Transcatheter aortic valve replacement and stroke: a comprehensive review
Periklis A Davlouros, Virginia C Mplani, Ioanna Koniari, Grigorios Tsigkas, George Hahalis
Department of Cardiology, Patras University Hospital, Rion, Patras, Greece
1 Introduction
Degenerative aortic stenosis (AS), is the most frequently encountered valvular heart disorder, as its incidence approaches 2.5%-7% in elderly patients.[1-3]In 2013, 50,222 deaths were related to valvular heart disease in USA. Of those, 67.5% were due to aortic valve disorders.[1]Indeed,after symptoms’ onset, AS carries a poor prognosis if left untreated.[2]Surgical aortic valve replacement (SAVR) has been the standard treatment for symptomatic aortic stenosis in patients at low and intermediate surgical risk [Risk assessment based on Logistic Euroscore, and/or Society of Thoracic Surgeons Predicted Risk of Mortality (STS Prom)].[3]Over the last few years, transcatheter aortic valve replacement (TAVR), emerged as a potential alternative to SAVR and the procedure of choice for patients who have a prohibitive surgical risk,[4]and more recently even for intermediate risk patients.[5]Hence, more than 200,000 TAVR procedures have been performed worldwide since 2002.[6]More importantly, some recent randomized trials and observational studies have reported similar, or even superior results with TAVR compared to SAVR in lower risk patients.[5,7,8]Therefore, an expansion of the use of this procedure might be anticipated in the near future. One of the most feared complications of TAVR is stroke because of its associated severe disability and high mortality,[9]being also one of the most feared complications by patients. With the expected broadening of the indications for TAVR in the future,the aim of this review is to enlighten the incidence, predictors, clinical impact, and potential strategies to avoid stroke following TAVR.
2 Stroke incidence following TAVR (Figure 1)
In an effort to adjust for discrepancies in the definitions of stroke used in various studies, the Valve Academic Research Consortium has provided definitions of transient ischemic attack (TIA) and stroke.[10,11]TIA is defined as a new neurological deficit that resolves rapidly, in less than 24 h (usually within 1-2 h), without evidence of tissue injury in neuroimaging. Stroke is defined as new focal or global neurological deficit that persists for more than 24 h and is thought to be embolic, ischemic or hemorrhagic.[11]The severity of stroke is usually categorized according to the modified Rankin Scale (mRS).
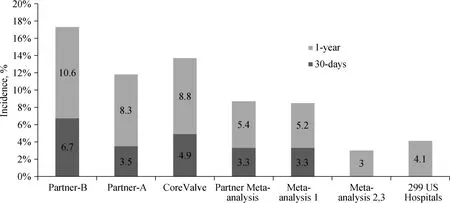
Figure 1. Incidence of stroke following TAVR in landmark studies and meta-analyses. PARTNER-B,[4] PARTNER-A,[12] Core-Valve,[7] PARTNER Meta-analysis,[11] meta-analysis 1,[15] meta-analyses 2,3,[9,23] and 299 US hospitals.[24] TAVR: transcatheter aortic valve replacement.
As will be discussed, the procedure of TAVR carries an inherent risk of stroke, which however continues to accrue with extended follow-up. Therefore, we should evaluate stroke risk with TAVR relative to the stroke risk of a similar patient population treated either medically, or with conventional SAVR, during the same follow-up period. Data from PARTNER 1B, study showed that medically treated patients had a 30-days stroke/TIA risk of 1.7%, and a 1-year risk of 4.5%, compared with corresponding risks of 6.7% and 10.6% in high risk TAVR patients.[4]Early experience with TAVR was associated with stroke rates nearly twice as high compared to SAVR.[12,13]Data from the PARTNER 1A study showed a risk of neurologic events with SAVR of 2.4% at 30 days and 4.3% at one year, with corresponding rates for TAVR of 5.5% and 8.3%, respectively.[12]Generally, in high risk patients, the risk of stroke following SAVR is approximately 2%-4%.[14]Hence, it is reasonable to speculate that violent manipulation of a calcified aorta and stenotic aortic valve with TAVR results in a higher frequency of cerebral events, through debris embolization,compared to SAVR during which debris removal and cross-clamping of the aorta is involved.[15]On the contrary,the long-term risk of stroke following TAVR is somewhat similar to that of medically and/or surgically treated patients,and depends on their profile.[15]In a much larger analysis of PARTNER trial including the continued access registry with 2621 patients, the 30-days, 1-year and 3-year stroke rate were 3.3% (85% of them within one week), 5.4% and 6.9%,respectively.[11]
