Controllable Synthesis of Mixed-Phase TiO2 with Small Anatase and Rutile Particle and Its Enhanced Photocatalytic Activity
Bai Yu; Li Kuan; Chen Xuebing; Zhang Jing
(School of Chemistry and Materials Science, Liaoning Shihua University, Fushun 113001)
1 Introduction
Significant volumes of wastewater with high concentration of aliphatic and aromatic hydrocarbons, generated in petroleum and petrochemical industries, are considered to be the one of the factors that pollutes the environment.The heterogeneous photocatalytic oxidation process using semiconductor photocatalysts for environmental remediation has been extensively studied[1-3]. In the past few decades, titanium dioxide (TiO2) has been a popular photocatalyst for the treatment of organic pollutants in water and air because of its unique advantages, such as nontoxicity, and chemical stability[4-6].
However, the ef ficiency of TiO2is still low for practical application. It is reported that the low ef ficiency of TiO2is mainly attributed to the fast recombination of the photoinduced electrons and holes[7]. Many strategies have been performed to improve the rate for separation of the photoinduced electrons and holes and the photocatalytic performance of TiO2. For example, suitable morphology design[8], doping[9-11], forming a semiconductor heterojunction by combining TiO2with other metal oxide semiconductors[12-15], and other approaches could be used,respectively.
Generally, TiO2exists mainly in three phases including rutile, anatase, and brookite. Moreover, anatase and brookite are usually transformed into rutile after calcination at high temperature. Our previous reports[16-18]have revealed that the formation of phase junction composed of anatase and rutile phases can significantly promote the spatial separation of photogenerated electrons and holes,which are capable of boosting the photocatalytic performance of TiO2.
Tiwari A, et al.[19]prepared 1-D anatase and rutile TiO2heterojunction nanorods using the solvothermal method.The heterojunction formed between anatase and rutile is beneficial to fast interfacial electron transfer and better
The sol–gel method[21-23], the chemical precipitation method[24], and many physical and chemical approaches[25-26]are usually used for the synthesis of the anatase TiO2.Moreover, the formation of pure rutile generally involves the transformation from anatase at a high calcination temperature of >700oC, which can result in sintering of the TiO2nanostructures. Thus, the obtained TiO2with mixed phases exhibits large rutile particles and anatase particles. We all know that the morphology, particle size,and surface area of TiO2have great in fluence on its photocatalytic activity. The large particle size and very low surface area usually induce a low photocatalytic activity.Therefore, synthesis of the mixed crystalline phases of TiO2with both small-particle-size anatase and rutile is an interesting topic of research.
Additionally, our previous results[27]indicate that the anatase-rutile ratio is one of the main factors that can in fluence the photocatalytic activity of TiO2with phase junction. Thus, it is still of significance to develop an effective method to control the phase composition of anatase-rutile in TiO2. However, to our knowledge, the study about controlling the TiO2with adjustment of both the particle size and the phase composition of anatase and rutile is rare.
The aim of this work is to develop an effective method toward the control over the crystalline phase and the particle size in TiO2by means of the hydrothermal approach.And the photocatalytic activity of TiO2samples is to be investigated by the photodegradation of Rhodamine B(RhB).
2 Experimental
2.1 Materials
Tetrabutyl titanate (TBOT, Ti[O(CH2)3CH3]4), triethanolamine (TEOA, N(CH2CH2OH)3), and nitric acid (HNO3)were purchased from the Sinopharm Chemical Reagent Co., Ltd (China). All the above chemicals were of analytical reagent grade and were directly used without further purification. Deionized water was prepared in our own laboratory.
2.2 Sample preparation
Anatase/rutile TiO2nanoparticles were prepared via the hydrothermal method. TBOT was mixed with TEOA at the same volume ratio under magnetic stirring to form a transparent solution. After the above transparent solution was placed in water bath at a specified curing temperature(T) for several hours (t), the pH value was adjusted to 0.2 by adding 2.5 mol/L HNO3. Then, it was transferred into a Teflon lined autoclave and was subject to ageing at 140 °C for 72 h. The precipitate was collected and washed several times with deionized water, and then dried at 60oC overnight. The obtained samples were labeled as TiO2-T-t, with T representing the curing temperature and t representing the curing time, respectively.
