Nutrient-enhanced n-alkanes biodegradation and succession of bacterial communities*
SUN Yanyu (孫延瑜) , WANG Hui (王慧) LI Junde (李俊德) , WANG Bin (王斌) ,QI Cancan (齊燦燦) , HU Xiaoke (胡曉珂)
1 Key Laboratory of Coastal Biology and Bioresource Utilization, Yantai Institute of Costal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract Bioremediation, is an effective and environment-friendly method of cleaning up crude oil pollution after an oil spill. However, the in situ bioremediation of crude oil is usually inhibited by de ficiency of inorganic nutrients. To understand the effects of nutrient addition on the bioremediation of crude oil and the succession of bacterial communities during process of bioremediation, microcosms containing oilcontaminated sediments were constructed and biodegradation of crude oil was assessed based on the depletion of different ingredients. We used two culture-independent methods, denaturing gradient gel electrophoresis and a 16S rRNA gene based clone library, to analyze the succession of bacterial communities. The results suggested n-alkanes were degraded after 30 days and that nutrient amendments signi ficantly improved the efficiency of their biodegradation. Moreover, oil contamination and nutrient amendments could dramatically change bacterial community structures. Lower diversity was detected after being contaminated with oil.For instance, bacterial clones affiliated with the phylum Armatimonadetes, Firmicutes, Gemmatimonadetes,and Planctomycetes and the class Deltaproteobacteria and Epsilonproteobacteria could not be identi fied after 30 days of incubation with crude oil. However, “professional hydrocarbonocastic bacteria” became abundant in samples treated with oil during the bioremediation period, while these clones were almost completely absent from the control plots. Interestingly, bioinformatics analysis showed that even when dramatic differences in oil biodegradation efficiency were observed, bacterial communities in the plots with nutrient amendments were not signi ficantly different from those in plots treated with oil alone. These findings indicated that nutrient amendments could stimulate the process of biodegradation but had less impact on bacterial communities. Overall, nutrient amendments might be able to stimulate the growth of n-alkane degrading bacteria.
Keyword: bioremediation; nutrient-enhanced; bacterial communities; n-alkane
1 INTRODUCTION
Oil spills are a major source of pollution that exert obvious negative impacts on various biota, including microorganisms, vegetation, fish and animals. The potential impacts of oil spills on biogeochemical processes, ecosystem services and the ultimate link to the health of humans have recently drawn increased attention (Mendelssohn et al., 2012). After the Deepwater Horizon oil spill in the Gulf of Mexico in 2010, several large-scale oil spills occurred in China,including the Penglai 19-3 oil spill in Bohai Bay in 2011, Binzhou oil spill in 2012, and Qingdao oil pipeline explosion in 2013. Signi ficant impacts on the environment were detected after these oil spills;accordingly, cost-effective and environmentally benign strategies are urgently needed to remediate spilled oil. Microorganisms, especially bacteria, play an important role in biodegradation of most organic pollutants (Head et al., 2006). The process of degrading hydrocarbon components is widely attributed to various microorganisms, and microorganismrelated bioremediation, such as phyto-microbial remediation, has been shown to be an effective method of removing residual oil from various environments (Atlas, 1995; Margesin, 2000; R?ling et al., 2004a, b; Zhao et al., 2011; Martin et al., 2013).
Bacteria that can degrade hydrocarbons affiliated with different phyla, including Flexibacter-Cytophaga-Bacteroides, Alpha-, Beta-, Gamma-Proteobacteria, Actinobacteria, Cyanobacteria and Firmicutes, have been cultured in the laboratory(Yakimov et al., 1998; Hennessee et al., 2009; Zhao et al., 2010; Szabó et al., 2011; Luo et al., 2012).However, laboratory cultured functional bacteria only play limited roles in the in situ bioremediation of crude oil. One of the most important reasons is that environmental factors, especially nutrients (nitric salts and phosphates), could be a signi ficant restricted parameter for the growth of functional bacteria, which vary dramatically between the in situ and laboratory environments (Wang et al., 2013).