In the randomized controlled trial of the self-expandable valve bioprosthesis (CoreValve, Medtronic, Minneapolis,MN) vs. SAVR in relatively high surgical risk patients(STS score 7.4%), the rates of stroke at 30 days and 1 year did not differ significantly between the two groups (4.9% vs.6.2%, P = 0.46, and 8.8% vs. 12.6%, P = 0.1, respectively).[7]A recent report including 3687 patients from the CoreValve US Extreme Risk and High Risk Pivotal Trials or Continued Access Study, the 1-year stroke rate after TAVR was 8.4%, with a frequency of major stroke 2.8% at 30 days, and 5% at 1 year.[16]The frequency of TIA within 30 days was 0.5%, and within the first year 2.1%. Following initial experience with TAVR, various studies reported a range of stroke of 0-3.9% vs. 0.5%-5.7% with SAVR.[7,17,18]Similarly, according to published registries, the overall incidence rate of stroke in high-risk patients after TAVR varied from 1.7% to 4.8%.[17,19-22]An earlier meta-analysis including 10,037 patients subjected to TAVR in various studies published between 2004/01 and 2011/11 reported a total 30-days stroke rate of 3.3% ± 1.8% (nearly all major strokes), which increased at 1-year to 5.2% ± 3.4%.[15]Two recent meta-analyses, one including seven European TAVR registries (9786 patients),[23]and another including 29,034 patients,[9]treated with both SAPIEN and CoreValve bioprostheses and involving both transfemoral (TF) and transapical (TA) approaches, reported a 1-year incidence of stroke of approximately 3%.[23]Additionally, a recent report from 299 US hospitals (12,182 patients), reported a stroke rate of 4.1% at 1 year following TAVR.[24]Finally, in a review of studies comparing TAVI vs. SAVR in patients with severe aortic stenosis and a mean risk score of 8% or less, the hazard for stroke was lower with TAVI (20 fewer per 1000 patients), however with a broad confidence interval (HR: 0.81,95% CI: 0.63-1.01).[25]Additionally, an analysis of patients with an STS PROM risk score < 7 revealed lower albeit not statistically significant stroke rates after TAVR compared with SAVR at 30 days (4.9% vs. 6.3%, P = 0.46).[26]
Thus, following the initial observation of a higher stroke rate with TAVR when compared to SAVR, latest studies,registries, and meta-analysis repetitively and constantly confirm that the incidence of stroke after TAVR decreased to rates comparable with those with SAVR.[7,15,19,23-28]This may be due to inclusion of lower risk patients compared to initial study cohorts, and to evolution of valve technology and implantation technique.
3 Timing and predictors of stroke following TAVR
The occurrence of TAVR related stroke demonstrates a bimodal pattern of distribution, which was evident in studies of the two most frequently implanted valves, namely the balloon expandable Edwards Sapien, and the self expandable CorValve.[11,13,16]Indeed, in the CoreValve US Extreme Risk and High Risk Pivotal Trials, and the Continued Access Study, with the self-expanding CoreValve bioprosthesis, strokes clustered in an early phase (0-10 days; 4.1% of strokes), and a late phase (11-365 days; 4.3% of strokes),for a total stroke rate at 1 year of 8.4%.[16]As mentioned, in the PARTNER trial, 3.3% of patients experienced a stroke within the first 30 days, the vast majority within the first week (85%), whereas the corresponding rate of TIA within 30 days was much less (0.5%).[11]More specifically, in the PARTNER trial, the stroke rate demonstrated a very early peak on the second day post-procedure, dropping thereafter to a low constant rate of 0.8% at 1 to 2 weeks.[11]A metaanalysis of 10,037 patients reported an incidence of stroke manifesting in < 24 h post TAVR of 1.5% ± 1.4%.[15]
The bimodal distribution of stroke post TAVR implies that different mechanisms and potential contributing factors are involved in its pathophysiology. Indeed, predictors of early stroke include patients’ demographics, clinical characteristics, and procedural factors.[11,16]The former two probably reflect the degree of patients’ illness, whereas the latter mainly refer to the duration and complexity of the procedure. On the other hand, predictors of late stroke seem to be principally related to patients’ frailty.[11,16]
4 Early stroke predictors (Table 1)
In general, early stroke (within the first seven days) is broadly considered to be related to the procedure due to particle embolization. These particles are comprised of tissue fragments from the aortic valve, aorta, and left ventricular myocardium, and thrombus, as shown in studiesusing embolic protection devices during TAVR.[29]A potential explanation for a delayed (up to 7 days) diagnosis of a stroke causally related to the procedure, might be lack of early imaging, and the requirement of additional time for thrombus formation on the embolized material and subsequent clinical presentation.[11]Indeed, the high rate of periprocedural stroke which seems to stabilize thereafter, highlights the paramount significance of procedural factors in stroke causality post TAVR.[11]However, it is also reasonable to assume that specific patient characteristics may relate with a higher predisposition for embolic events. However, despite the inherent complexity of analyzing and identifying two interrelated different groups of potential predictors of early stroke (i.e., procedural and baseline patient characteristics), it is important to realize that the former group of predictors (procedural) may be modifiable, whereas identification of the latter (patient characteristics), although theoretically not modifiable, could help us to avoid or improve certain procedural details which may confer a higher risk of stroke in specific patient subsets.
