Impact of seawater carbonate variables on post-larval bivalve calcification*
LI Jiaqi (李加琦) , MAO Yuze ( 毛玉澤) , JIANG Zengjie ( 蔣增杰) ,ZHANG Jihong ( 張繼紅) , BIAN Dapeng ( 卞大鵬) , FANG Jianguang ( 方建光) ,
1 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266071, China
3 Function Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
4 National Engineering and Research Center of Marine Shellfish, Weihai 264316, China
1 INTRODUCTION
Marine calcifiers, such as corals, coralline algae,and shellfish that produce calcium carbonate are vulnerable to ocean acidification (OA) (Anthony et al., 2008; Kuff ner et al., 2008; Pandolfiet al., 2011).The calcification rate of coral reefs (Maier et al.,2009; Jokiel, 2013; Maier et al., 2016) and coralline algae (Kuff ner et al., 2008; Semesi et al., 2009b) will decrease under acidified conditions based on the carbon dioxide partial pressure (pCO2) levels predicted at the end of this century. Larval shell growth and hardness of eastern oysters (Crassostrea virginica) decreases under highpCO2conditions(Anthony et al., 2008). Larval shells in several species of shellfish, such as mussels (Kurihara et al., 2009;Gazeau et al., 2010; Kelly et al., 2016), oysters(Kurihara et al., 2007; Parker et al., 2010; Waldbusser et al., 2016), abalone (Byrne et al., 2011; Crim et al.,2011; Li et al., 2013), and scallops (Talmage and Gobler, 2009; White et al., 2013; Wang et al., 2016)are malformed by OA. Larval (Miller et al., 2009;Talmage and Gobler, 2010) and adult bivalves(Gazeau et al., 2007; Pfister et al., 2016; Ries et al.,2016) will also suff er reduced calcification under the pH conditions predicted for the end of this century.The calcification response by these marine calcifiers to acidified environments is a main issue for OA-related studies to explore. However, the variables regulating calcification rate are controversial.
It is well known that marine calcifiers useto deposit their calcareous skeleton by the following reaction:(Frankignoulle et al., 1995). Therefore, a decline in calcification rates of calcifiers under acidified conditions might be attributed to increased [H+] or a decrease ofor dissolved inorganic carbon (DIC). Some researchers have proposed that calcification rate of coral (Madracisauretenra)responds to variations in bicarbonate concentration rather than carbonate concentration or pH (Jury et al.,2010). They observed a normal or even elevated calcification rate of corals at pH 7.6–7.8 when bicarbonate concentration was >1 800 μmol/L, but the calcification rate decreased if the bicarbonate concentration was lower under a normal pH condition.Other researchers believe that coral calcification is determined by the availability of therather than pH orˉ (Kleypas et al., 1999; Silverman et al.,2007; Marubini et al., 2008). Gazeau et al. (2007)reported that the calcification rates of mussels (Mytilus edulis) and Pacific oysters (Crassostreagigas) decline linearly with increasingpCO2, but they did not differentiate the roles of pH, bicarbonate, carbonate,or DIC concentration when determining the calcification rate.
To identify the key factors involved in biomineralization, calcification rates of calcifiers have been measured in manipulated CO2-carbonate systems. Gattuso et al. (1998) manipulated calcium concentration under constant pH and carbonate conditions to adjust the aragonite saturation level of seawater and found that the coral calcification rate increased if the aragonite saturation level was also increased. Langdon et al. (2000) manipulated both calcium and carbonate concentrations to obtain the required aragonite saturation level of seawater and found consistent regulated changes in calcification rate of coral to the manipulated aragonite saturation.Waldbusser et al. (2015) manipulated seawater DIC with mineral acids and bases and reported that shell development of two bivalve larvae,C.gigas, andMytilusgalloprovincialis, are dependent on carbonate saturation state, and not onpCO2or pH. Another study reported that limiting DIC strongly reduced calcification, despite a high(Thomsen et al.,2015). They believed that mussels utilizerather thanas the inorganic carbon source for biomineralization. To distinguish the key factors determining calcification rate of post-larval shellfish,conditions with decreased pH or lowered DIC were created by bubbling CO2-enriched air or adding a hydrochloride solution to natural seawater.Calcification rates of juvenile blue mussels (M.edulis)and Zhikong scallops (Chlamysfarreri) were measured in these different carbonate systems.Significant correlations were observed between calcification rate and DIC/[H+] andin both species. We also found that calcification rate of these two bivalve species increased under elevated pH conditions.
