. Beginning at the ninth week of exposure, the aquaria were monitored 3 times daily for newly hatched offspring.
After 90 days of E2exposure, the guppies were anesthetized in 75 mg/L MS-222 (Sigma-Aldrich, St.Louis, MO, USA), and efforts were made to minimize suffering. The phenotypic sex of each guppy was identi fied to calculate the phenotypic sex ratio.Twenty males from each treatment were randomly sampled for the following experiments. The body length and body weight were recorded, and the condition factor was calculated as follows:
Condition factor= 100×body weight (g)/body length (cm)3.
All fish were imaged for the determination of the GPI and CI, and then sperm cells were collected and counted. Subsequently, the skin, testis, and liver tissues were each dissected, in order to measure the carotenoid content, determine the GSI (the ratio of the testis weight to the body weight)/detect the sex hormone levels, and calculate the hepatosomatic index (HSI, the ratio of the liver weight to the body weight)/con firm the presence of VTG, respectively.
2.2 Radioimmunoassay
The homogenate of testis tissues was prepared using the method described by Tian et al. (2012). The E2and testosterone ( T) levels were detected by radioimmunoassay using commercial kits from the Tianjin Nine Tripods Medical and Bioengineering Co. Ltd.,China. For E2, the assay detection limit was 0.50 pg/mL.For T, the assay detection limit was 0.10 ng/mL.
2.3 Western blot analysis
Each sample of livers from 6 male fish per treatment was homogenized in 4 μL of homogenization buffer(50 mmol/L Tris-HCl, 0.02% aprotinin, and 1 mmol/L phenylmethanesulfonyl fluoride) per mg of liver tissue, and 6 livers of the untreated female guppies were used as a positive control.
The presence of VTG in the liver samples was veri fied by western blot analysis, mainly according to the protocol described by Tian et al. (2009). A polyclonal antiserum against gold fish VTG was used,the bond ability of which to VTG produced by other teleosts had been proved (Li, 2006; Shi, 2007).
2.4 Analysis of the sexual characteristics
The sperm cell suspension was prepared using the method described by Baatrup and Junge (2001), and the sperm number was counted following the general guidelines for human sperm (WHO, 1992). The area and intensity of the orange-colored spots were first observed macroscopically, and then the GPI (the length of the gonopodium related to the body length)and CI (the orange-colored area as a percentage of the whole body area) values were calculated using the method described by Baatrup and Junge (2001). To determine the direct cause of the decreased coloration index, the carotenoid content in the skin was tested(Britton, 1985). The total carotenoid content of each male was divided by the skin weight to calculate the carotenoid content per mg of skin, and this value was further divided by the CI to calculate the carotenoid content per mg of orange-colored skin.
2.5 Calculation of the phenotypic sex ratio
The phenotypic gender was visually identi fied by external sexual characteristics, including the presence of gravid spots in the females and orange coloration and gonopodia in the males, and con firmed by the macroscopic observation of the gonad structure.Accordingly, the number of males and females was counted, and the sex ratio for both the treatment and the control was calculated.
2.6 Production of offspring and calculation of the survival rate
The offspring were counted to calculate the number of offspring produced per female from each aquarium.The survival of the offspring was observed under microscope. Accordingly, another apical endpoint,the survival rate of the offspring, was recorded.
2.7 Statistics
The differences in the phenotypic sex ratios were assessed using an x2-test. All of the other data were presented as the means±standard deviations. The signi ficant differences between the E2-treated group and the control were found using a t-test analysis. The values were considered to be signi ficant when 0.01< P <0.05 and highly signi ficant when P <0.01.Pearson’s correlation coefficient was used to calculate the relationship between testicular T levels and GSI,testicular T levels and sperm count, sperm count and carotenoid content in the total skin, carotenoid content in the total skin and CI.