Nitrogen and phosphorus, which are the two major limiting nutrients for all organisms on earth, are essential to synthesis of DNA, RNA, proteins,phospholipids and many other important biomolecules.In oil contaminated environments, nitrogen and phosphorus are generally insufficient to support the growth of in situ microorganisms. Indeed, considering the high carbon content of oil, the low levels of nitrogen and phosphorus are the factors limiting hydrocarbon degradation in oxic soil environments(Mohn and Stewart, 2000). Therefore, it is suggested that the addition of nitrogen and phosphorus to a contaminated environment could stimulate the growth of functional microorganisms, leading to an increase in the biodegradation rate of crude oil. McKew suggested that biostimulation with nutrients (N and P)produced a signi ficant increase (29%; P <0.05) in degradation of n-alkanes after 15 days, and an increase of one order of magnitude in the concentration of Thalassolituus (McKew et al., 2007). However,excessive nutrient concentrations can also inhibit the biostimulation progress. Several authors have reported the negative effects of high nutrient levels on the biodegradation of oil (Carmichael and Pfaender,1997; Oudot et al., 1998; Cha?neau et al., 2005). A better understanding of nutrient enhancement of crude oil biodegradation is essential to monitoring of bioremediation projects. In this experiment, changes in the microbial community structure during crude oil biodegradation with nutrient enhancement rather than a single strain were investigated. The results agreed with those of the actual restoration project and therefore have the potential for use as an improved reference relative to existing data.
A discarded oil wellhead located at the oilproducing city Binzhou in northern China blew out on November 15, 2012, resulting in thousands of square meters of farmland and a stream flowing through the area being badly contaminated. Additionally, more than 3 000 ducks on a farm near the wellhead were killed by the spilled oil. Accordingly, this contaminated environment was in urgent need of remediation. In this case, sediments from the contaminated stream were collected immediately after the oil spill, after which microcosms were constructed in the laboratory using the sampled sediments to investigate: 1, the capacity for bioremediation of crude oil by indigenous bacterial communities; 2, enhancement of biodegradation in response to nutrient addition; 3,changes in bacterial communities during crude oil bioremediation.
2 MATERIAL AND METHOD
2.1 Sediments and crude oil collection
Sediments for microcosm construction were collected from an oil contaminated stream in Binzhou,Shandong Province, China (37°24′293″N,118°06′767″E). The field studies did not involve endangered or protected species. Field work in this study was only conducted to sample oil contaminated soil and sediments; thus, no special permission was needed. In the field, sediments were kept in a cooler with insulation and ice packs, then transported to the laboratory within 24 hours for further analysis. The crude oil was obtained from Shengli Oil field,Dongying, China and stored at room temperature. The API (American Petroleum Institute) gravity of the crude oil was 25.6 and the viscosity was 4 896 mPa-s when measured by a rotary viscometer (Shanghai Changji Instrument Tech. Co. Ltd., China).
2.2 Experimental setup for nutrient-enhanced bioremediation of crude oil
Ten-gram sediments and aliquots (2% w/v) of crude oil were co-incubated in 100 mL mineral solution (4 mmol/L MgSO4, 0.5 mmol/L CaCl2,85 mmol/L NaCl) to conduct the oil bioremediation experiment. To evaluate the stimulation of bioremediation by nutrient amendments, an experimental setup designated as TON (treatments with both oil and nutrient) was conducted. Inorganic nutrients consisting of nitrate (as sodium nitrate,82 g/L) and phosphorus (as potassium dihydrogen phosphate, 6 g/L) were added at the beginning of the bioremediation process and at 10 days and 20 days. A parallel experiment, designated as TO (treatments with oil only), was conducted without the addition of nutrients. All treatments were performed in duplicate and maintained using 250 mL Erlenmeyer flasks in a 170 r/min shaker at 30°C for 30 days. Samples were vortexed for 10 min using a vortex mixer to ensure that the soil and solution phases were mixed well,after which 10 mL aliquots were collected from the homogenized mixture. Aliquots were collected on day 0, 10, 20, and 30, then stored at -80°C until further analysis of crude oil composition.