2.3 Characterization
The crystalline structures of the as-synthesized samples were characterized by X-ray diffraction, using a Rigaku Rotaflex Ru-200B diffractometer with Cu Kα radiation(λ=0.15418 nm) at a speed of 5 (°)/min. The particle size and morphology of samples were obtained on a HITACHI HT7700 transmission electron microscope. The optical properties of samples were tested on the assembled surface photovoltage equipped with a SR830 DSP lock-in amplifier of Stanford Research Systems.
2.4 Photocatalytic reaction
The photocatalytic activity of the TiO2-T-t samples was investigated through photocatalytic degradation of Rhodamine B (RhB). Typically, 0.05 g of TiO2-T-t sample were dispersed into 60 mL of 10 mg/L RhB solution in a beaker. Prior to the light irradiation, the suspension was kept in dark for 30 min at room temperature under magnetic stirring for achieving the adsorption-desorption equilibrium. A 250-W Hg lamp (λ= 365 nm, made by the Shanghai Yaming Lighting Co., Ltd.) was used as the illumination source for the reaction system. Then 3 ml of the suspension liquid were collected every 30 min and centrifuged for obtaining the absorbance of RhB analyzed by a spectrophotometer (UV mini-1240 spectrophotometer,made by the Shimadzu (China) Co., Ltd.) at a wavelength of 553 nm. The blank study carried out with no radiation showed that a merely insignificant photolysis can be neglected for RhB.
3 Results and Discussion
3.1 Effect of curing temperature on the crystalline structure
The influence of the curing temperature (20oC, 40oC,60oC, and 80oC) on the crystalline phase was investigated by XRD, with the results shown in Figure 1. At a curing temperature of 20oC, the broad peaks at 2θ=25.5°,37.9°, 48.2°, and 53.8° were observed. These peaks represent the indices of (101), (004), (200), and (105) planes of anatase phase, respectively. This indicates that the TiO2-20oC-48 h is in the anatase phase. When the curing temperature was increased to 40oC and 60oC, two tiny peaks at 2θ=27.4° and 36.1° were observed. These two peaks were ascribed to (110) and (101) planes of rutile phase.These results showed that the TiO2-40oC-48 h or TiO2-60oC-48 h was dominated by the anatase phase with a small amount of rutile phase.
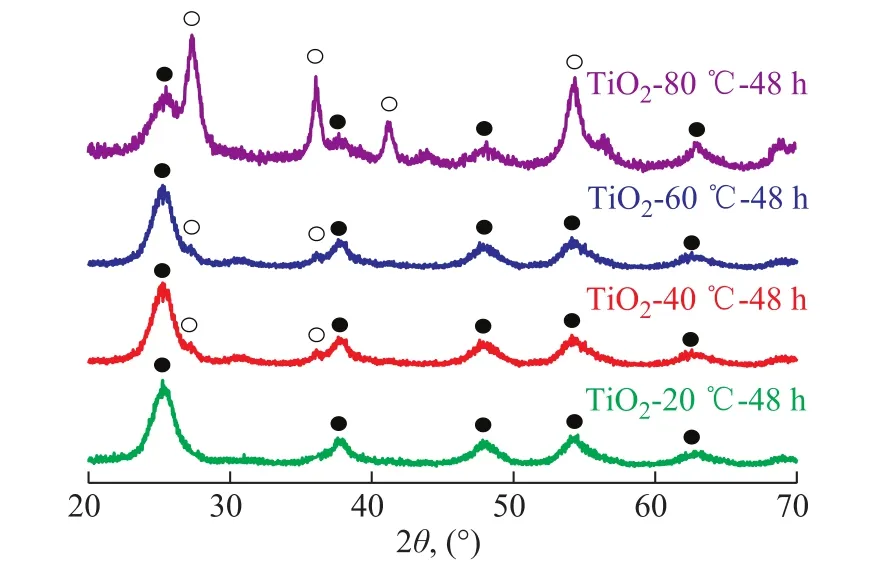
Figure 1 XRD patterns of TiO2-T-48 h
The relative weight fractions of rutile phase, WR, are calculated using the integrated intensity of the (101) peak of anatase and the (110) peak of rutile according to the following formula[28]:
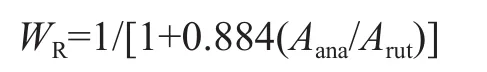
where Aanaand Arutrepresent the X-ray integrated intensity of anatase (101) and rutile (110) diffraction peaks, respectively. According to the above method, the rutile content of the TiO2-40oC-48 h, and TiO2-60oC-48 h samples was calculated to be 2%, and 4%, respectively. As shown in Figure 1, the diffraction patterns of rutile phase became predominant with the increase of curing temperature to 80oC, and the rutile content increased to 83%. Thus, it can be concluded that the curing temperature has the influence on the crystalline structure and the crystalline composition. The high curing temperature is bene ficial to the formation of the rutile phase.
