Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage
FENG Yun-xiao , CHENG Yu-dou , HE Jin-gang , LI Li-mei , GUAN Jun-feng
1 Institute of Genetics and Physiology, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang 050051, P.R.China
2 Plant Genetic Engineering Center of Hebei Province, Shijiazhuang 050051, P.R.China
1. Introduction
The Yali pear (Pyrus bretschneideri Rehd. cv. Yali) is a famous Chinese pear variety with delicious and juicy flesh. However, it is predisposed to browning scald in the peel during prolonged cold storage. This phenomenon is commonly known as superficial scald. Pathogenesis of superficial scald in pears is similar to that in apples. Studies have shown that superficial scald is closely associated with the accumulation of α-farnesene and conjugated trienes(CTols) in the peel as well as the reduction of antioxidative enzyme activity and an increase of reactive oxygen in fruits(Ahn et al. 2001; Rupasinghe et al. 2001; Whitaker 2007;Sabban-Amin et al. 2011; Bordonaba et al. 2013; Gao et al.2015). Many postharvest methods have been established to inhibit the development of superficial scald; however,the mechanism through which it occurs is not completely understood (Lurie and Watkins 2012).
In recent years, increasing applications of 1-methycyclopropene (1-MCP) in the preservation of freshness for harvested fruits have been reported. Studies have shown that 1-MCP effectively delays fruit senescence and inhibits α-farnesene biosynthesis and the subsequent accumulation of its oxidation products (CTols), reducing the incidence of superficial scald in pears (Ekman et al.2004; Isidoro and Almeida 2006; Xie et al. 2014). Modified atmosphere packaging (MAP) can keep fruit fresh, suppress respiration and delay the aging process (Li et al. 2013;Wang and Sugar 2013; Cheng et al. 2015). In addition,the combination of 1-MCP and MAP treatment has been shown to be more effective in the preservation of some fruits compared to either treatment alone (Reuck et al. 2009;Sabir and Agar 2011; Li et al. 2013). However, to date, no report has shown the effects of combined MAP and 1-MCP treatment on the storage quality of Yali pears. The objective of this study was to investigate the effects of 1-MCP, MAP,and their combined treatment (MAP+1-MCP) on fruit quality as well as superficial scald during cold storage and at shelf life, especially with respect to changes in superficial scaldrelated physiological and biochemical indices in Yali pears.
2. Materials and methods
2.1. Materials
Yali pears (Pyrus bretschneideri Rehd. cv. Yali) were harvested at 163 days after full bloom from an orchard in Zhao County, Hebei Province, China, and delivered to the laboratory within 2 h. Pears (average single-fruit weight:(280.49±8.06) g; soluble solids content: (12.47±0.80)%) of similar size and no signs of pest damage or disease were selected and evenly divided into two groups. The first group was sealed with 1.0 μL L–11-MCP (SmartFreshTMAgro Fresh Inc., Dow Agro Sciences, Philadelphia, PA,USA) for 24 h at room temperature ((20±2)°C). The second group was placed in sealed containers with air at the same temperature for 24 h. Afterwards, each of the two groups was further divided into two subgroups. The first subgroup was directly put into an ordinary paper box, and the second subgroup was first packed with microporous plastic film (with O2and CO2permeabilities of 10 955 and 75 200 mL m–2d 24 h–10.1 Mpa–1at 20°C, respectively, made by the National Engineering Technology Research Center for Preservation of Agricultural Products, Tianjin, China) and subsequently placed into a paper box. The treated subgroups were labeled control (no 1-MCP treatment or packaging), 1-MCP(1-MCP treatment alone), MAP (microporous plastic film packaging alone), and MAP+1-MCP (MAP combined with 1-MCP treatment). Each box contained 30 pears and was stored at 0°C. All fruits were removed after 210 days of cold storage, and packaging bags were unwrapped for MAP treatments and then stored at (20±2)°C for seven days over the shelf life period. The fruit quality indices were tested on days 0, 60, 120, 180, and 210 during cold storage and at shelf life. The peel samples were immediately frozen in liquid nitrogen and subsequently stored at –80°C for further experiments. Three replicates of 10 fruit each were measured for each treatment at every sampling time.
2.2. Analysis the O2, CO2 and ethylene contents inside the packaging films
The O2and CO2contents inside the packaging films were measured with a handheld O2/CO2analyzer (Model:OXYBABY M+O2/CO2, WITT-Gasetechnik GmbH & Co KG,Witten, Germany). For ethylene measurement, 1 mL of gas inside the packaging film was withdrawn with a gas-tight syringe, which was then injected into a gas chromatograph(Model: GC9790II, Fuli Instruments Technology Co., Ltd.,Wenling, China) equipped with a GDX-502 column and a flame ionization detector (FID). The O2and CO2contents were expressed as %, while the ethylene content was expressed as μL L–1. Three replicates were measured for each treatment.
2.3. Fruit quality evaluation
Fruit firmness was determined using a digital fruit hardness meter (Model: GY-4, Top Instruments Co., Ltd., Hangzhou,China). The soluble solids content (SSC) was determined with a PAL-1 pocket digital refractometer (Atago Co., Ltd.,Tokyo, Japan).
2.4. Determination of the superficial scald index and the contents of α-farnesene and CTols
The severity of superficial scald was evaluated according to an index based on the percentage of the fruit surface area affected: 0, 1, 2, and 3 suggested no superficial scald and superficial scald covering <25%, 25–50%, and >50%,respectively (Zanella 2003), with the superficial scald index calculated using the following formula:
Superficial scald Index=∑(Scald level×Number of fruits at the level)/(3×Total number of fruits)
The α-farnesene and CTols contents were analyzed by the hexane-extraction method (Xie et al. 2014; Gao et al.2015). Four discs (1 cm in diameter) of peel tissue were removed from the equatorial portion of each pear and were placed in tubes containing 20 mL of hexane for 2 h.After incubation, 2 mL of the extract was filtered through a Florisil column and eluted using 3 mL of hexane. The absorbances at 232, 281 and 290 nm were recorded using a spectrophotometer (Model: UV-2100, UNICO Instrument,Dayton, USA). The α-farnesene and conjugated trienols were calculated using the molar extinction coefficients ε232=27 740 for α-farnesene and ε281–290=25 000 for the conjugated trienols, expressed as nmol cm–2.
2.5. Determination of the relative conductivity, production rate and the malondialdehyde (MDA) and phenolic compound contents
To assess relative conductivity, fifteen peel discs (1 cm in diameter) were immersed in 20 mL of mannitol (100 mmol L–1) and gently shaken at 20°C for 2 h (Zhao et al.2009). The conductivity of the solution (L1) was measured with a conductivity meter (Model: DDS-307, INESA Auto Electronics System Co., Ltd., Shanghai, China). Solutions were boiled for 10 min and then cooled to 20°C, and the conductivity of dead tissues (L2) was measured. Relative conductivity was calculated as the ratio of L1 to L2.