2 MATERIAL AND METHOD
2.1 Animal collection and cultivation
All animals used in the experiment were collected from Sangou Bay, Weihai, China. The shell heights of the Zhikong scallops and blue mussels sampled from the local aquaculture populations were 3.53±0.25 cm and 3.55±0.41 cm, respectively. Individuals were acclimated to experimental conditions for 2 weeks before being used to measure calcification rate.Animals were reared together withUlvapertusaKjellman in 1-L beakers placed in an illumination incubator. The incubation temperature was 20°C and the light-dark photoperiod was 6 min light: 3 min dark. The pH of the experimental seawater was maintained at 8.20±0.10 by adjusting the amount ofU.pertusain the beaker. No aeration was used, as the mollusks obtained sufficient oxygen from the photosynthetic activity ofU.pertusa(dissolved oxygen [DO]>6.5 mg/L). Animals in each beaker were fed about a 50 mL suspension ofPlatymonassp.(about 2×106/mL seawater) twice daily.
2.2 Manipulation of the seawater carbonate system
The pH level or DIC was adjusted in the seawater carbonate system. The seawater was bubbled with CO2-enriched air, and pH was adjusted down to the required level.U.pertusawas added and incubated in the illumination incubator to increase seawater pH. A two-step method was applied to decrease DIC of seawater while the pH remained constant. In the first step, seawater pH was increased by incubating withU.pertusain the illumination incubator. Then, a mixture of seawater and concentrated hydrochloric acid (1:1) was added to the experimental seawater to reduce pH down to pH 8.2 in the second step. After repeating these steps twice, we acquired experimental seawater with an extremely low DIC level and normal pH (8.2). Experimental seawater with the requiredDIC level was prepared by mixing seawater with a low level of DIC and natural seawater.

Table 1 Initial water temperature, salinity, total alkalinity (TA), dissolved inorganic carbon (DIC), pH, and carbonate saturation state (Ω Cal) of the experimental seawater
Water temperature and salinity were measured using a combined electrode (YSI ProPlus; YSI,Yellow Springs, OH, USA), and pH was measured using a pH electrode (Thermo Scientific 3-Star,ORION; Waltham, MA, USA). The pH electrode was calibrated daily with buff ers traceable to the NIST(NBS) standard. The DO concentration in the experimental seawater was measured using a DO electrode (Multi 3420; WTW, Weilheim, Germany).Total alkalinity (TA) was measured within hours after sampling using an automatic titrator (848 Titrino plus;Metrohm, Riverview, FL, USA).
2.3 Calcification rate measurement
We used the following equation to calculate the calcification rate (Gazeau et al., 2007):
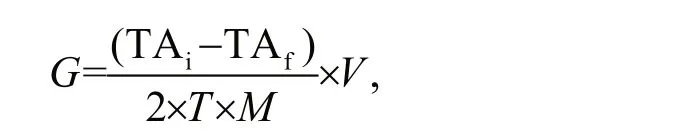
whereGis calcification rate (μmol/(g·h)), TAiis initial TA of the experimental seawater (μmol/L), TAfis the final TA of the experimental seawater (μmol/L),Tis the experimental duration (h),Mis whole wet weight of the experimental animals (g), andVis the volume of the experimental seawater (L).
Four to six animals and about 3 g ofU.pertusawere placed in a 500-mL beaker filled with 400 mL of seawater and the required carbonate system. The beaker was placed in the illumination incubator with a light-dark photoperiod of 6 min light: 3 min dark. The animals were incubated in the beaker for 1 hour while the amount ofU.pertusawas adjusted every 15 min to maintain a stable pH. Before measuring the calcification rate, the incubating seawater was replaced with fresh seawater. During the 2 h incubation for the calcification rate measurement, the amount ofU.pertusawas adjusted every 30 min to maintain pH within ±0.1 units. Seawater that was used to measure pH, salinity, and TA was sampled before and immediately after the incubation. Mean pH, TA, water temperature, and salinity were used to calculate thepCO2,DIC, calcite, and aragonite saturation values (Table 1) using CO2SYS (Pierrot et al., 2006). Four replicate groups were employed for the calcification rate measurements of each species.