Based on the XRD patterns, the crystallite sizes of TiO2-T-48 h samples are calculated by using the Scherrer formula. The crystallite sizes of the TiO2-T-48 h samples are shown in Table 1. It can be seen from Table 1 that the particle size of the TiO2-20oC-48 h with anatase phase is 5.1 nm. Interestingly, the anatase phase shows approximately the same size (~5 nm) as that of rutile phase in the TiO2-80oC-48 h sample containing the mixed phases.
3.2 Effect of curing time on the crystalline phase
We tried to change the curing time in order to control the phase composition in the TiO2-80oC-t sample. Figure 2 displays the XRD patterns of TiO2-80oC-t samples. Only the typical peaks of the anatase phase were observed in the TiO2-80oC-24 h sample. As the curing time was prolonged to 47 h, the typical peaks of rutile phase at 2θ=27.4° and 36.1° were observed, and the rutile mass ratio was calculated to be 18 %. When the curing temperature was further increased to 48 h, the rutile content was increased to 83 %. These results indicate that curing time plays a role in the formation of TiO2crystalline phase.Additionally, it was found that the particle size in the TiO2-80oC-t samples almost unchanged when the curing time increased from 24 h to 48 h as evidenced by the data listed in Table 1.

Figure 2 XRD patterns of TiO2-80 oC-t
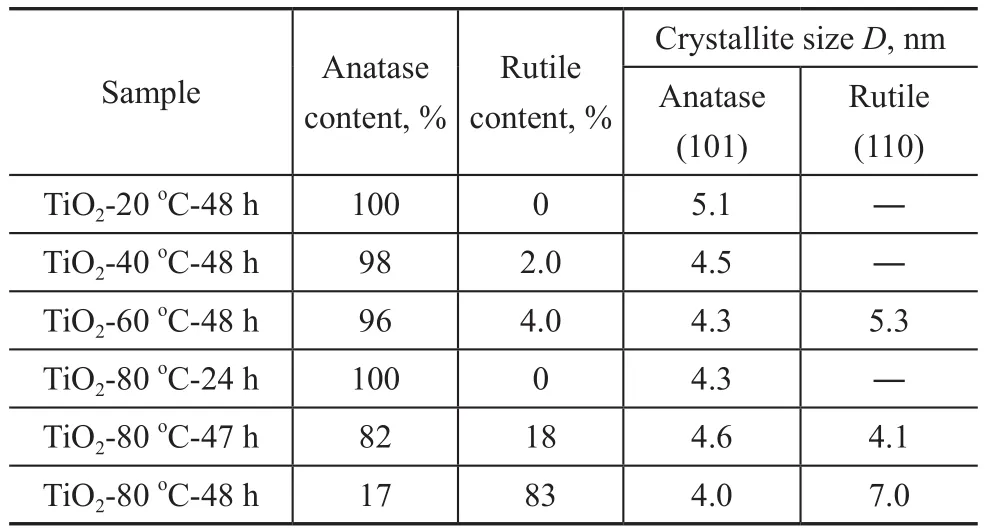
Table 1 Crystallite size and rutile content identified by XRD data
3.3 Morphology analysis
TEM was used to investigate the morphology of the TiO2-80oC-48 h sample, with the TEM images shown in Figure 3. The TiO2-80oC-48 h sample showed agglomeration,and the TEM image indicated that the particle size of the TiO2-80oC-48 h sample was about 5 nm, which was in agreemeent with the results of XRD analysis. Moreover,the particle size of the TiO2-80oC-48 h sample was uniform, although anatase and rutile particles could not be distinguished clearly from Figure 3. Upon combining with the results of XRD analyses (Figure 1 and Figure 2),the small TiO2particles with mixed phases of anatase and rutile were successfully prepared by the simple method.