To measure the rate of· production, 0.5 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended in 5 mL of 50 mmol L–1phosphate buffer (pH 7.8), containing 1 mmol L–1EDTA, 0.3% (v/v) Triton X-100,and 2% (w/v) polyvinylpyrrolidone, and then the extract was centrifuged at 12 000×g for 20 min at 4°C. After centrifuging,1 mL of the supernatant was blended thoroughly with 1 mL of 50 mmol L–1phosphate buffer (pH 7.8) and 1 mL of 1 mol L–1hydroxylamine hydrochloride and then was incubated at 4°C for 1 h. After incubating, the extract was added to 1 mL of 17 mmol L–1p-aminobenzene sulfonic acid and 1 mL of 7 mmol L–1naphthylamine, after which it was incubated at 25°C for 20 min (Wang and Luo 1990). The absorbance at 530 nm was recorded using a spectrophotometer (Model:UV-2100, UNICO Instrument, Dayton, USA). A standard curve for KNO was used to quantify the· content, and
2the production ratio· was represented as μmol min–1g–1frozen weight (FW).
To measure the MDA content, 2 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended with 10 mL of 10% (w/v) trichloroacetic acid, which was then centrifuged at 12 000×g for 15 min at 4°C. After centrifuging, 2 mL of the supernatant was added to 3 mL of 10% (w/v) trichloroacetic acid aqueous containing 0.67%(w/v) thiobarbituric acid. The mixture was boiled at 100°C for 15 min. After rapid cooling, the mixture was centrifuged at 5 000×g for 10 min at 4°C. The absorbance of supernatant was measured at 450, 532, and 600 nm. The MDA content was calculated as 6.45×(OD532–OD600)–0.56×OD450(Xu et al.2012) and was represented as μmol g–1FW.
To measure the total phenolic content, 0.5 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended in 5 mL 70% ethanol (v/v) and extracted with an ultrasonic instrument at 40°C for 1 h. Next, the mixture was centrifuged at 10 000×g for 15 min at 4°C, and 0.5 mL of the supernatant was withdrawn and diluted eight fold.Afterwards, 0.1% (w/v) Fast Blue RR salt (4-benzoylamino-2,5-dimethoxybenzenediazonium chloride hemi-[zinc chloride] salt) and 5% (w/v) NaOH were added to the diluted supernatant (4 mL) at a ratio of 1:10, after which the mixture was incubated at 25°C for 90 min. The absorbance of reaction was determined at 420 nm (Lester et al. 2012).A standard curve for gallic acid was used to quantify the total phenolic content, and the results were expressed as mg g–1FW.
2.6. Measurement of enzyme activity
To extract and determine the activity of superoxide dismutase(SOD, EC 1.15.1.1) and catalase (CAT, EC 1.11.1.6), 2 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended in 5 mL of extract solution containing 100 mmol L–1phosphate buffer (pH 7.8), 2 mmol L–1DTT,5% (w/v) polyvinylpolypyrrolidone (PVPP), 0.1 mmol L–1EDTA and 1.25 mmol L–1polyethylene glycol, after which the extraction was centrifuged at 12 000×g for 30 min at 4°C. The supernatant was collected as the crude enzyme extract to measure SOD and CAT activity (Gao et al. 2015).For the SOD activity assay, a reaction mixture containing 1.5 mL of 50 mmol L–1phosphate buffer (pH 7.0), 0.3 mL of 130 μmol L–1L-methionine, 0.3 mL of 750 μmol L–1nitroblue tetrazolium (NBT), 0.3 mL of 100 μmol L–1EDTA-Na2, 0.3 mL of 20 μmol L–1riboflavin, 200 μL of deionized H2O,and 100 μL of the crude enzyme extract was initiated by illumination with 60 mol m–2s–1white fluorescent light at 25°C. After 8 min, the reaction was stopped by removing the light source. A control tube with the reaction mixture and enzyme was maintained in the dark, while another tube without the enzyme was maintained in light conditions to serve as the control for the reduction of NBT by light.The absorbance of the reaction was read at 560 nm. One unit of SOD activity was defined as the amount of enzyme that inhibited the photoreduction of NBT by 50% under the assay conditions and was represented as U g–1FW. For the CAT activity assay, a 3-mL reaction mixture containing 50 mmol L–1phosphate buffer (pH 7.0) and 100 μL of the crude enzyme extract was initiated by the addition of 100 μL of 100 mmol L–1H2O2. The decomposition was directly assessed by observing the decrease in absorbance at 240 nm every 30 s for 2 min at 25°C. The CAT activity was defined as the amount of enzyme that reduced the absorbance and was represented as ΔOD240min–1g–1FW.
To extract and measure lipoxygenase (LOX, EC 1.13.11.12) activity, 1 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended in 10 mL of 100 mmol L–1phosphate buffer (pH 6.8) containing 1.5% polyvinylpyrrolidone (PVP). Next, the mixture was centrifuged at 12 000×g for 20 min at 4°C, after which the supernatant was collected as the crude enzyme extract.The 3 mL reaction mixture contained 25 μL of 10 mmol L–1sodium linoleic and 2.775 mL of 100 mmol L–1sodium acetate buffer (pH 4.4) and was initiated by the addition of 200 μL of the crude enzyme extract. The decomposition was directly assessed by observing the increase in absorbance at 234 nm for 2 min at 25°C (Axelrod et al. 1981). The LOX activity was defined as the amount of enzyme that raised the absorbance in 1 min and was expressed as ΔOD234min–1g–1FW.
For polyphenol oxidase (PPO, EC 1.14.18.1) extraction and activity measurements, 0.5 g of frozen peel tissue was powdered in liquid nitrogen with a mortar and blended in 5 mL of 100 mmol L–1phosphate buffer (pH 7.0) containing 0.3 g PVP. Next, the solution was centrifuged at 20 000×g for 15 min at 4°C, after which the supernatant was collected as the crude enzyme extract. The reaction mixture contained 100 mmol L–1catechol in 50 mmol L–1phosphate buffer (pH 6.0) (Cheng et al. 2015). Changes in the absorbance at 420 nm were measured by using a spectrophotometer(Model: UV-2100, UNICO Instrument, Dayton, USA). The PPO activity was expressed as ΔOD420min–1g–1FW.
2.7. Statistical analysis
The results were statistically analyzed using SPSS 18(SPSS Inc., Chicago, IL, USA). Least significant differences(LSD) at a significance level of 0.05 were generated by ANOVA.