2.3 Analysis of crude oil composition
Residual crude oil was extracted three times with 10 mL of n-hexane and 30 min ultrasonic treatment.The extract was then combined and dried with anhydrous sodium sulfate (Bost et al., 2001). The volume of the extract was adjusted to 25 mL after air drying, after which the components of extracts were fractionated by column chromatography (Head et al.,2006) and analyzed using a Gas Chromatography-Mass Spectrometer (GC-MS, Agilent 7890-5975c)equipped with a HP-5 capillary column (Agilent Technologies, U.S.A., 60 m×0.25 mm×0.25 μm).High purity helium (99.999%) was applied as the carrier gas at a flow rate of 1 mL/min. The temperature program was as follows: 50°C–120°C at 20°C/min,120°C–250°C at 4°C/min, 250°C–310°C at 3°C/min,and then held at 310°C for 30 min. The transfer line and injector temperatures were both set at 300°C and the remaining hydrocarbon concentrations in the cultures were calculated according to the peak area.Anthracene (20 mg/L) was used as an internal standard to calibrate the GC-MS measurement. The recovery was 62.38%±3.68% and the RSD was 9.87%.
2.4 DNA extraction
Aliquots (5 mL) of samples collected at the end of the incubation period for both remediation experiments were centrifuged at 6 000× g (Thermo Scienti fic, Waltham, MA, USA) to collect microbial flora. A PowerSoil? DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA, USA) was then applied to extract the total genomic DNA from collected pellets and original contaminated sediments(OCS). We used two culture-independent methods,denaturing gradient gel electrophoresis (DGGE) and 16S rRNA gene based clone library analysis bacterial communities from samples before and after bioremediation (Mohamed et al., 2008; Wang et al.,2012).
2.5 DGGE analysis of bacterial communities
For DGGE analysis of the bacterial community, a variable region (~195-bp) corresponding to positions 341 and 534 in the 16S rRNA gene of Escherichia coli was PCR ampli fied from total genomic DNA using primers P2 and P3 (Mohamed et al., 2008;Wang et al., 2012). The PCR ampli fication was conducted in a 50-μL reaction system that included 37.8 μL of sterilized distilled water, 5 μL of 10× highfidelity PCR buffer, 2 μL of template DNA, 1 μL of 100 μmol/L of P2 and P3 primer, 1 μL of a mixture of dNTPs (2.5 mmol/L each), 0.2 μL of Platinum? Taq DNA (Invitrogen Life Technologies, Carlsbad, CA,USA) and 2 μL of MgSO4(50 mmol/L). PCR ampli fication was conducted as follows. A DCode system (BioRad, Hercules, CA, USA) was applied for DGGE analysis of the bacterial communities and a 5-min initial denaturing period at 97°C was followed by 29 cycles of 92°C for 30 s, 52°C for 2 min, and 72°C for 90 s, and then final extension at 72°C for 30 min. To separate different bacterial amplicons, 6%(w/v) polyacrylamide gel with a denaturing gradient of 40% to 70% in 1×Tris-acetate-EDTA was used.Electrophoresis was applied at 60 V and 60°C for 16 h. The gel was then stained with SYBR green I(diluted 1:10 000, Invitrogen) for twenty minutes and visualized with a Molecular Imager? Gel Doc?XR+ System (BioRad, Hercules, CA, USA). Scanned negative DGGE gels were analyzed using Quantity One 4.6 (BioRad) for statistical analysis of DGGE tracks. Similarities between samples were calculated using the Dice coefficient (band based).
2.6 Cloning and phylogenetic analysis of 16S rRNA gene

Fig.1 Percentage of remaining n-alkanes during the microcosm experiment after 30 days of incubation
16S rRNA gene based clone libraries were constructed to investigate bacterial community succession. Brie fly, the bacterial universal primers 27F and 1492R were used to amplify the 16S rRNA gene fragments of total genomic DNA extracted from the bacterial communities as previously described(Enticknap et al., 2006). PCR products were then ligated into the PCR-XL-TOPO vector, after which the TOPO XL PCR cloning kit (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to transform the PCR-XL-TOPO vector into One Shot TOP 10 chemically competent Escherichia coli cells.Colonies were then randomly selected from selective plates (Luria-Bertani agar plates with 50 μg/mL kanamycin), after which they were incubated in 2×YT medium (NaCl 5 g/L, tryptone 16 g/L, yeast extract 10 g/L, with 50 μg/mL kanamycin) overnight. Plasmid DNA was sequenced using primer 27F.