3 RESULT
3.1 Bioavailability of E 2 in vivo
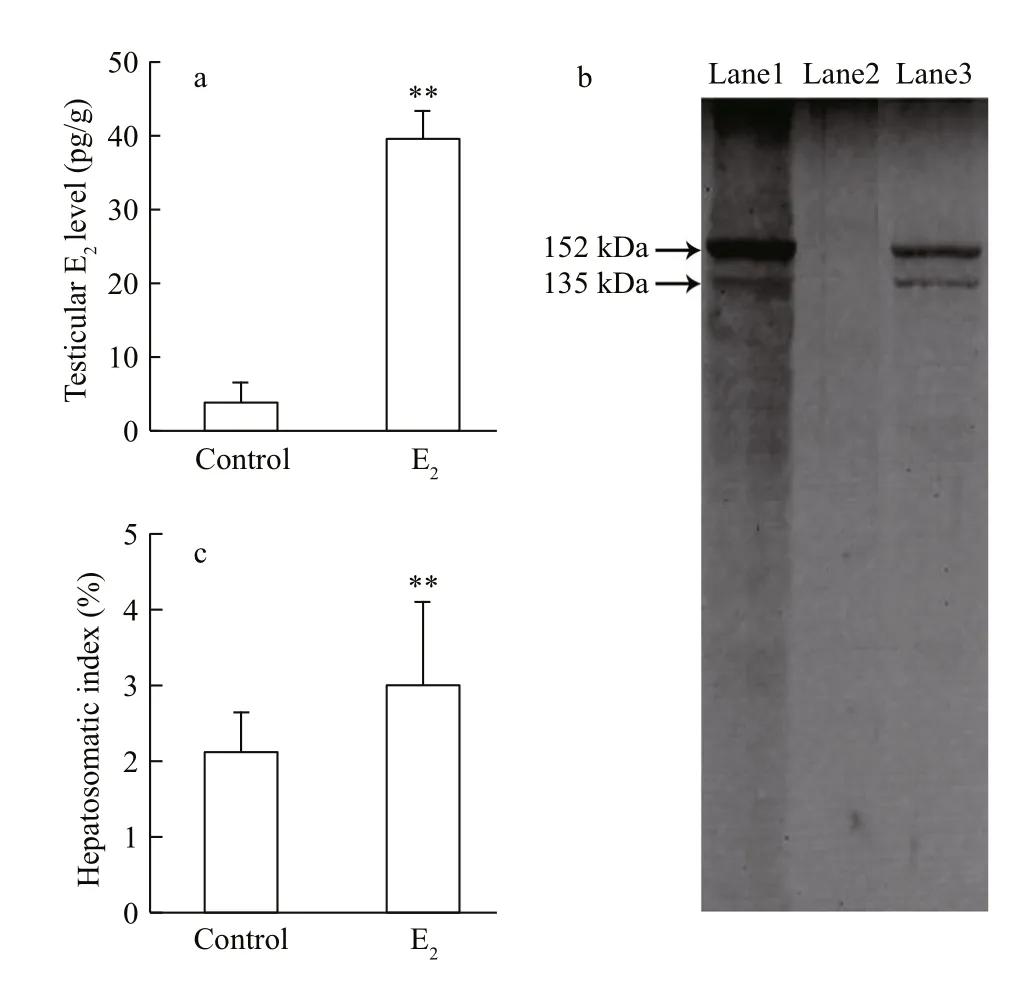
Fig.1 The bioavailability of 17 β-estradiol (E 2) in male guppies after 90-days of waterborne exposure
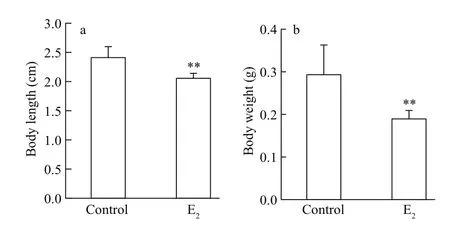
Fig.2 The decrease in body length and weight of male guppies after E 2 exposure
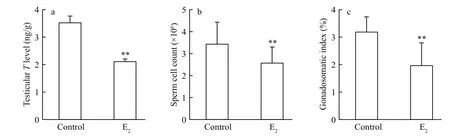
Fig.3 Changes of the primary sexual characteristics in male guppies after E 2 exposure
Mortality rates were lower than 10% for both the treatment and the control. The effects of waterborne E2exposure on the testicular E2levels and hepatic VTG presence in male guppies are shown in Fig.1. As shown in Fig.1a, the E2concentration in testis tissue of guppies exposed to exogenous E2was signi ficantly higher than the concentration observed in the control male individuals, with a resulting ratio of 10:1( P < 0.01). Western blot analysis revealed the presence of two VTG protein subunits with estimated molecular masses of 152 kDa and 135 kDa in the livers of the control females. These subunits were not observed in the livers of the control males but were observed in the E2-exposed males, indicating that VTG protein synthesis was induced by waterborne exposure to E2in the male guppies (Fig.1b). Concomitantly, the E2treatment induced a signi ficant increase of HSI values in the males ( P < 0.01, Fig.1c).