2.4 Statistics
The correlation coefficient between carbonate variables and the calcification rates of blue mussels and Zhikong scallops and its significance was calculated using Pearson’s correlation analysis and SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A two-tailed procedure was used to test the significance of the correlation coefficient, and aP<0.05 was considered significant for all tests.
3 RESULT
Calcification rate of juvenile blue mussels was associated with the seawater carbonate system (Fig.1).However, different results were found between the two different carbonate systems. Positive correlations were observed between calcification rate of juvenile blue mussels and pH, DIC/[H+] orwhereas negative correlations were observed between calcification rate and DIC orduring incubations under high or low pH conditions (Fig.1,column a). Positive correlations were observed between calcification rate and DIC,, DIC/[H+], andduring the incubation with low DIC and normal pH, whereas no correlation was detected between calcification rate and pH (Fig.1, column b).

Fig.1 Plot of seawater pH,, dissolved inorganic carbon (DIC), DIC/[H+], andvs. calcification rate of blue mussels
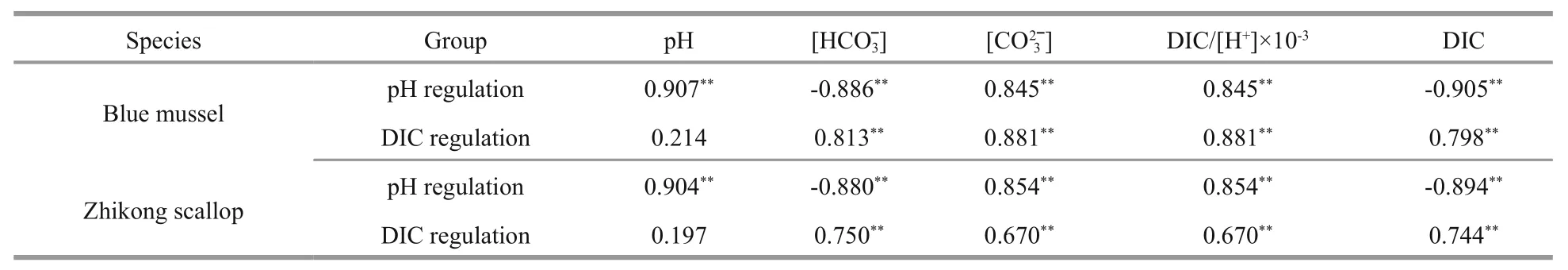
Table 2 Correlation coefficients between carbonate variables and calcification rate of blue mussels and Zhikong scallops
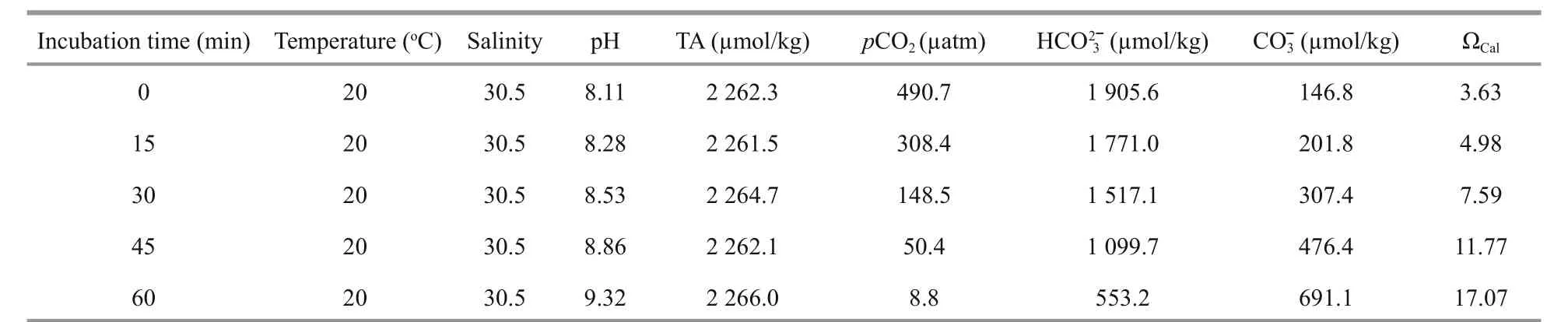
Table 3 Variations in pH, total alkalinity (TA), and the carbonate system variables in the experimental seawater incubated with Ulva pertusa for 60 min
It was clear that the calcification rate of juvenile mussels was positively correlated (P<0.05) with and DIC/[H+] andin both carbonate systems (Table 2).