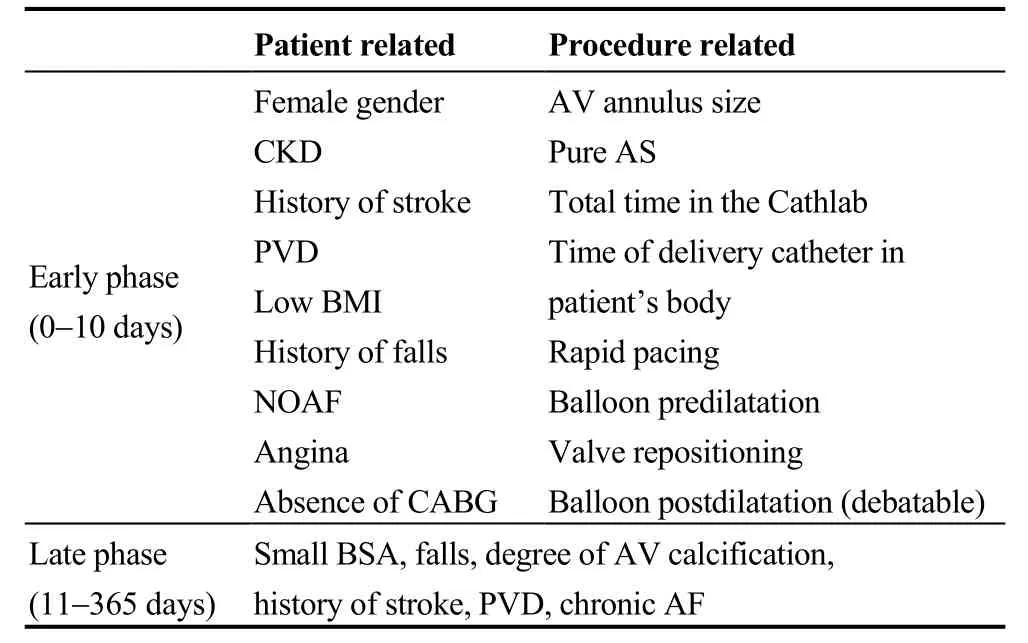
Table 1. Early and late stroke predictors.
In the CoreValve trials, patient related multivariable predictors of stroke were a total NIHSS score > 0, history of stroke, or TIA, peripheral vascular disease, absence of prior coronary artery bypass surgery, presence of angina, low body mass index, and falls within the past six months.[16]Interestingly, there were no significant imaging predictors of early stroke (echocardiographic, or computerized tomographic).[16]In a recent meta-analysis by Krassopoulos, et al.,[23]the mean Logistic EuroSCORE was not associated with the incidence of stroke.
Procedural related multivariable predictors of stroke in the CoreValve trials were the total time in the catheteriza-tion laboratory, the total time that the delivery catheter time in the patient’s body, rapid pacing during valvuloplasty, and repositioning of the CoreValve with a snare.[16]In the PARTNER trial, predictors of stroke were stratified according to the access strategy, namely TF vs. TA.[11]A higher aortic valve peak gradient pre-TAVR was a predictor of early strokes in TF access, and pure aortic stenosis without regurgitation, along with more valve post-dilatations for the TA approach.[11]More rapid pacing during valvuloplasty was also a weak predictor of early stroke in the TA approach, whereas longer procedure time was weakly associated with an increasing number of late strokes in the TF approach.[11]
At first glance, TA-TAVR might confer a lower risk of stroke compared to TF-TAVR, as it involves minimal manipulation of the ascending aorta and arch, which in elderly patients with severe atherosclerosis may be a source of embolic material.[15,30]However, in the PARTNER trial, a slightly higher rate of stroke was noted in the transapical group of patients, potentially related to the higher vascular risk of the latter.[11]On the other hand, in the meta-analysis by Eggebrecht, et al.,[15]stroke rates at 30-days were significantly lower with the TA approach using the Edwards SAPIEN valve (2.7% ± 1.4%), compared to the retrograde transarterial (TF and subclavian) implantation of the same valve (4.2% ± 2.2%). However, retrograde transarterial implantation of the 18 Fr Medtronic/CoreValve prosthesis compared to the bulkier 22-24 Fr Edwards SAPIEN valve was associated with a lower 30-days stroke rate (3.1% ±2.2% vs. 4.2% ± 2.2%).[15]Hence, it may be that the valve type and the bulkiness of the delivery system play a significant role in determining stroke risk when it comes to TF access. In the meta-analysis of seven European TAVR registries, the pooled estimate for the incidence of stroke was 0.03 [95% CI: 0.03-0.04], 0.03 [95% CI: 0.02-0.05] and 0.03 [0.02-0.04] for TF implanted SAPIEN?, TA implanted SAPIEN and CoreValve, respectively (test for subgroup difference, P = 0.79).[23]In general, the majority of evidence does not verify an association between type of access (TA vs. retrograde TF) and stroke.[11,28,31,32]Despite lack of significant differences in stroke rates between the two types of access, stroke predictors seem to differ between them as pointed above.[11]The potential risk of stroke with new approaches like transcarotid has not been studied in detail.[32]
Although balloon valvuloplasty with valve pre-dilatation has been associated with a higher risk for stroke,[9,16,32]balloon post-dilation of the prosthesis used to reduce severe paravalvular leak, is not universally recognized as predictor of stroke post TAVR.[11,16,32,33]It has been proposed that this probably relates to the different design of valves used for TAVR. For example, the CoreValve prosthesis is surrounded by a relatively large metal scaffold, which may prevent particle embolization from the ascending aorta during post-dilatation.[16]Additionally, balloon post-dilatation may actually reflect more extensive vascular disease, leading to the decision for the need of further valve dilatation.[16]On the other hand, the type of valve per se seems to be not crucial as there was a similar stroke risk between them across a broad spectrum of studies.[9,32,34,35]
The potential relationship of the aortic valve area with stroke post-TAVR is also debated.[11,16,36]In the PARTNER trial, a higher rate of stroke was demonstrated in patients with smaller aortic valve area (6.3% vs. 2.8%).[36]It has been shown that women demonstrate smaller aortic annuli dimensions than men, irrespective of age or body surface area.[37]It may be that a smaller aortic annulus may increase the mechanical interaction between the native valve and the prosthesis, and this may partly explain the higher risk of early stroke demonstrated by women in some studies.[32]
It would appear reasonable that operator, and center experience may also be predictors of stroke post TAVR. This was suggested by the meta-analysis by Athappan, et al.,[28]who found a 1.5% drop in stroke risk between early and late experience within high-volume centers, and this has been reproduced in a later meta-analysis.[32]However, in the CoreValve trials, this was not evident.[16]The latter observation may relate to the “sterile” environment of a clinical study, but the concept that the risk of stroke due to instrumentation of the aorta, arch, and aortic valve, may not be centre/operator dependent cannot be excluded.[16]In the PARTNER trial, valve implantation during the “earlier date” of TAVR experience, had a weak and unreliable association with early stroke only in the TA group of patients.[11]In essence, analyses based on chronology may be influenced by numerous patient and procedure related factors like patient selection, and procedural and device evolution, which make difficult to reach to definite conclusions regarding any potential relationship between stroke and the TAVR learning curve.[32]