3.2 Decrease in body length and body weight following E 2 exposure
The body length and the body weight were decreased after E2exposure ( P <0.01, Fig.2), whereas the condition factor was not signi ficantly affected(data not shown).
3.3 In fluences on the male sexual characteristics of E 2 exposure
The testicular T concentration, 3.52±0.23 ng/g testis in the control guppies, was signi ficantly decreased by E2exposure ( P < 0.01, Fig.3a). The GSI values were signi ficantly reduced from 3.18%±0.54%in the control fish to 1.96%±0.82% in the E2treatment( P <0.01, Fig.3c). Signi ficant positive correlation of GSI values with T levels was found ( R=0.705,P=0.000). The total sperm count was signi ficantly reduced by about 25% after E2treatment(0.01< P < 0.05, Fig.3b), and it was also positive correlated with T levels ( R=0.371, P=0.024).
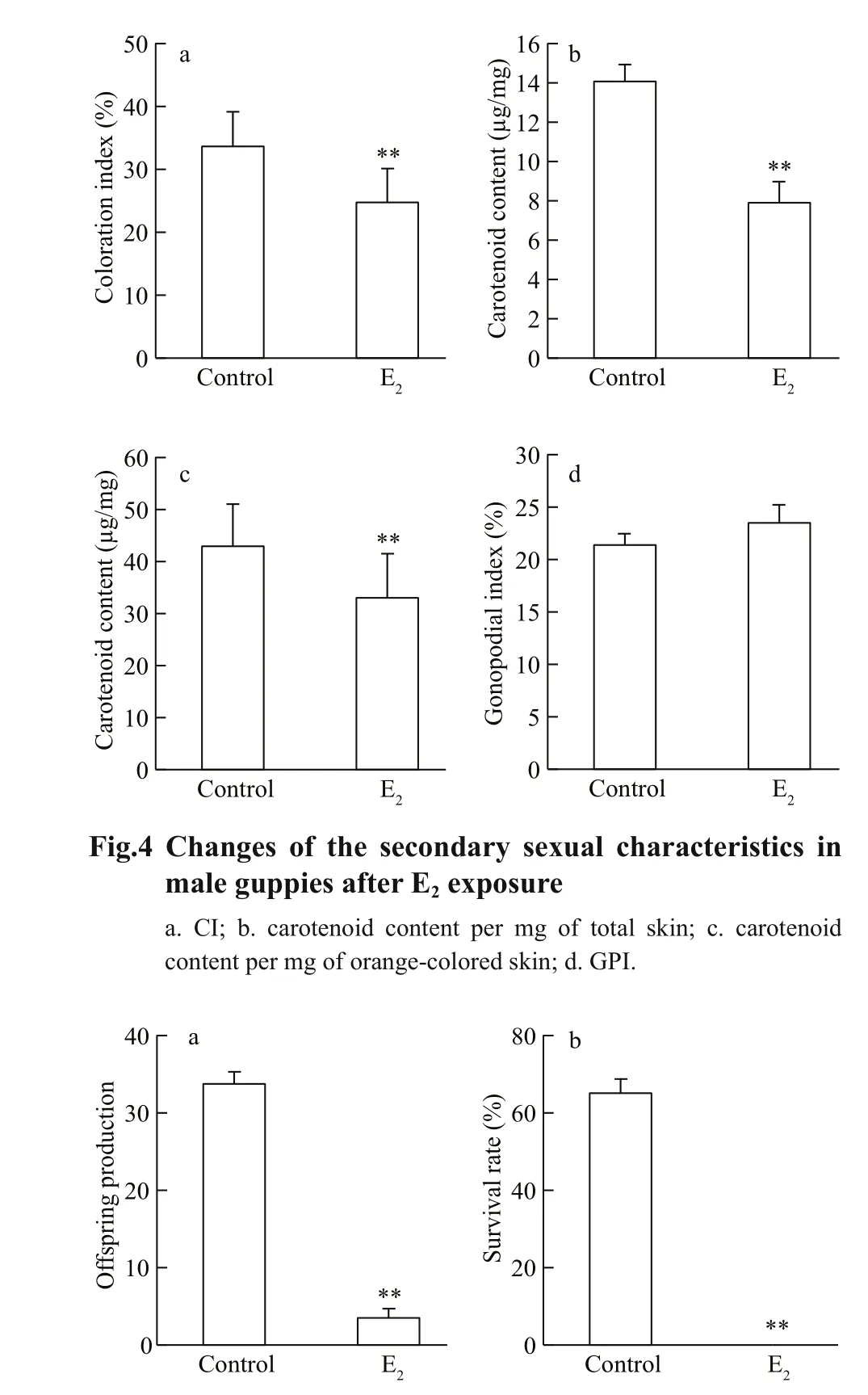
Fig.5 The reduction in the reproductive output of guppiesafter E 2 exposure
Even without magni fication, a reduction in the area and intensity of the orange coloration in the males treated with E2was obvious. The CI analysis quantitatively revealed that 33.65%±5.36% of the body area was covered with orange spots in the control male guppies, while this index was reduced in the individuals treated with E2( P <0.01, Fig.4a). The mean carotenoid content in the total skin was signi ficantly decreased by 44% after E2exposure( P <0.01, Fig.4b). Signi ficant positive correlation of CI with carotenoid content ( R=0.574, P=0.000), as well as that of carotenoid content with sperm count( R=0.402, P=0.014) was found. The pigment concentration was expressed as the carotenoid content per mg of orange-colored skin to enable the distribution area-adjusted measurement of pigment content. Using this method, a marked reduction remained evident in the E2treatment (by 23%, P <0.01,Fig.4c), suggesting that the reduction in the total carotenoid concentration is a consequence of the inhibitory action on the pigment distribution area combined with the pigment concentration in each orange spot. The exposed males had a shorter gonopodium than the control ones. However, the length of the gonopodium relative to the body length of the fish (GPI) was unaffected by E2treatment( P >0.05, Fig.4d), suggesting that the reduction in gonopodium length may be a result of the general effects on body growth, rather than the speci fic inhibition of gonopodium development.
3.4 The unaffected phenotypic sex ratio upon E 2 exposure
The phenotypic sex ratio of the control guppies was approximately 1:1, which was not different from that of the E2treatment.
3.5 Reduction in reproductive output following E 2 exposure
The offspring production was signi ficantly decreased by E2exposure ( P <0.01), and the survival rate of the offspring in the E2treatment was 0 ( P <0.01),collectively indicating a severe reduction in reproductive output (Fig.5).