Chimeric sequences from clone libraries were identi fied using the CHECK_CHIMERA program of the Ribosomal Database Project. Sequences that were<400 bp in length were removed from the file using the MEGA 5.0 software package (Tamura et al.,2011). We then used the BLASTn tool at the National Center for Biotechnology Information website to aid in selection of the closest reference sequences, after which phylogenetic analysis of the 16S rRNA gene sequences from clone libraries was conducted.Different phylotypes were classi fied using the Mothur software (Schloss et al., 2009).
2.7 Nucleotide sequence accession numbers
The 16S rRNA gene sequences from clones newly determined in this study have been deposited in GenBank under accession numbers BankIt1966583:(330).
3 RESULT AND DISCUSSION
3.1 Bioremediation of crude oil
The GC-MS analysis suggested that n-alkanes, a major component in crude oil, could be signi ficantly degraded with/without nutrient amendments after incubation for 30 days. Depletion of n-alkanes with chain lengths of C 16 to C 30 was evaluated following treatment with oil (TO) or oil and nutrient (TON). The depletion was characterized by comparison of percentages of remaining n-alkanes with levels in the original contaminated sediments (OCS). Chemical analysis demonstrated signi ficant differences between TO treatments and TON treatments after 30 days of incubation. As shown in Fig.1, when crude oil was added to the OCS as the stress factor (TO treatments),the depletion of n-alkanes was chain-length dependent.Shorter n-alkanes (C 16 to C 23) showed a relatively higher depletion rate, with homogeneous values ranging from 57.16% to 60.52%. For longer n-alkanes(C 24 to C 30), percentages of remaining n-alkanes increased from 46.60% for C 24 to 99.73% for C 30. In contrast, dramatic degradation rates were observed in TON treatments. However, only C 16–C 30 were detected in our experiment. This may have occurred because concentrations of other n-alkanes were below the detection limit. All ingredients of measured nalkanes were reduced to less than 5% of their initial value.
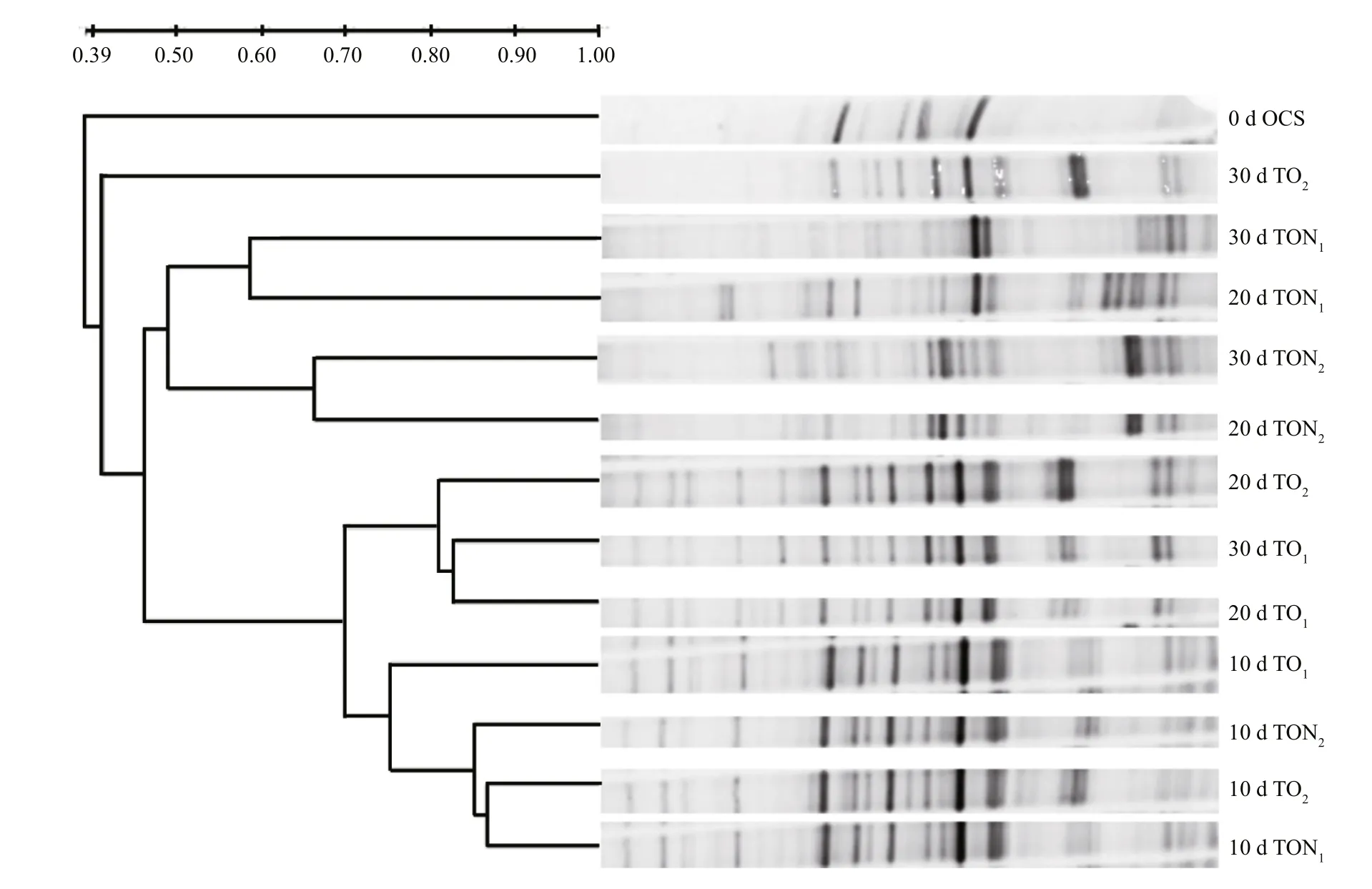
Fig.2 DGGE analysis of bacterial community dynamics during n-alkane bioremediation in microcosm treatments