A recent meta-analysis of sixty-four studies involving 72,318 patients suggested that female sex, chronic kidney disease (CKD), performance of TAVR during the first half of centers’ experience, and post-procedural new onset of atrial fibrillation (AF), are associated with an increased risk of early stroke following TAVR.[32]Most importantly, in this study, no association was found between early stroke following TAVR and other baseline risk factors, valve type and implantation approach. In contrast to other datasets,[16]only CKD and not other markers of advanced atherosclero-sis such as peripheral vascular disease, or cardiovascular disease (CAD), was associated with stroke post-TAVR in this analysis.[32]This may suggest the existence of a “renal factor” leading to an excess risk of stroke regardless of the existence of other traditional risk factors. This includes advanced atherosclerosis and calcification demonstrated by CKD patients, and the administration of suboptimal antithrombotic treatment due to bleeding risks. Finally, the strongest predictor of early stroke in this analysis was new onset AF, which happens in up to 30% TAVR patients, especially with the TA approach.[32]The pivotal role of new onset AF in predicting subacute (1-30 days), stroke following TAVR has also been pointed by earlier studies.[38]
5 Late stroke predictors (Table 1)
Predictors of late stroke seem to be principally related to patients’ atherosclerotic risk and frailty.[11,16]In the Core-Valve trials, predictors of late strokes (between 11 days and 1 year) were small body surface area, severe aortic calcification, and falls within the past six months, with the latter being the only significant predictor of major stroke.[16]The degree of aortic calcification, however, is rather considered a marker of a severely diseased vascular system, with the potential for aorto-arterial embolism.[16]Predictors of late stroke in the PARTNER trial were dementia, and a smaller prosthetic valve size (23 vs. 26 mm) for the TF approach,and race (non-white), lower left ventricular ejection fraction(LVEF), and atrial fibrillation (AF), for the TA approach.[11]Markers of patients’ atherosclerotic risk and frailty such as prior cerebrovascular disease, peripheral vascular disease,and chronic AF, have also been shown to predict late stroke risk by others.[38]
In general, it seems that the baseline predictors of early stroke post-TAVR including both clinical and procedural factors, whereas after the first 10 days or so, post TAVR,stroke risk is mainly dependent on patient characteristics and not to specific valvular anatomic features.[16,39]
6 Clinical implications of stroke following TAVR
The occurrence of major stroke following TAVR is associated with increased early and late mortality.[11,38]However,mortality from stroke does not seem to differ between TAVR and SAVR.[28]In the CoreValve studies, the oneyear mortality following a stroke was quite high and similar for patients who sustained the primary event either during the early or late phase of stroke distribution post TAVR(46.2% and 44.6% for all strokes).[16]The highest mortality was confined in patients with a major stroke. An alarming observation was that 7% of patients with a stroke within the first year experienced a recurrent stroke during the first year of follow-up.[16]In the PARTNER trial, both TIA and stroke had a major impact compared to expect one year survival rates (64% and 47% vs. 83% and 82%, respectively).[11]In the meta-analysis by Eggebrecht, et al.,[15](10,037 patients,81.5 ± 1.8-years, mean logistic EuroSCORE 24.77% ± 5.60%),the average 30-day mortality was more than 3.5- fold higher in patients with stroke (25.5% ± 21.9% vs. 6.9% ± 4.2%).Similarly, in a recent meta-analysis of 29,034 patients with a mean age 81.37 years, mortality following a stroke within the first 30-days of implantation was 12.27%, and stroke related mortality was 28.22%, compared to 6.4% for patients without a stroke.[9]This fourfold increase in mortality corresponded to an OR of 6.45 (95% CI: 3.9- 10.66, P <0.0001).[9]
7 Silent stroke
There is a growing body of evidence that the real stroke rate following TAVR is underestimated.[40]This is due to the fact that the clinical definition of stroke and TIA, may be a low sensitivity measure. With increasing use of neuroimaging (CT and MRI), the definition of stroke has changed over time. Hence, the 2013 AHA/ASA expert consensus document proposed an “Updated Definition of Stroke for the 21stCentury”, which updated the definition of cerebral infarction (based on imaging, and/or pathology),but also proposing a definition for “silent” central nervous system (CNS) infarction.[41]The latter constitutes imaging or pathological evidence of CNS infarction without acute clinical neurological dysfunction.[41]Subsequently, studies that used brain imaging with MRI [diffusion weighted imaging (DWI)] early post TAVR, found a more than tenfold increase in the rate of “silent infarction” compared to the rate of clinical evident ischemic stroke as reported in the PARTNER trial.[42,43]However, even with such sensitive contemporary brain imaging, different acquisition protocols,post-processing, and interpretation, may lead to a wide variation in the reported incidence of “silent infarction”,underlining the need for standardization of brain imaging protocols post TAVR.[40]
Inherent to the “silent” nature of such findings, is their largely unknown long-term implications. It may be that such brain lesions detected with MRI have an impact on patients’neurocognitive function, which in one study not involving imaging was shown to decline in 5% of patients post TAVR.[44]In the well designed, albeit small neuro-TAVI trial, DWI-MRI imaging was used with serial systematic neurologic and cognitive assessments in 44 consecutive patients.[45]Brain lesions were detected in 94% of patients,with worsening of NIHSS score in 22.6% at discharge and 14.8% at 30-days, whereas cognitive deviation from baseline using the Montreal cognitive assessment was shown in 33% and 41% of patients, respectively.[45]This study implies that cerebral insult may be much more frequent than reported, and it may be associated with neurological impairment not routinely evaluated with current scoring systems. A similar high rate (84%), of new brain lesions on DWI-MRI was reported in an earlier study of 32 patients undergoing TAVR, which is much higher compared to a 48% incidence in control patients undergoing surgical AVR.[42]However, no TAVR patient in this study developed clinical neurological events, or a measurable impairment of neurocognitive function at 3-months follow-up.Hence, the potential implications of this nearly universal imaging finding post TAVR needs clarification in larger scale studies.