4 DISCUSSION
The guppy is an OECD recommended fish species for testing bioconcentration of chemicals. In this study, a 10-fold increase in the testicular levels of E2was observed after a 90-day waterborne exposure.The lipophilic nature of xenoestrogens leads to an accumulation in the lipids and membranes of the organism, principally in the livers and gonads(Ekelund et al., 1990; Ahel et al., 1993; Hertl and Nagel, 1993). In addition to chemical analysis, the estrogen-sensitive bioassay (such as exposure biomarker VTG induction in male fish) provides another measurement re flecting the deposition of E2and speci fically indicates the bioavailability in vivo(Emmersen et al., 1979; Kishida and Specker, 1993;Sumpter and Jobling, 1995; Koya et al., 1997; Susca et al., 2001; Marin and Matozzo, 2004; Nilsen et al.,2004; Duong et al., 2009).
Numerous studies have evaluated the utility of detectable changes in androgen concentration and/or production as indicators of contaminants that impact male reproduction (Dubé and MacLatchy, 1999,2001; Karels et al., 2001; Lavado et al., 2004). T plays the main role in the regulation of male reproduction within the Poeciliinae subfamily (Kime and Groves,1986; Borg, 1994; Bayley et al., 2002). In the present study, increasing E2levels in the testis tissues resulted in decreased T levels in male guppies, as in other teleosts (Lambert and Pot, 1975; Trudeau et al., 1991,1993; Chang and Lin, 1998; Sharpe et al., 2007).Accordingly, reduced GSI was observed in the E2treatment, and there was also a reduction in the total number of sperm cells in the provoked ejaculates.Sperm count is a fundamentally important predictor of male fertility and is considered a sensitive indicator in the analyses of the effects of chemicals on male reproduction (Haubruge et al., 2000; Baatrup and Junge, 2001). Therefore, the reduction in the sperm cell count of the E2-exposed males can be interpreted as a reduction in reproductive output. However, it is worth to notice that the decrease in sperm count is not sufficient to account for the observed decrease in reproductive success.
A positive association between the orange pigmentation of the male guppies and sperm count was observed in this study. Namely, the E2-exposed males with signi ficantly smaller sperm loads also had a reduced area and intensity of orange pigmentation.Guppies show remarkable sexual dichromatism. The males of Red Albino guppies have sexually attractive orange coloring that is absent in females. Male Red Albino guppies with the brightest colors are presumed to be those that have sufficient carotenoids for meeting coloration feature, as well as antioxidative and immunological functions (Vershinin, 1999; McGraw and Ardia, 2003). Thus, color intensity is not only a marker of good health but also indicates a sufficient quantity of high quality sperm (Matthews et al., 1997;Pitcher and Evans, 2001; Locatello et al., 2006).Therefore, females copulate more often with the brightly colored fish (Houde and Torio, 1992;Nicoletto, 1993; Hudon, 1994; Houde, 1997; Grether,2000). We suggested that the long-term exposure to E2during the juvenile period of male guppies led to a decrease in the amount of carotenoids deposited in the skin, which caused the fading of the display coloration.In accordance with the phenotype-linked fertility hypothesis, this is also likely to decrease reproductive output (Houde, 1997; Evans et al., 2004).
The sexual pigmentation of male guppies has been previously reported to be discolored after exposure to the anti-androgens vinclozolin, p, p’-DDE, and flutamide (Baatrup and Junge, 2001; Bayley et al.,2002). The bright coloration of the male-speci fic secondary sexual characteristics in guppies can easily be quanti fied to indicate the effects of EDCs on male reproductive phenotypes. Interestingly, the conspicuous coloration is directly related to the reproductive fitness of the individual and is associated with population-level adverse outcomes (Houde and Torio, 1992; Nicoletto, 1993; Hudon, 1994; Houde,1997; Grether, 2000). Such a sensitive and visual effect biomarker could potentially be used in both in situ biomonitoring and in vivo laboratory assays for the testing of potential estrogenic and anti-androgenic EDCs.