The GC-MS analysis conducted in the present study indicated that nutrient addition dramatically stimulated the biodegradation process. Trials in which nutrients were added to various oil-contaminated environments have con firmed that nutrients, mainly nitrogen and phosphorus, are key factors in fluencing biodegradation of crude oil. Studies were performed successively to investigate the effects of oil degradation by addition of different values of nutrients in crude oil contaminated intertidal zone of Stert Flats, Somerset, UK (R?ling et al., 2002; R?ling et al., 2004a, b). All three studies demonstrated that adequate levels of nutrient addition could enhance the biodegradation of crude oil, while higher levels did not show further enhancement. After conducting a nutrient amendment in soil microcosms, Cha?neau et al. (2005) suggested that the C/N/P ratio should be considered when designing the bioremediation of crude oil polluted soils. In this study, the C/N/P ratio(C:N:P=100:10:1) was calculated based on the concentration of crude oil in collected sediments.With the addition of nutrients, n-alkane was fully degraded, while an obviously lower degrading rate was observed when nutrients were not supplemented.The other phenomenon observed in this study was that shorter-chain-length n-alkanes showed higher degrading rates than longer-chain-length ones,especially in the treatments without added nutrients.These findings were consistent with the finding that small hydrocarbon molecules were more readily biodegraded than longer ones (Harayama et al., 1999;Sei et al., 2003).
3.2 Changes in bacterial communities associated with oil degradation
Monitoring the dynamics of microbial communities could facilitate identi fication of functional bacteria responsible for the biodegradation of crude oil, which in turn would be bene ficial to development of new strategies for bioremediation of crude oil contamination by these bacteria (Wang et al., 2013).In this study, 16S rRNA gene based DGGE and clone library analysis were used to investigate succession of bacterial communities under the stress of oil contamination, and functional bacterial communities relevant to oil degradation were predicted.