Despite all these uncertainties, DWI-MRI may prove a useful research tool for evaluation of embolic protection devices, as a clearly smaller number of patients will be needed in such studies.[40]
8 Stroke prophylaxis (Figure 2)
It is evident that a strategy to reduce the probability of stroke following TAVI should include modification of procedural factors associated with the former, development of protection devices against debris embolization, and antithrombotic treatment during the procedure and thereafter.Unfractionated heparin (UFH) administration is the current standard of treatment during TAVR. The latter, is administered as a parenteral bolus, followed by additional doses until an activated clotting time (ACT) of ≥ 300 s is achieved,with complete reversal of anticoagulation by administration of protamine sulphate (milligram-to-milligram neutralisation dose), being available but not always necessary with the TF approach.[46]As major bleeding has a reported incidence of up to 17% post TAVR,[4]administration of bivallirudin could be an attractive alternative to UFH due to its lower incidence of major bleeding in coronary stenting trials.[47]An equal rate and type of early stroke with administration of bivalirudin instead of UFH during TAVR has been shown.[48]However, the effect of Bivalirudin on Aortic Valve Intervention Outcomes 2/3 (BRAVO 2/3) study, in which 802 patients undergoing TF-TAVR were randomized to bivalirudin versus UFH, failed to demonstrate superiority of bivalirudin as the latter did not result in significantly less major bleeding episodes 48 h [6.9% vs. 9.0%; relative risk:0.77; 95% confidence interval (CI): 0.48-1.23; P = 0.27],with similar net adverse clinical events at 30 days.[49]Despite the fact that the noninferiority hypothesis was met regarding effectiveness, the authors pointed that due to cost issues, UFH should remain the standard of care, with bivalirudin reserved for patientsunable to receive the former.
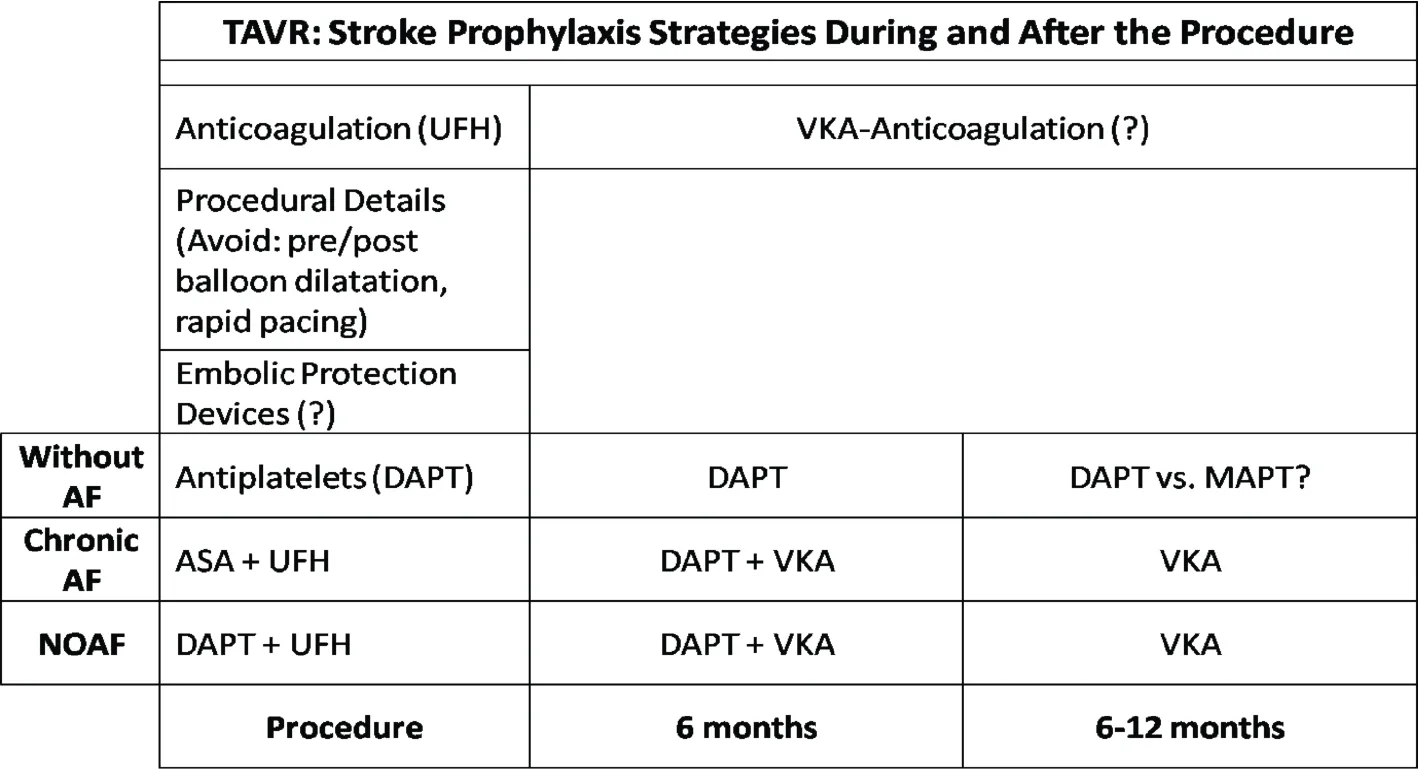
Figure 2. Stroke prophylaxis strategies. During TAVR, antithrombotic treatment includes anticoagulation with UFH, and antiplatelets.DAPT with low dose ASA and Clop is administered thereafter for six months, although the exact time needed is not known. Certain procedural details like avoiding balloon pre-dilatation and rapid pacing, minimal contact with the aortic arch etc, may contribute to stroke prophylaxis. For patients with chronic AF low dose ASA and UFH is administered during the procedure, whereas VKA with low dose ASA or Clopidogrel are administered for 6 months, and VKA thereafter. Similarly, for NOAF, switching from DAPT to ASA/clopidogrel plus VKA is preferred instead of triple antithrombotic treatment. The role of extended DAPT or MAPT, or VKA-anticoagulation treatment is not known. AF: atrial fibrillation; ASA: aspirin; Clop: clopidogrel; DAPT: dual antiplatelet therapy; MAPT: monoantiplatelet; NOAF: new onset atrial fibrillation; TAVR: transcatheter aortic valve replacement; UFH: unfractionated heparin; VKA: vitamin-k antagonists.
There is no consensus regarding the best antithrombotic treatment strategy post TAVR regarding both the antithrombotic regimen, and the duration of such treatment due to lack of properly designed and powered studies thus far.[50]In the CoreValve trials, approximately 95% of patients were discharged on aspirin, 85% on aspirin with a P2Y12 inhibitor, and 61% on anticoagulants.[16]The antithrombotic regimen, did not differ between patients with or without stroke.Current AHA/ACC guideline recommendations suggest empirical therapy with ASA and clopidogrel for 6 months after TAVI (class IIb, level of evidence C).[51]Surprisingly a systematic review and meta-analysis of studies comparing dual antiplatelet (DAPT) with mono-antiplatelet (MAPT)therapy following TAVR, showed a trend toward an increase in 30-day stroke, spontaneous MI, all-cause mortality,and combined lethal and major bleeding with DAPT.