3. Results
3.1. Change in gas composition within the fruit packaging
The gas composition within the packaging bags varied dynamically during cold storage. There was no significant difference in the oxygen content inside the packaging bags between the MAP and MAP+1-MCP treatments except for day 60 (Fig. 1-A). However, compared with MAP alone, the carbon dioxide content at days 60 and 120, and the ethylene content during whole storage period for the MAP+1-MCP treatment were lower (Fig. 1-B and C). This observation suggested that 1-MCP reduced the contents of carbon dioxide and ethylene within the packaging bag and more significantly inhibited the accumulation of ethylene.
3.2. Change in fruit quality
The firmness declined gradually during storage, which was significantly higher in different treatments than that in control at later storage time (Fig. 2-A). The SSC in the MAP treatment was lower than in the control after day 60, while it had no significant difference between the 1-MCP treatment and the control. Moreover, the SSC in the MAP+1-MCP treatment was lower than in the control on day 210 and at shelf life (Fig. 2-B).
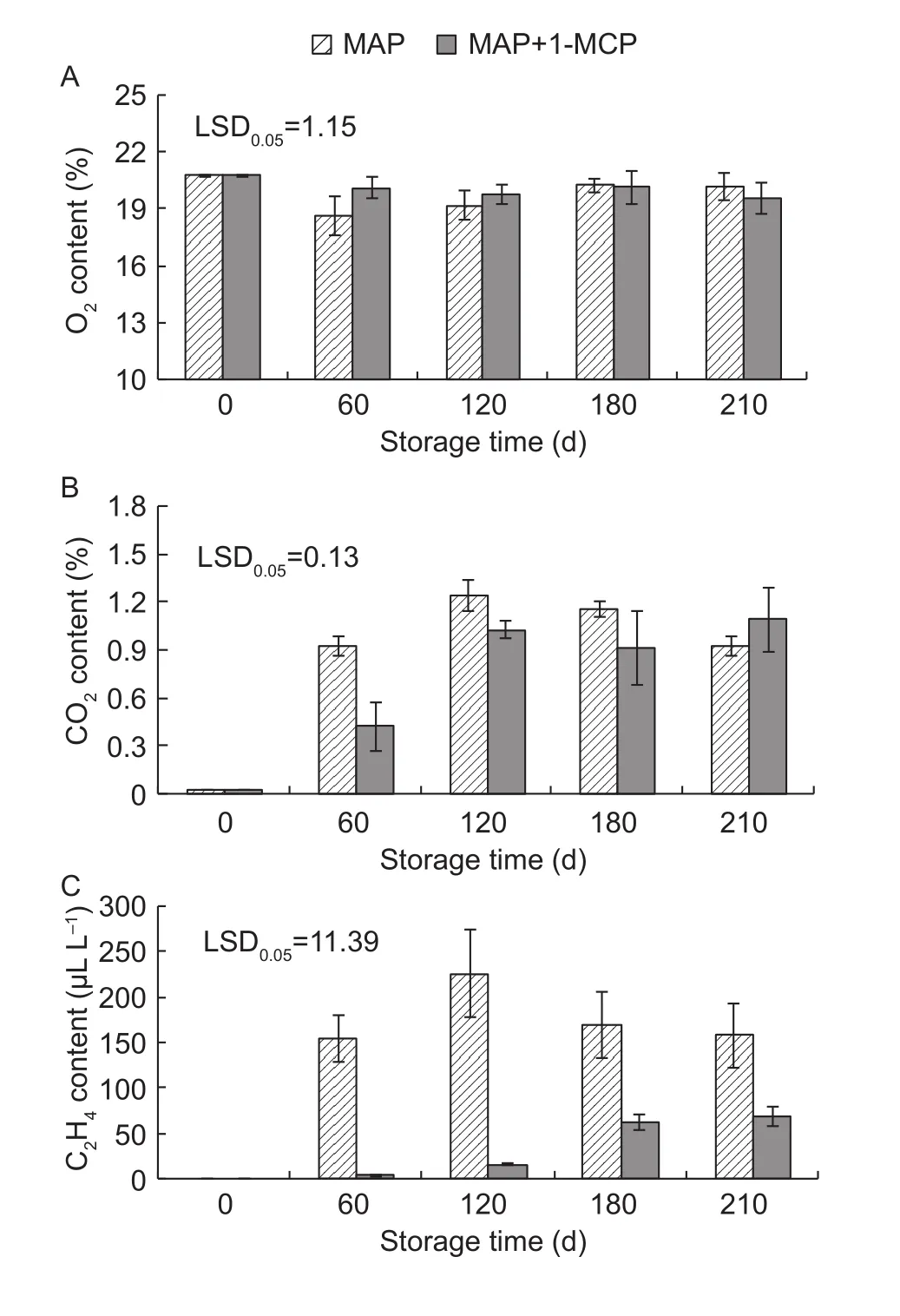
Fig. 1 Oxygen (A), carbon dioxide (B), and ethylene (C) contents inside the packaging films. 1-MCP, 1-methylcyclopropene;MAP, modified atmosphere packaging. LSD, least significant differences. Error bars represent ±SE from three replicates.
3.3. Changes of the superficial scald index, the contents of α-farnesene and CTols in peels
Superficial scald was first observed at day 210 of cold storage and was more obvious over the shelf life period in Yali pear. Compared with the control, 1-MCP, MAP and MAP+1-MCP significantly reduced the superficial scald index, while the MAP+1-MCP treatment showed the lowest index (Fig. 3-A). The α-farnesene content first increased to maximum value at day 180 and subsequently decreased,and the values were significantly lowered by the 1-MCP and MAP+1-MCP treatments (Fig. 3-B). The CTols content increased to maximum value at day 210 during cold storage and decreased over the shelf life period. Compared with the control, different treatments significantly reduced the CTols content of the peel, with the MAP+1-MCP treatment showing the lowest CTols content (Fig. 3-C).