Similar results were found in Zhikong scallops(Fig.2). Positive correlations were observed between calcification rate of Zhikong scallops and pH, DIC/[H+], andwhereas negative correlations were observed between calcification rate and DIC andunder low and high pH conditions (Fig.1,column a). Positive correlations were detected between calcification rate and DIC,, DIC/[H+], andwhereas no significant correlation was observed between calcification rate and pH in seawater with a low DIC level and normal pH (Fig.1,column b). Only DIC/[H+] andshowed a positive dominant effect (P<0.05) when determining calcification rate of juvenile Zhikong scallops in both carbonate systems (Table 2).
4 DISCUSSION
It has been reported that ocean surface seawater pH will drop to about 7.7 (IPCC, 2007) by the end of this century. Marine calcifiers, such as mollusks, may face a stressful future as it will be more difficult for them to generate calcareous shells (Gazeau et al., 2007). It seems that the decrease in calcification rate under acidified conditions is directly attributed to higher[H+]. However, higher [H+] changes the seawater carbonate system, which may play a determining role in biological calcium carbonate deposition. Therefore,it is important to isolate the main factor from acidified seawater that is responsible for the decrease in calcification rate. We measured calcification rate of blue mussels and Zhikong scallops under conditions created in different carbonate systems. The results support that the calcifying ability of post-larval shellfish was correlated withorrather than pH, DIC, or
Experimental animals continuously released CO2into seawater during the calcification rate measurement. If this CO2is absorbed, experimental seawater pH would decrease. Thus, we introducedU.pertusainto the incubation system to remove the CO2. However, this raised concern about the accuracy of the calcification measurement, as the seawater carbonate system would change in response to the photosynthetic activity ofU.pertusa. The carbonate system variables and pH of the experimental seawater changed in response to the photosynthetic activity ofU.pertusa, but the TA of seawater remained unchanged (Table 3). As a result, addingU.pertusato the incubation system did not affect the calcification rate calculation.

Fig.2 Plot of seawater pH, ˉ], dissolve inorganic carbon (DIC), DIC/[H+ ], and [ vs. calcification rate of Zhikong scallops
Several studies have investigated the dominant variables in carbonate systems while calcium carbonate deposition rate was determined in marine calcifiers. Jury et al., (2010) reported that calcification rate of coral (Madracisauretenra) responds to variations in bicarbonate concentration rather than carbonate concentration. In contrast, Jokiel (2013)found that the calcification rates of the coralPorites rusand the crustose coralline algae (CCA)Hydrolithon onkodesare correlated with the DIC/[H+] andMoreover, Gattuso et al. (1998) manipulated a carbonate chemistry system by adjusting calcium concentration and found that calcification rate of the coralStylophorapistillatawas affected by carbonate saturation state. Waldbusser et al. (2015) reported that larval bivalve shell development and growth are dependent on seawater carbonate saturation state, but not onpCO2or pH. They did not discuss the correlation between calcification rate of larval bivalves with DIC/[H+]. Jokiel (2013) reported that calcification of coral reefs is driven by the DIC/[H+] ratio and thathas no physiological relevance. However, the carbonate saturation state orin natural seawater is tightly correlated with the DIC/[H+] ratio, and carbonate saturation state is determined byTherefore, it is difficult to determine whetheror the DIC/[H+] ratio is the key variable when calculating calcification rate. Our findings support that calcification rates of post-larval blue mussels and Zhikong scallops are correlated withand DIC/[H+] ratio rather than DIC,or pH.