[52]However, the authors pointed that this was driven by increased events in observational studies, with no difference in randomized studies. Nevertheless, despite current recommendations, there is no robust evidence that DAPT may be superior to MAPT in preventing ischemic complications post-TAVR, whereas it may increase bleeding complications.[52]MAPT with clopidogrel may be equally efficacious to aspirin, and clopidogrel 300 mg loading before the procedure, may increase periprocedural bleeding complications.[52]More importantly, data from the PARTNER trial showed a rate of 5.9% major late bleeding (≥ 30 days)post-TAVR at a median time 132 days.[53]These included gastrointestinal (40.8%), intracranial (15.5%), and traumatic fall (7.8%) bleedings. These late bleeding events were strong predictors of mortality between 30 days and 1 year(adjusted hazard ratio: 3.91; 95% CI: 2.67-5.71; P <0.001).[53]Data from the CoreValve US Extreme Risk and High Risk Pivotal Trials or Continued Access Study, designate 8.9% of strokes post-TAVR as hemorrhagic.[16]These occurred solely in patients on double antiplatelet therapy(aspirin and clopidogrel), aspirin and anticoagulant, and patients on triple antithrombotic therapy, with nearly half of hemorrhagic strokes occurring in the latter, highlighting the bleeding risk with combination of antithrombotic treatments in this fragile population. However, frequent comorbidities in this patient population may require addition of anticoagulation to antiplatelet therapy, with a history of, or new onset AF being the most frequent indication for the latter.New onset AF may happen in up to 30% of patients early post-TAVR as mentioned above.[32,38]In patients with a history of AF, an older expert opinion document suggests continuing anticoagulation and adding low dose aspirin without clopidogrel post-TAVR.[46]The recent ACC/AHA guidelines do not comment specifically on anticoagulation post-TAVR.[51]The Canadian perspective is against triple antithrombotic therapy post-TAVR unless definite need exists.[54]Finally, European Guidelines suggest use of vitamin-K antagonists with either aspirin, or clopidogrel,weighting the risk of bleeding.[55]
Procedural improvements (smaller size and better flexibility of delivery catheters, avoidance of balloon pre-dilatation), may have contributed to the decrease in the incidence of stroke following TAVR, as already mentioned.Although the role of balloon post-dilatation in early stroke post TAVR is still debated,[11,16,32,33]proper imaging of the aortic annulus for valvular size selection, and emphasis on device features which may reduce paravalvular leak (external skirt of the Sapien 3 valve, or repositionable prostheses),are advised so as to avoid the former.[32]Continuous in hospital and Holter outpatient monitoring for capturing new onset AF may also be important in TAVR patients as the latter seems to be a significant predictor of stroke.[32]
Finally, the use of cerebral protection devices has not shown any positive clinical results yet.[40,56]These devices usually employ a filter membrane used to either capture, or deflect debris away from cerebral circulation during the procedure. However, a significant reduction of total ischemic lesion volume, and number of new ischemic cerebral lesions has been reported with some devices.[45-57]
9 Conclusions
Stroke is a devastating complication being confined mainly in the periprocedural and 30-day period following TAVR, with a lower and relatively constant frequency thereafter. Early stroke is mainly due to debris embolization during the procedure, whereas later events are associated with patient specific factors. Despite the fact that the rate of clinical stroke has been constantly decreasing compared to initial TAVR experience, modern neuro-imaging with MRI suggests that new ischemic lesions post-TAVR are almost universal. The impact of the latter is largely unknown,however they seem to correlate with a reduction in neurocognitive function. Because TAVR is set to