The DGGE pro file analyzed from the different microcosms revealed a signi ficant difference between oil-amended sediments with or without nutrients and the bacterial community of the natural sediment(Fig.2). DGGE patterns from all microcosms contaminated by crude oil for a period of ten days showed high similarity to each other and unambiguous changes compared to the natural sediment. The development of clearly different microbial communities was detected after ten days of domestication. All TO samples, except TO2at 30 d,were grouped into a distinct cluster with 70%similarity. The four TON samples were found to be combined into one group, but signi ficant differences were detected between individual samples. TON1at 20 d and TON1at 30 d showed 60% similarity, while the value for TON220 d and TON2at 30 d was 65%.The lower similarity between TON1and TON2was observed with a value of 50%.
3.3 Phylogenetic analysis and diversity of bacterial communities
Bacterial communities in the original contaminated sediment, treatments amended with crude oil, and treatments amended with crude oil and nutrients were analyzed using a 16S rRNA gene based clone library,and six clone libraries were generated based on analysis of the DGGE pro files. Two clone libraries were constructed for the original contaminated sediment (designated as OCS1and OCS2) used as a control to investigate bacterial communities in the original contaminated sediment. The other four clone libraries were constructed for treatments at 30 d,including two for samples amended with crude oil(designated as TO32and TO34), and two for samples amended with both crude oil and nutrients (designated as TON31and TON33).
Phylogenetic analysis was applied to a total of 330 sequences from the six clone libraries (55, 56, 61, 59,48, and 51 sequences for OCS1, OCS2, TO32, TO34,TON31and TON33, respectively). This generated 117 phylotypes affiliated with the following phyla: Acidobacteria (13), Armatimonadetes (1), Bacteroidetes(9), Cyanobacteria (1), Firmicutes (2), Gemmatimonadetes (1), Nitrospirae (3), Planctomycetes (2),Proteobacteria (72), Verrucomicrobia (9) and unclassi fied bacterial group (4). Few changes were detected for bacteria affiliated with Acidobacteria,Bacteroidetes, and Verrucomicrobia. Diversity of bacterial communities decreased dramatically after 30 days of co-incubation with crude oil and nutrients(Fig.3). Cyanobacteria, Fusobacteria, Gemmatimonadetes, and epsilon-Proteobacteria were detected in OCS clone libraries, but not in clone libraries for TO and TON samples, which were treated with oil.Members of the Proteobacteria, especially gamma-Proteobacteria, dominated the bacterial group in all six clone libraries. Different classes in Proteobacteria showed various trends after being treated with oil and nutrients. Proportions of bacterial colonies affiliated with alpha-Proteobacteria increased in all TO and TON treatments compared to OCS clone libraries,while increases in delta- and beta-Proteobacteria were only found when nutrients and oil were amended together. The ratio of colonies in beta-Proteobacteria accounted for up to 19.47%±2.35% of the total population in the TON treatments, compared to 4.38%±0.96% and 5.73%±1.53% in the OCS and TO treatments, respectively. Bacterial colonies affiliated with gamma-Proteobacteria decreased dramatically in the two clone libraries for TON treatments compared to OCS and TO treatments. For instance,60.64%±1.96% and 58.65%±4.70% of recovered colonies were affiliated with gamma-Proteobacteria in the clone libraries constructed for samples OCS and TO, while 45.86%±2.87% of the total colonies were grouped into gamma-Proteobacteria in the two TON clone libraries.
The 12 most dominant phylotypes recovered from all six clone libraries were selected to analyze the succession of bacterial communities (Table 1). These phylotypes possessed 328 colonies, which accounted for 68.62% of colonies from all six clone libraries.The results indicated that degradation related bacteria were selected with the addition of crude oil, while bacterial communities with other functions were eliminated after 30 days of incubation. As shown in Table 1, colonies affiliated with the unclassi fied genus in the family of Comamonadaceae in beta-Proteobacteria, unclassi fied genus in the class PYR10d3 in gamma-Proteobacteria, the genus of Hydrocarboniphaga in gamma-Proteobacteria were only detected or showed dramatic increases in clone libraries for samples treated with oil (TO and TON).For example, the phylotypes grouped into the genus Hydrocarboniphaga were not recovered from the original oil samples (OCS), while they reached 3.04%±1.01% and 13.19%±0.46% after treatment with oil and oil/nutrients, respectively. Two cultured species in this genus, H. effuse (Palleroni et al., 2004)and H. daqingensis (Liu et al., 2011), were reportedly capable of degrading n-alkanes. Similar results were also reported for the phylotype affiliated with the family Comamonadaceae, which contained functional genera capable of degrading n-alkanes and cyclic alkanes (Brzostowicz et al., 2005; Mattes et al., 2008).Bacterial colonies were affiliated with the class PYR10d3, an uncultured bacterial class, also showed the aforementioned trend. By applying DNA-based stable-isotope probing (SIP) and quanti fication by real-time quantitative PCR analysis, Singleton et al.(2006) demonstrated that PYR10d3 was involved in the process of degrading pyrene. These findings indicated that functional bacteria capable of degrading PAHs also existed in the contaminated environment.Unfortunately, no dramatic biodegradation of PAHs was detected.