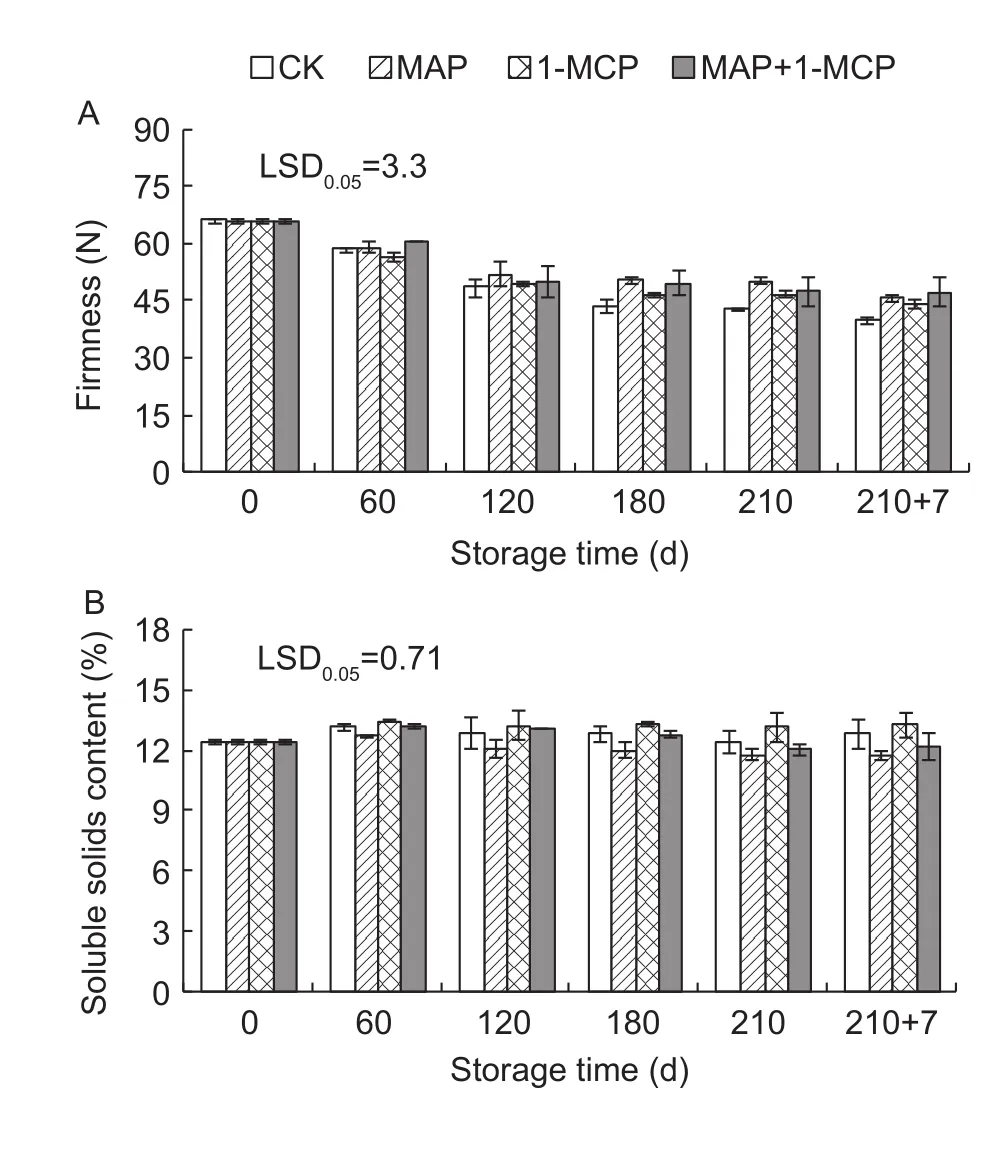
Fig. 2 Changes in fruit firmness (A) and soluble solids content (SSC, B) in Yali pears during storage. 1-MCP,1-methylcyclopropene; MAP, modified atmosphere packaging.210+7 indicates 7 days at shelf life after 210 days of cold storage. Error bars represent ±SE from three replicates.
3.4. Changes in the rate of · production and the activities of CAT and SOD in peels
The rate of· production in the peel was increased with prolonged storage, which was significantly lower in the MAP, 1-MCP, and MAP+1-MCP treatments after day 180 compared with the control (Fig. 4-A). The CAT activity first declined and subsequently increased in all treatments,showing significantly higher CAT activities in the 1-MCP and MAP+1-MCP treatments than in control after day 120(Fig. 4-B). Compared with control, the MAP, 1-MCP and MAP+1-MCP treatments had significantly higher SOD activities after day 180 (Fig. 4-C).
3.5. Changes in LOX activity, MDA content, and relative conductivity of the peels
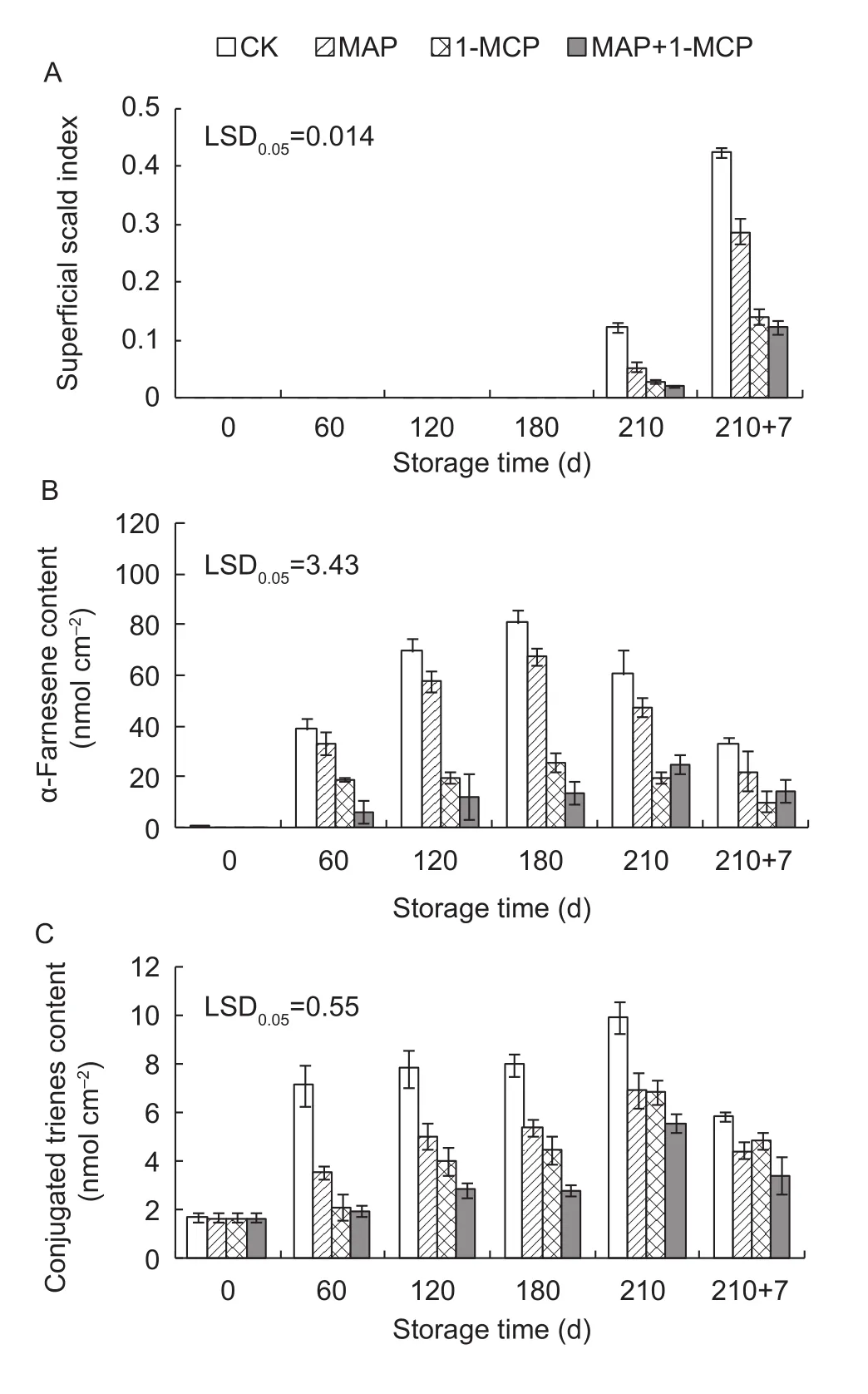
Fig. 3 Changes in the superficial scald index (A), α-farnesene(B) and conjugated trienes (CTols, C) contents of Yali pear peels during storage. 210+7 indicates 7 days at shelf life after 210 days of cold storage. 1-MCP, 1-methylcyclopropene;MAP, modified atmosphere packaging. LSD, least significant differences. Error bars represent ±SE from three replicates.