expand its indication to lower surgical-risk patients, stroke prophylaxis during and after TAVR becomes of paramount importance.Based on clinical and pathophysiological evidence, three lines of research are actively employed towards this direction: improvement in valve and delivery system technology with an aim to reduce manipulations and contact with the calcified aortic arch and native valve, antithrombotic therapy, and embolic protection devices. Careful patient selec-tion, design of the procedure, and tailored antithrombotic strategies respecting the bleeding risks of this fragile population appear crucial in further reducing stroke rate following TAVR.
References
1 Writing Group M, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016; 133: 447-454.
2 Baumgartner H. Aortic stenosis: medical and surgical management. Heart 2005; 91: 1483-1488.
3 Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451-2496.
4 Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New Eng J Med 2010; 363: 1597-1607.
5 Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. New Eng J Med 2016; 374: 1609-1620.
6 Cerrato E, Nombela-Franco L, Nazif TM, et al. Evaluation of current practices in transcatheter aortic valve implantation:The WRITTEN (WoRldwIde TAVI ExperieNce) survey. Int J Cardiol 2016; 228: 640-647.
7 Adams DH, Popma JJ, Reardon MJ. Transcatheter aorticvalve replacement with a self-expanding prosthesis. New Engl J Med 2014; 371: 967-968.
8 Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J A m Coll Cardiol 2015; 65: 2184-2194.
9 Muralidharan A, Thiagarajan K, Van Ham R, et al. Metaanalysis of perioperative stroke and mortality in transcatheter aortic valve implantation. Am J Cardiol 2016; 118:1031-1045.
10 Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013; 145: 6-23.
11 Kapadia S, Agarwal S, Miller DC, et al. Insights into timing,risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER Trial (Placement of Aortic Transcatheter Valves).Circ Cardiovasc Interv 2016; 9: e002981.
12 Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. New Engl J Med 2011; 364: 2187-2198.
13 Miller DC, Blackstone EH, Mack MJ, et al. Transcatheter(TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg 2012;143: 832-843.
14 Daneault B, Kirtane AJ, Kodali SK, et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis: a comprehensive review. J Am Coll Cardiol 2011; 58: 2143-2150.
15 Eggebrecht H, Schmermund A, Voigtlander T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012; 8: 129-138.
16 Kleiman NS, Maini BJ, Reardon MJ, et al. Neurological events following transcatheter aortic valve replacement and their predictors: a report from the CoreValve trials. Circ Cardiovasc Interv 2016; 9: e003551.
17 Grabert S, Lange R, Bleiziffer S. Incidence and causes of silent and symptomatic stroke following surgical and transcatheter aortic valve replacement: a comprehensive review.Interact Cardiovasc Thorac Surg 2016; 23: 469-476.
18 Barbanti M, Ussia GP, Cannata S, et al. 3-year outcomes of self-expanding Corevalve prosthesis―The Italian Registry.Ann Cardiothorac Surg 2012; 1: 182-184.
19 Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States.JAMA 2013; 310: 2069-2077.
20 Werner N, Zeymer U, Schneider S, et al. Incidence and clinical impact of stroke complicating transcatheter aortic valve implantation: results from the German TAVI registry. Catheter Cardiovasc Interv 2016; 88: 644-653.
21 Holmes DR, Jr., Nishimura RA, Grover FL, et al. Annual Outcomes With transcatheter valve therapy: from the STS/ACC TVT registry. Ann Thorac Surg 2016; 101: 789-800.
22 Gilard M, Eltchaninoff H, Donzeau-Gouge P, et al. Late outcomes of transcatheter aortic valve replacement in high-risk patients: The FRANCE-2 registry. J Am Coll Car diol 2016;68: 1637-1647.
23 Krasopoulos G, Falconieri F, Benedetto U, et al. European real world trans-catheter aortic valve implantation: systematic review and meta-analysis of European national registries. J Cardiothorac Surg 2016; 11: 159.