Several biological processes of calcifying organisms are stressed under acidified conditions (Orr et al., 2005). We also found that biological calcification of bivalves is susceptible to seawater acidification.No signs of acclimation to a saturated state by a coral reef have been observed, as no significant difference in calcification rate was observed between short-term and long-term incubation (Pandolfiet al., 2011). This result reminds us that some species of marine calcifiers may lack the capacity to generate a calcium carbonate skeleton under acidified conditions even after longterm acclimation. These calcifying organisms may face severe challenges in the acidified future.However, blue mussels generated a calcareous shell under extremely low levels of carbonate saturation in a long-term incubation experiment (Thomsen and Melzner, 2010). This finding is markedly different from short-term research (White et al., 2013), in which blue mussels nearly lost their calcifying ability under similar conditions to that of the long-term incubation. It seems that some bivalves may possess the mechanisms to acclimate to lowor DIC/[H+] conditions. As a result, more studies are required to understand the response of shellfish calcification to the seawater carbonate system.
This study may provide some novel insight about the benefit that shellfish can acquire in integrated shellfish-algae aquaculture. Algae are helpful for producing oxygen and absorbing waste in integrated aquaculture systems (Mao et al., 2009; Tang et al.,2011; Chopin et al., 2012). Algae may also be helpful in creating a comfortable carbonate environment for shellfish to calcify. Algae provide oxygen, absorb nutrients, and are capable of adjusting the carbonate system. Seawater pH in a low water exchange bay can increase from 7.9 to 8.9 because of the photosynthetic activity of seaweeds (Semesi et al., 2009a), and a high seaweed biomass can easily elevate the pH of seawater in confined tidal pools (Clements and Chopin, 2016).This higher seawater pH would then increase the level ofor the DIC/[H+] ratio, which is beneficial for biomineralization of shellfish. Therefore, algae can help enhance the calcium carbonate deposition efficiency of shellfish in an integrated aquaculture system.
5 CONCLUSION
Our findings show that calcification rates of postlarval blue mussels and Zhikong scallops are correlated withand the DIC/[H+] ratio rather than DIC,or pH. However, the calcification rate differed in short-term and long-term experiments.As a result, more studies are required to understand the response of shellfish calcification to the seawater carbonate system.
6 ACKNOWLEDGEMENT
We thank Mr. James Yang Xie from Hong Kong Baptist University (HKBU) for editing our manuscript.
Anthony K R N, Kline D I, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders.Proceedings oftheNationalAcademyofSciencesoftheUnitedStates ofAmerica,105(45): 17 442-17 446.
Byrne M, Ho M, Wong E, Soars N A, Selvakumaraswamy P,Shepard-Brennand H, Dworjanyn S A, Davis A R. 2011.Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean.Proceedingsofthe RoyalSocietyB:BiologicalSciences,278(1716): 2 376-2 383.
Chopin T, Cooper J A, Reid G, Cross S, Moore C. 2012. Openwater integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture.Reviewsin Aquaculture,4(4): 209-220.
Clements J C, Chopin T. 2016. Ocean acidification and marine aquaculture in North America: potential impacts and mitigation strategies.ReviewsinAquaculture,56(3): 182-196
Crim R N, Sunday J M, Harley C D G. 2011. Elevated seawater CO2concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana).JournalofExperimentalMarineBiology andEcology,400(1-2): 272-277.
de Putron S J, McCorkle D C, Cohen A L, Dillon A B. 2011.The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals.CoralReefs,30(2): 321-328.
Frankignoulle M, Pichon M, Gattuso J P. 1995. Aquatic calcification as a source of carbon dioxide.In: Beran M A ed. Carbon Sequestration in the Biosphere: Processes and Prospects. Springer, Berlin Heidelberg. p.265-271.
Gattuso J P, Frankignoulle M, Bourge I, Romaine S,Buddemeier R W. 1998. effect of calcium carbonate saturation of seawater on coral calcification.Globaland PlanetaryChange,18(1-2): 37-46.
Gazeau F, Gattuso J P, Dawber C, Pronker A E, Peene F, Peene J, Heip C H R, Middelburg J J. 2010. effect of ocean acidification on the early life stages of the blue musselMytilusedulis.Biogeosciences,7(7): 2 051-2 060.