In all treatments, the LOX activity first increased, reaching maximal activity at day 180, and then subsequently declining, showing significantly lower in the 1-MCP and MAP+1-MCP treatments than that in control after 180 days of cold storage, while no significant difference was observed between the MAP and control (Fig. 5-A). The MDA content increased during storage. Compared with the control, all treatments significantly inhibited the increase in MDA content in the peel after 120 days of cold storage, and the MAP+1-MCP treatment had the lowest MDA content at shelf life (Fig. 5-B). The relative conductivity also increased during storage and was significantly lower in the 1-MCP,MAP and MAP+1-MCP treatments than that in control after day 210 (Fig. 5-C).
3.6. Changes of total phenolic content and PPO activity of the peels
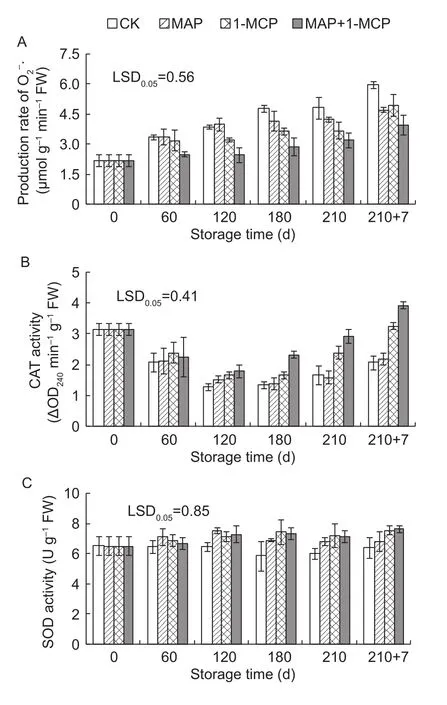
Fig. 4 Changes in the production rate of (A), activities of catalase (CAT, B) and superoxide dismutase (SOD, C) in pear peels during storage. 210+7 indicates 7 days at shelf life after 210 days of cold storage. 1-MCP, 1-methylcyclopropene;MAP, modified atmosphere packaging. LSD, least significant differences. Error bars represent ±SE from three replicates.
Total phenolic content decreased during storage, which was significantly higher in the MAP+1-MCP treatment than that in the control after 120 days of cold storage. However,no significant difference was observed between the MAP treatment and the control (Fig.6-A). The change in the PPO activity was rather low during cold storage but significantly increased at the shelf life. Compared with the control, the 1-MCP and MAP+1-MCP treatments exhibited significantly lower PPO activities on day 180 of cold storage and at the shelf life, but MAP had no significant effect on PPO activity(Fig. 6-B).
3.7. Correlation between the superficial scald index and physiological and biochemical indices of the peels of Yali pears
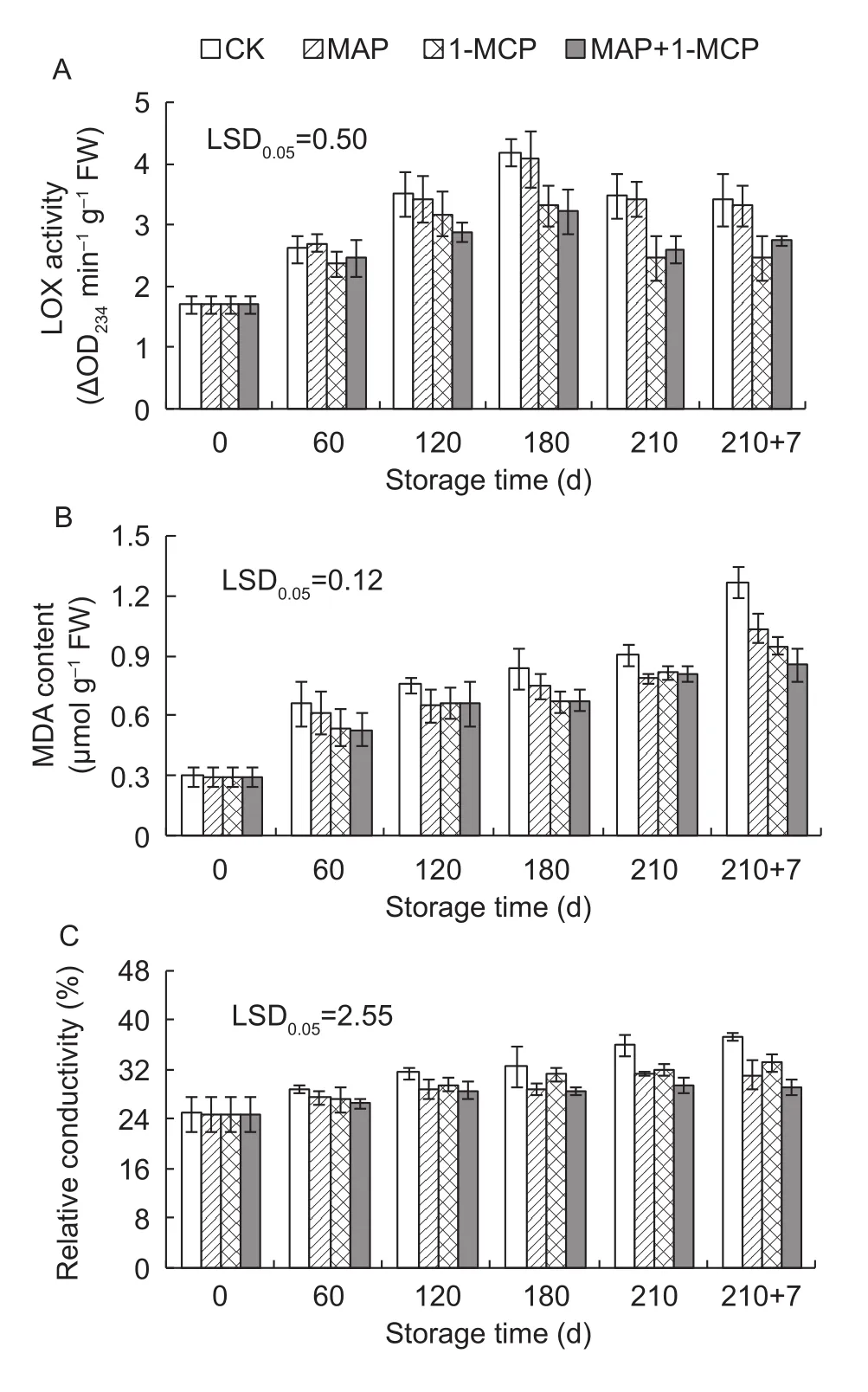
Fig. 5 Changes in lipoxygenase (LOX) activity (A), malondialdehyde (MDA) content (B), and relative conductivity(C) in Yali pear peels during storage. 210+7 indicates 7 days at shelf life after 210 days of cold storage. 1-MCP,1-methylcyclopropene; MAP, modified atmosphere packaging.LSD, least significant differences. Error bars represent ±SE from three replicates.