Considering the above mentioned factors comprehensively, we suggest that the reduced reproductive output of the E2treatment may be due to the changes of the male sexual characteristics,including discolored orange spots and a reduced sperm count.
The development of a gonopodium, which is critical for sperm transfer, is another male-speci fic secondary sexual characteristic regulated by androgen. A smaller gonopodium has been observed in male Poeciliinae exposed to sewage treatment effluent (Batty and Lim, 1999). However, in the present study, no alteration was found in the GPI values between the 0.5 μg/L E2treatment and the control. Similarly, Nielsen and Baatrup (2006)demonstrated that after being exposed to 10 and 50 ng/L of E2and 10, 50, and 200 ng/L of 17 βethinylestradiol for 3.5 months from birth to adulthood, male guppies did not show signi ficant differences in GPI when compared to the control. The exact hormonal pathways controlling the development of the gonopodium in the male guppy are not fully understood. It is possible that both androgens and estrogens are necessary for normal gonopodium growth (Pandey, 1969a, b; Bayley et al., 2002; Toft and Baatrup, 2003). In this regard, even if male guppies exhibited reduced GPI values in response to exogenous chemical exposure, it could be a consequence of the exposure to EDCs, including estrogenic, androgenic, anti-estrogenic, and antiandrogenic substances, whereas the reduced GSI,sperm count, and display coloration could indicate a speci fic anti-androgen or estrogen response.Regarding gonopodium length, a relationship to male fitness is less clear (Brooks and Caithness, 1995;Bayley et al., 2002). Thus, although possibly useful for detecting environmental contaminants, changes of GPI values do not offer assistance in targeting detailed chemical(s) nor do they provide insight about the potential adverse effects and speci fic causative MOA.
In our previous study, exposure to 0.5 μg/L E2from the time of fertilization to 40 days post-hatching caused a skewed nature of the phenotypic sex ratio towards females in zebra fish (Zhang et al., 2013),suggesting that E2acts as the natural inducer of ovarian differentiation in teleost fish (Nakamura et al., 1998). However, in this study the results showed that the phenotypic sex ratio of guppies remained 1:1 after the exposure to 0.5 μg/L E2from birth to adulthood. We consider that in addition to the interspecies differences in the sensitivity of sex differentiation (Toft and Baatrup, 2003; Nielsen and Baatrup, 2006; Larsen et al., 2008), the timing and duration of exposure directly relates to the severity of the effects by estrogenic compounds. Guppies is an ovoviviparous species in which sex differentiation starts in the last 10 days of gestation (Goodrich et al.,1934; Houde, 1997), while zebra fish are oviparous fish whose sex differentiation period begins from approximately 23–25 days post-hatching (Uchida et al., 2002). Therefore, a possible explanation as to why the phenotypic sex ratio of guppies was unaffected by E2exposure in this study could be that the exposure was not conducted during gonadal differentiation but rather from birth, when gonadal differentiation may have progressed to a point where the xenoestrogens have reduced effects. Such fundamental differences in developmental strategies are likely to have a major bearing on the potential impacts of EDCs.
5 CONCLUSION
The male guppy is a recognized model species in aquatic toxicology, and there is growing interest in its application in endocrine-disruption research. The results from the present study show that E2not only induces signi ficant changes of several male sexual characteristics at the cellular and organ levels but also causes ecologically relevant effects by adversely affecting individual fertility in guppies. Our study proposed the potential of several sex characteristics of male guppies serving as in vivo effect biomarkers of EDCs on reproduction. However, a single dose of 0.5 μg/L E2was adopted in this study. For the practical and scienti fically credible use of sex characteristics of male guppies as effect biomarkers for the ecological risk assessment of EDCs, a series of environmentally relevant concentrations should be adopted to study the dose-dependent response of E2and other xenoestrogens with weak estrogen activities.
6 DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin?) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