24 Holmes DR, Jr., Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 2015; 313: 1019-1028.
25 Siemieniuk RA, Agoritsas T, Manja V, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis. BMJ 2016; 354: i5130.
26 Reardon MJ, Kleiman NS, Adams DH, et al. Outcomes in the randomized CoreValve US pivotal high risk trial in patients with a society of thoracic surgeons risk score of 7% or less.JAMA Cardiol 2016; 1: 945-949.
27 Burrage M, Moore P, Cole C, et al. Transcatheter aortic valve replacement is associated with comparable clinical outcomes to open aortic valve surgery but with a reduced length of in-patient hospital stay: a systematic review and meta-analysis of randomised trials. Heart Lung Circ 2017; 26: 285-295.
28 Athappan G, Gajulapalli RD, Sengodan P, et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta-analysis and systematic review of literature. J Am Coll Cardiol 2014; 63: 2101-2110.
29 Van Mieghem NM, El Faquir N, Rahhab Z, et al. Incidence and predictors of debris embolizing to the brain during transcatheter aortic valve implantation. JACC Cardiovasc Interv 2015; 8: 718-724.
30 Walther T, Schuler G, Borger MA, et al. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement.Eur Heart J 2010; 31: 1398-1403.
31 Rodes-Cabau J, Dumont E, Boone RH, et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol 2011; 57: 18-28.
32 Auffret V, Regueiro A, Del Trigo M, et al. Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J A m Col l Cardiol 2016; 68: 673-684.
33 Nombela-Franco L, Rodes-Cabau J, DeLarochelliere R, et al.Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloonexpandable valve. JACC Cardiovasc Interv 2012; 5: 499-512.
34 Abdel-Wahab M, Richardt G. Reply: Outcomes After transcatheter aortic valve replacement with balloon-expandable versus self-expandable valves: CHOICE trial results. J Am Coll Cardiol 2016; 67: 236-237.
35 Athappan G, Gajulapalli RD, Tuzcu ME, et al. A systematic review on the safety of second-generation transcatheter aortic valves. EuroIntervention 2016; 11: 1034-1043.
36 Rodes-Cabau J, Pibarot P, Suri RM, et al. Impact of aortic annulus size on valve hemodynamics and clinical outcomes after transcatheter and surgical aortic valve replacement: insights from the PARTNER trial. Circ Cardiovasc Interv 2014;7: 701-711.
37 Buellesfeld L, Stortecky S, Kalesan B, et al. Aortic root dimensions among patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv 2013; 6: 72-83.
38 Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing,predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012; 126: 3041-3053.
39 Tay EL, Gurvitch R, Wijesinghe N, et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2011; 4:1290-1297.
40 Mokin M, Zivadinov R, Dwyer MG, et al. Transcatheter aortic valve replacement: perioperative stroke and beyond. Expert Rev Neurother 2016: 1-8.
41 Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 2064-2089.
42 Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010; 121: 870-878.
43 Astarci P, Glineur D, Kefer J, et al. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans-apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg 2011; 40: 475-479.
44 Ghanem A, Kocurek J, Sinning JM, et al. Cognitive trajectory after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2013; 6: 615-624.
45 Lansky AJ, Brown D, Pena C, et al. Neurologic Complications of unprotected transcatheter aortic valve implantation(from the Neuro-TAVI Trial). AmJ Cardiol 2016; 118:1519-1526.
46 Holmes DR, Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012; 59: 1200-1254.
47 Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374: 1149-1459.
48 Van Belle E, Hengstenberg C, Lefevre T, et al. Cerebral embolism during transcatheter aortic valve replacement: The BRAVO-3 MRI study. J Am Coll Cardiol 2016; 68: 589-599.
49 Dangas GD, Lefevre T, Kupatt C, et al. Bivalirudin versus heparin anticoagulation in transcatheter aortic valve replacement: the randomized BRAVO-3 Trial. J Am Coll Cardiol 2015; 66: 2860-2868.
50 Gargiulo G, Collet JP, Valgimigli M. Antithrombotic therapy in TAVI patients: changing concepts. EuroIntervention 2015;11 Suppl W: W92-W95.
51 Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: e57-185.
52 Gandhi S, Schwalm JD, Velianou JL, et al. Comparison of dual-antiplatelet therapy to mono-antiplatelet therapy after transcatheter aortic valve implantation: systematic review and meta-analysis. Can J Cardiol 2015; 31: 775-784.
53 Genereux P, Cohen DJ, Mack M, et al. Incidence, predictors,and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol 2014;64: 2605-2615.
54 Webb J, Rodes-Cabau J, Fremes S, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol 2012; 28: 520-528.
55 Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS).Eur J Cardiothorac Surg 2012; 42: S1-S44.
56 Giustino G, Mehran R, Veltkamp R, et al. Neurological outcomes with embolic protection devices in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2016; 9: 2124-2133.
57 Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis:The CLEAN-TAVI randomized clinical trial. JAMA 2016;316: 592-601.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- Restrictive perimembranous ventricular septal defect with left to right Shunt post urgent aortic balloon valvuloplasty and transcatheter aortic valve replacement
- The role of echocardiography and CT angiography in transcatheter aortic valve implantation patients
- Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis
- Antithrombotic therapy in TAVI
- Endocarditis after transcatheter aortic valve implantation: a current assessment
- TAVR in 2017―What we know? What to expect?