Correlation analysis of different indices was conducted during fruit storage. By linear stepwise regression, we concluded that the 5 indices (PPO activity, CAT activity,relative conductivity, α-farnesene content, and MDA content)are closely related to the superficial scald index, and the scald index can be well predicted through the following regression equation:
Scald index=–0.360+0.749×PPO activity+0.228×CAT activity+0.190×Relative conductivity–0.148×α-farnesene content+0.161×MDA content, r=0.9607 (P<0.01)
Furthermore, path analysis showed that the direct path coefficient of PPO to the scald index was the largest (0.749).Thus, PPO was an important direct determinant, and the direct path coefficient of the α-farnesene content to the scald index was negative (–0.148), showing that the substance has a slightly negative correlation with the scald index. The indirect path coefficient showed that PPO activity, MDA content and relative conductivity were important indices for influencing the scald index (Table 1). This suggested that the PPO activity, MDA content, and cell membrane permeability were closely correlated with the superficial scald index for Yali pears.
4. Discussion
In this study, the MAP and MAP+1-MCP treatments maintained higher firmness (Fig. 2-A), which was similar to a previous study of the Laiyang pear (Li et al. 2013). However,the MAP, 1-MCP, and MAP+1-MCP treatments had a relatively lower effect on the SSC in Yali pear (Fig. 2-B),and the 1-MCP treatment had inconsistent effects on SSC in apples, peaches, plums, and other fruits (Watkins 2006).The 1-MCP treatment reduced the ethylene content in the packaging to a much lower level than that in the MAP alone(Fig. 1), which may be due to 1-MCP inhibiting the ethylene production of the pears.
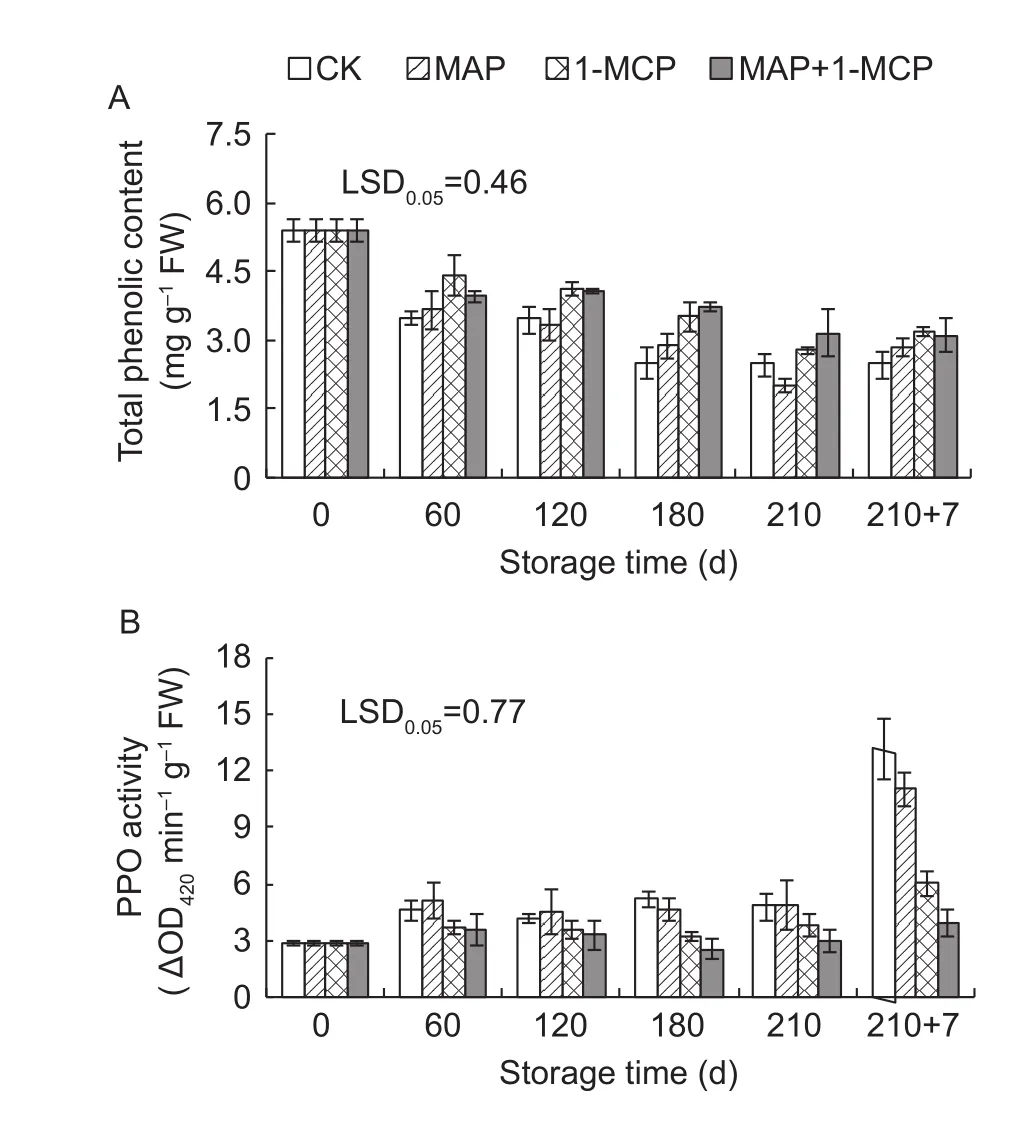
Fig. 6 Changes in total phenolic content (A) and polyphenol oxidase (PPO) activity (B) in Yali pear peels during storage.210+7 indicates 7 days at shelf life after 210 days of cold storage. 1-MCP, 1-methylcyclopropene; MAP, modified atmosphere packaging. LSD, least significant differences.Error bars represent ±SE from three replicates.