Fig.3 Successions of bacterial communities (phylum level) in the process of n-alkanes degradation based on 16S rRNA gene clone library analysis
In contrast, colonies affiliated with the genera Sulfuricurvum, Marinobacter, and Pseudomonas showed obvious decreasing trends after being treated with oil or oil and nutrients. For instance,10.65%±2.84% of the total recovered colonies were affiliated with the genus Sulfuricurvum in the clone libraries constructed for OCS samples, while no colonies were grouped into this phylotype in the four clone libraries for TO and TON samples. The newly proposed genus Sulfuricurvum was acknowledged to be responsible for oxidation of reduced sulfur compounds (Kodama and Watanabe, 2004; Haaijer et al., 2008). Even the type strain Sulfuricurvum kujiense YK-1T was isolated from an underground crude-oil storage cavity, while there was no evidence that the genus could degrade n-alkane. Similarly,45.66%±3.46% of the recovered colonies were grouped into the genus Pseudomonas before being treated with oil, but the ratio dropped to 22.88%±0.53%and 6.27%±1.23% after being treated with oil and oil/nutrient, respectively.
The results of this study indicated that the addition of nutrients could greatly stimulate changes in bacterial communities. As shown in Table 1, TON treatments resulted in more abundant colonies being affiliated into the genus Hydrocarboniphaga and the unclassi fied genus family Comamonadaceae when compared to TO treatments. Additionally,13.19%±0.46% of the recovered colonies belonged to the genus Hydrocarboniphaga for the TON treatments,while the ratio was only 3.04%±1.01% for the TO treatments. The two ratios for colonies affiliated intothe unclassi fied genus in the family Comamonadaceae were 17.43%±3.63% and 5.45%±1.13%, respectively.In contrast, TON treatments possessed less abundant bacterial colonies affiliated with the genus Pseudomonas. The aim of bioremediation is to stimulate pollutant-degrading functional microorganisms to speed the recovery of contaminated ecosystems (R?ling et al., 2002). In the present study,bacteria grouped into phylotypes Hydrocarboniphaga and Comamonadaceae were promoted along with notable biodegradation of n-alkanes. Nutrient amendments could be considered a factor in fluencing the succession of bacterial communities as changes in bacterial communities were more closely related to the stress of crude oil and the degrading process.These findings are consistent with the conclusions raised by R?ling et al. (2002), who found remarkable changes in bacterial communities, even without nutrients being amended. Alcanivorax/ Fundibacterlike sequences, which were closely-related to oildegradation, were found to be dominant in the corresponding clone library. The role that nutrientamendments played could be interpreted as stimulating the growth of functional bacteria that were responsible for oil-degradation.

Table 1 The 12 most dominant bacterial phylotypes recovered from all six clone libraries
4 CONCLUSION
Microcosm experiments in this study demonstrated that bioremediation of crude oil could be enhanced by amendment with inorganic nutrients. Additionally,nutrient amendments showed a substantial impact on succession of bacterial communities. Efficient bioremediation was closely related to dramatic changes in bacterial communities when nutrients were supplied. n-alkanes degrading bacteria became more abundant during the biodegradation process.This study provides a deep understanding of nutrient enhancement of bioremediation of n-alkanes and bacterial communities associated with the process that could be helpful for assisting recovery of oilcontaminated environments.
5 ACKNOWLEDGEMENT
We acknowledge critical review of the manuscript by Dr. Roy Bonnette from Southeastern Louisiana University.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin?) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