Gazeau F, Quiblier C, Jansen J M, Gattuso J P, Middelburg J J,Heip C H R. 2007. Impact of elevated CO2on shellfish calcification.GeophysicalResearchLetters,34(7):L07603.
IPCC. 2007. Climate Change 2007: The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press,Cambridge, UK and New York, USA.
Jokiel P L. 2013. Coral reef calcification: carbonate,bicarbonate and proton flux under conditions of increasing ocean acidification.ProceedingsoftheRoyalSocietyB:BiologicalSciences,280(1764): 20130031.
Jury C P, Whitehead R F, Szmant A M. 2010. effects of variations in carbonate chemistry on the calcification rates ofMadracisauretenra(=MadracismirabilissensuWells,1973): bicarbonate concentrations best predict calcification rates.GlobalChangeBiology,16(5): 1 632-1 644.
Kelly M W, Padilla-Gami?o J L, Hofmann G E. 2016. HighpCO2aff ects body size, but not gene expression in larvae of the California mussel (Mytiluscalifornianus).ICES JournalofMarineScience,73(3): 962-969.
Kleypas J A, Buddemeier R W, Archer D, Gattuso J P, Langdon C, Opdyke B N. 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs.Science,284(5411): 118-120.
Kuff ner I B, Andersson A J, Jokiel P L, Rodgers K S, Mackenzie F T. 2008. Decreased abundance of crustose coralline algae due to ocean acidification.NatureGeoscience,1(2):114-117.
Kurihara H, Asai T, Kato S, Ishimatsu A. 2009. effects of elevatedpCO2on early development in the musselMytilus galloprovincialis.AquaticBiology,4(3): 225-233.
Kurihara H, Kato S, Ishimatsu A. 2007. effects of increased seawater pCO2on early development of the oysterCrassostreagigas.AquaticBiology,1(1): 91-98.
Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J,Marubini F, Aceves H, Barnett H, Atkinson M J. 2000.effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef.Global BiogeochemicalCycles,14(2): 639-654.
Li J Q, Jiang Z J, Zhang J H, Qiu J W, Du M R, Bian D P, Fang J G. 2013. Detrimental effects of reduced seawater pH on the early development of the Pacific abalone.Marine PollutionBulletin,74(1): 320-324.
Maier C, Hegeman J, Weinbauer M G, Gattuso J P. 2009.Calcification of the cold-water coralLopheliapertusa,under ambient and reduced pH.Biogeosciences,6(8):1 671-1 680.
Maier C, Popp P, Sollfrank N, Weinbauer M G, Wild C,Gattuso J P. 2016. effects of elevatedpCO2and feeding on net calcification and energy budget of the Mediterranean cold-water coralMadreporaoculata.TheJournalof ExperimentalBiology,219(20): 3 208-3 217.
Mao Y Z, Yang H S, Zhou Y, Ye N H, Fang J G. 2009. Potential of the seaweedGracilarialemaneiformisfor integrated multi-trophic aquaculture with scallopChlamysfarreriin North China.JournalofAppliedPhycology,21(6): 649-656.
Marubini F, Ferrier-Pagès C, Furla P, Allemand D. 2008. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism.Coral Reefs,27(3): 491-499.
Miller A W, Reynolds A C, Sobrino C, Riedel G F. 2009.Shellfish face uncertain future in high CO2world:influence of acidification on oyster larvae calcification and growth in estuaries.PLoSOne,4(5): e5661.
Orr J C, Fabry V J, Aumont O, Bopp L, Doney S C, Feely R A,Gnanadesikan A, Gruber N, Ishida A, Joos F, Key R M,Lindsay K, Maier-Reimer E, Matear R, Monfray P,Mouchet A, Najjar R G, Plattner G K, Rodgers K B,Sabine C L, Sarmiento J L, Schlitzer R, Slater R D,Totterdell I J, Weirig M F, Yamanaka Y, Yool A. 2005.Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Nature,437(7059): 681-686.
PandolfiJ M, Connolly S R, Marshall D J, Cohen A L. 2011.Projecting coral reef futures under global warming and ocean acidification.Science,333(6041): 418-422.