It is generally believed that the accumulation of α-farnesene,and more significantly, CTols, is important for the induction of superficial scald in apples and pears. Some studies have shown that 1-MCP significantly reduced the concentrations of α-farnesene and CTols in the peels of pear (Ekman et al. 2004; Gapper et al. 2006; Isidoro and Almeida 2006;Xie et al. 2014; Gao et al. 2015). In this study, the 1-MCP and MAP+1-MCP treatments reduced the contents of α-farnesene and CTols (Fig. 3-B and C) and lowered the superficial scald index (Fig. 3-A). These results further indicated that the accumulation of α-farnesene and CTols in the peel had a positive effect on the onset of superficial scald. However, the α-farnesene and CTols contents in the peel were reduced at shelf life (Fig. 3-B and C), this might be related to the metabolic exhaustion of α-farnesene and CTols during the development of superficial scald. Therefore,the promoting roles of α-farnesene and CTols on scald development were weakened at the shelf life period in Yali pear. In addition, based on path analysis results (Table 1),α-farnesene content was shown to have a slightly negative correlation with the scald index.
It is thought that O2–· together with H2O2could form the radical hydroxyl ·OH, after which the ·OH might cause lipid peroxidation and result in damage to the cell membrane and physiological disorders, which could induce the onset of superficial scald (Lu et al. 2011; Sabban-Amin et al.2011; Tian et al. 2013). SOD and CAT could enhance the resistance to various abiotic stresses by scavenging the reactive oxygen species (ROS) in cells (Gao et al.2015). In this work, lower production of O2–· and higher activities of CAT and SOD were observed in the 1-MCP and 1-MCP+MAP treatments (Fig. 4), in agreement with previous studies in the Blanquilla and Wujiuxiang pears and in Granny Smith apples (Larrigaudière et al. 2004; Sabban-Amin et al.2011; Gao et al. 2015). These results suggested that the 1-MCP and 1-MCP+MAP treatment could reduce ROS accumulation by improving the activities of SOD and CAT,helping to maintain the cell membrane integrity.
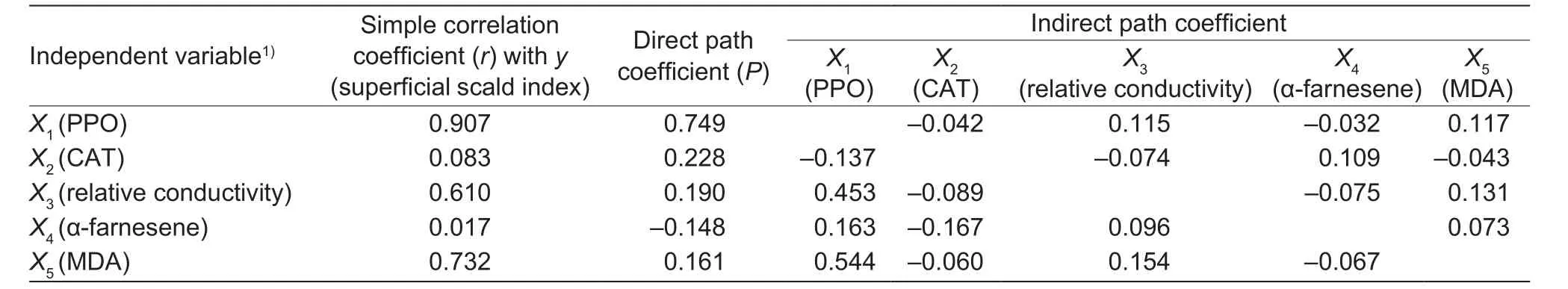
Table 1 Path coefficient of the scald index and related indices
MDA is one of the primary products of membrane lipid peroxidation. Accumulation of MDA causes specific damage to cell membranes and is important in the development of superficial scald in pears (Hui et al. 2011). LOX can activate membrane lipid peroxidation, and its activity was closely correlated with lipid peroxidation during fruit ripening (Li and Wang 2009; Singh et al. 2012). In this study, the 1-MCP and MAP+1-MCP treatments significantly reduced LOX activity, MDA content and relative conductivity preceding the onset of superficial scald (Fig. 5), suggesting that 1-MCP and MAP+1-MCP treatments could protect cell membrane integrity by inhibiting LOX activities and MDA accumulation.
PPO catalyzes the oxidation reaction of phenolic compounds to form quinones, resulting in tissue browning(Franck et al. 2007). In this study, the 1-MCP and MAP+1-MCP treatments markedly delayed the decrease in phenolic content and inhibited the increase in PPO activity(Fig. 6). Linking the reduced superficial scald indices in 1-MCP and MAP+1-MCP treatments, and the result of correlation analysis of different indices (Table 1), it is proposed that PPO might play an important role in the development of superficial scald, and the 1-MCP and MAP+1-MCP treatments may inhibit this process by decreasing PPO activity of peel in Yali pears.
5. Conclusion
The 1-MCP, MAP, and MAP+1-MCP treatments delayed fruit softening but had a low effect on the SSC and significantly reduced the superficial scald index in Yali pears. The overall positive effect of the MAP+1-MCP treatment was more effective. A possible mechanism of MAP+1-MCP treatment on inhibiting superficial scald development might include delaying α-farnesene and CTols accumulation before the onset of superficial scald and suppressing the increase in the MDA and O2–· content. This would maintain the cell membrane integrity and higher SOD and CAT activities and decrease the LOX and PPO activities of the peel, thereby reducing peel browning and superficial scald severity in Yali pears.
Acknowledgements
This research was supported by the emarked fund for the China Agriculture Research System for National Technology System for Pear Industry (CARS-28-22), the Innovation Project of Modern Agricultural Sciences and Technology of Hebei Province, China (494-0402-YSN-C8RA), the Youth Fund of Hebei Academy of Agriculture and Forestry Sciences,China (A2015110106), and the Finance Special Foundation of Hebei Province, China (494-0402-YBN-0G4L).
Ahn T, Paliyath G, Murr D P. 2007. Antioxidant enzyme activities in apple varieties and resistance to superficial scald development. Food Research International, 40, 1012–1019.
Axelrod B, Cheesbrough T M, Laakso S. 1981. Lipoxygenase from soybeans: EC 1.13.11.12 Linoleate: Oxygen oxidoreductase. Methods in Enzymology, 71, 441–451.
Bordonaba J G, Matthieu-Hurtige V, Westercamp P, Coureau C, Dupille E, Larrigaudière C. 2013. Dynamic changes in conjugated trienols during storage may be employed to predict superficial scald in ‘Granny Smith’ apples. LWTFood Science and Technology, 54, 535–541.
Cheng Y, Liu L, Zhao G, Shen C, Yan H, Guan J, Yang K.2015. The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. LWT-Food Science and Technology, 60, 1243–1248.
Ekman J H, Clayton M, Biasi W V, Mitcham E J. 2004.Interactions between 1-MCP concentration, treatment interval and storage time for ‘Bartlett’ pears. Postharvest Biology and Technology, 31, 127–136.
Isidoro N, Almeida D P F. 2006. α-Farnesene, conjugated trienols, and superficial scald in ‘Rocha’ pear as affected by 1-methylcyclopropene and diphenylamine. Postharvest Biology and Technology, 42, 49–56.
Franck C, Lammertyn J, Ho Q T, Verboven P, Verlinden B, Nicola? B M. 2007. Browning disorders in pear fruit.Postharvest Biology and Technology, 43, 1–13.
Gao M, Zhou S, Guan J, Zhang Y. 2015. Effect of 1-methylcyclopropene on superficial scald and related metabolism in ‘Wujiuxiang’ pear during cold storage. Journal of Applied Botany and Food Quality, 88, 102–108.
Gapper N E, Bai J, Whitaker B D. 2006. Inhibition of ethylene-induced alpha-farnesene synthase gene PcAFS1 expression in ‘d’Anjou’ pears with 1-MCP reduces synthesis and oxidation of alpha-farnesene and delays development of superficial scald. Postharvest Biology and Technology,41, 225–233.
Hui W, Niu R X, Song Y Q, Li D Y. 2011. Inhibitory effects of 1-MCP and DPA on superficial scald of ‘Dangshansuli’ pear.Agricultural Sciences in China, 10, 1638–1645.
Larrigaudière C, Vilaplana R, Soria Y, Recasens I. 2004.Oxidative behaviour of Blanquilla pears treated with 1-methylcyclopropene during cold storage. Journal of the Science of Food and Agriculture, 84, 1871–1877.
Lester G E, Lewers K S, Medina M B, Saftner R A. 2012.Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: Assay interference by ascorbic acid. Journal of Food Composition and Analysis,27, 102–107.
Li F, Zhang X, Song B, Li J, Shang Z, Guan J. 2013. Combined effects of 1-MCP and MAP on the fruit quality of pear (Pyrus bretschneideri Rehd cv. Laiyang) during cold storage.Scientia Horticulturae, 164, 544–551.
Li Z Q, Wang L J. 2009. Effect of 1-methylcyclopropene on ripening and superficial scald of Japanese pear (Pyrus pyrifolia Nakai, cv. Akemizu) fruit at two temperatures. Food Science and Technology Research, 15, 483–490.
Lu X G, Liu X H, Li S F, Wang X J, Zhang L H. 2011. Possible mechanisms of warming effects for amelioration of superficial scald development on ‘Fuji’ apples. Postharvest Biology and Technology, 62, 43–49.
Lurie S, Watkins C B. 2012. Superficial scald, its etiology and control. Postharvest Biology and Technology, 65, 44–60.
Reuck K D, Sivakumar D, Korsten L. 2009. Integrated application of 1-methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharvest Biology and Technology, 52, 71–77.
Rupasinghe H P V, Almquist K C, Paliyath G, Murr P D. 2001.Cloning of hmg1 and hmg2 cDNAs encoding 3-hydroxy-3-methylgatary coenzyme A reductase and their expression and activity in relation to α-farnesene synthesis in apple.Plant Physiology Biochemistry, 39, 933–947.
Sabban-Amin R, Feygenberg O, Belausov E, Pesis E. 2011.Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species(ROS) accumulation in stored ‘Granny Smith’ apples.Postharvest Biology and Technology, 62, 295–304.
Sabir F K, Agar I T. 2011. Effects of 1-methylcyclopropene and modified atmosphere packing on postharvest life and quality in tomatoes. Journal of Food Quality, 34, 111–118.
Singh S P, Singh Z, Swinny E. 2012. Climacteric level during fruit ripening influences lipid peroxidation and enzymatic and non-enzymatic antioxidative systems in Japanese plums (Prunus salicina Lindell). Postharvest Biology and Technology, 65, 22–32.
Tian S P, Qin G Z, Li B Q. 2013. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Molecular Biology, 82, 593–602.
Wang A G, Luo G H. 1990. Quantitative relation between the reaction of hydroxylamine and superoxide anion redicals in plants. Plant Physiology Communications, 6, 55–57. (in Chinese)
Wang Y, Sugar D. 2013. Internal browning disorder and fruit quality in modified atmosphere packaged ‘Bartlett’pears during storage and transit. Postharvest Biology and Technology, 83, 72–82.
Watkins C B. 2006. The use of 1-methylcyclopropene (1-MCP)on fruits and vegetables. Biotechnology Advances, 24,389–409.
Whitaker B D. 2007. Oxidation products of α-farnesene associated with superficial scald development in d’Anjou pear fruits are conjugated trienols. Journal of Agricultural and Food Chemistry, 55, 3708–3712.
Xie X, Song J, Wang Y, Sugar D. 2014. Ethylene synthesis,ripening capacity, and superficial scald inhibition in 1-MCP treated ‘d’Anjou’ pears are affected by storage temperature.Postharvest Biology and Technology, 97, 1–10.
Xu M, Dong J, Zhang M, Xu X, Sun L. 2012. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage.Postharvest Biology and Technology, 65, 5–12.
Zanella A. 2003. Control of apple superficial scald and ripening- A comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra low oxygen storage. Postharvest Biology and Technology, 27, 69–78.
Zhao D, Shen L, Fan B, Yu M, Zheng Y, Lv S, Sheng J. 2009.Ethylene and cold participate in the regulation of LeCBF1 gene expression in postharvest tomato fruits. FEBS Letters,583, 3329–3334.
 Journal of Integrative Agriculture2018年7期
Journal of Integrative Agriculture2018年7期
- Journal of Integrative Agriculture的其它文章
- Characterisation of pH decline and meat color development of beef carcasses during the early postmortem period in a Chinese beef cattle abattoir
- Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits
- Insertion site of FLAG on foot-and-mouth disease virus VP1 G-H loop affects immunogenicity of FLAG
- Update of Meat Standards Australia and the cuts based grading scheme for beef and sheepmeat
- Light shading improves the yield and quality of seed in oil-seed peony (Paeonia ostii Feng Dan)
- Effects of leaf removal and cluster thinning on berry quality of Vitis vinifera cultivars in the region of Weibei Dryland in China