Parker L M, Ross P M, O’Connor W A. 2010. Comparing the effect of elevatedpCO2and temperature on the fertilization and early development of two species of oysters.Marine Biology,157(11): 2 435-2 452.
Pfister C A, Roy K, Wootton J T, McCoy S J, Paine R T,Suchanek T H, Sanford E. 2016. Historical baselines and the future of shell calcification for a foundation species in a changing ocean.ProceedingsoftheRoyalSocietyB:BiologicalSciences,283(1832): 20160392.
Pierrot D, Lewis E, Wallace D W R. 2006. MS Excel program developed for CO2system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN.
Ries J B, Ghazaleh M N, Connolly B, Westfield I, Castillo K D. 2016. Impacts of seawater saturation state (ΩA=0.4-4.6) and temperature (10, 25°C) on the dissolution kinetics of whole-shell biogenic carbonates.Geochimicaet CosmochimicaActa,192: 318-337.
Semesi I S, Beer S, Bj?rk M. 2009a. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow.MarineEcologyProgressSeries,382: 41-47.
Semesi I S, Kangwe J, Bj?rk M. 2009b. Alterations in seawater pH and CO2affect calcification and photosynthesis in the tropical coralline alga,Hydrolithonsp. (Rhodophyta).Estuarine,CoastalandShelfScience,84(3): 337-341.
Silverman J, Lazar B, Erez J. 2007. effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef.JournalofGeophysical Research:Oceans,112(C5): C05004.
Talmage S C, Gobler C J. 2009. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenariamercenaria),bay scallops (Argopectenirradians), and Eastern oysters(Crassostreavirginica).LimnologyandOceanography,54(6): 2 072-2 080.
Talmage S C, Gobler C J. 2010. effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish.Proceedingsofthe NationalAcademyofSciencesoftheUnitedStatesof America,107(40): 17 246-17 251.
Tang Q S, Zhang J H, Fang J G. 2011. Shellfish and seaweed mariculture increase atmospheric CO2absorption by coastal ecosystems.MarineEcologyProgressSeries,424: 97-105.
Thomsen J, Haynert K, Wegner K M, Melzner F. 2015. Impact of seawater carbonate chemistry on the calcification of marine bivalves.Biogeosciences,12(14): 4 209-4 220.
Thomsen J, Melzner F. 2010. Moderate seawater acidification does not elicit long-term metabolic depression in the blue musselMytilusedulis.MarineBiology,157(12): 2 667-2 676.
Waldbusser G G, Gray M W, Hales B, Langdon C J, Haley B A, Gimenez I, Smith S R, Brunner E L, Hutchinson G.2016. Slow shell building, a possible trait for resistance to the effects of acute ocean acidification.Limnologyand Oceanography,61(6): 1 969-1 983.
Waldbusser G G, Hales B, Langdon C J, Haley B A, Schrader P, Brunner E L, Gray M W, Miller C A, Gimenez I. 2015.Saturation-state sensitivity of marine bivalve larvae to ocean acidification.Nat.ClimateChang,5(3): 273-280.
Wang W M, Liu G X, Zhang T W, Chen H J, Tang L, Mao X W. 2016. effects of elevated seawater pCO2on early development of scallopArgopectenirradias(Lamarck,1819).JournalofOceanUniversityofChina,15(6):1 073-1 079.
White M M, McCorkle D C, Mullineaux L S, Cohen A L.2013. Early exposure of bay scallops (Argopecten irradians) to high CO2causes a decrease in larval shell growth.PLoSOne,8(4): e61065.
 Journal of Oceanology and Limnology2018年2期
Journal of Oceanology and Limnology2018年2期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Hydroacoustic estimates of fish biomass and spatial distributions in shallow lakes*
- A comparison between benthic gillnet and bottom trawl for assessing fish assemblages in a shallow eutrophic lake near the Changjiang River estuary*
- Morphological beak differences of loliginid squid, Uroteuthis chinensis and Uroteuthis edulis, in the northern South China Sea*
- Muelleria pseudogibbula, a new species from a newly recorded genus (Bacillariophyceae) in China*
- Planaxidae (Mollusca, Gastropoda) from the South China Sea*