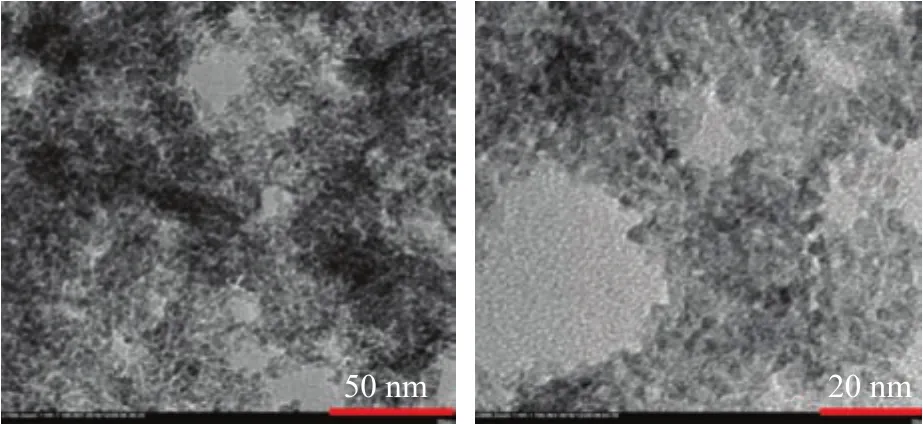
Figure 3 TEM images of TiO2-80 oC-48 h
3.4 Photocatalytic activity for the degradation of RhB
RhB is an important chemical used widely in industrial production, which often causes environmental pollution[29].Generally, the semiconductor photocatalysis involves the generation of electrons in the conduction band and holes in the valence band within a semiconductor upon light irradiation at energies equal to or greater than the band gap of the semiconductor[30]. The photocatalytic reaction is initiated by the band gap excitation of TiO2, followed by generation of various reactive species (Eqs. 1-8)[31].
The formed hydroxyl radical ?OH can oxidize organic pollutants, and the electrons in the CB can participate in the reduction process.
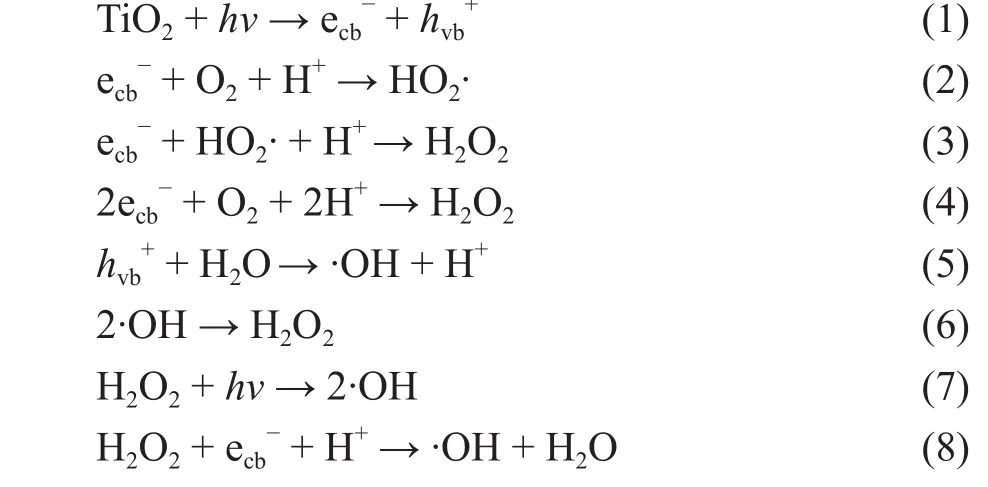
The photocatalytic activity for the degradation of RhB on the various samples was evaluated under UV light irradiation, with the results presented in Figure 4. Only 36% of RhB were degraded by TiO2-20oC-48 h sample as shown in Figure 4a. The photocatalytic activity of TiO2-40oC-48 h sample and TiO2-60oC-48 h sample was comparable and increased to 57% and 60%, respectively. The maximum photocatalytic activity was observed for the TiO2-80oC-48 h sample (80%). The above-mentioned samples exhibited the similar particle size as depicted in Table 1. According to the XRD results (Figure 1), TiO2-20oC-48 h sample was mostly composed of the anatase phase, while TiO2-40oC-48 h,TiO2-60oC-48 h, and TiO2-80oC-48 h samples were dominated by the mixed phases of anatase and rutile. Therefore,the particle size might not the main reason leading to the difference in the photocatalytic activity, while the higher photocatalytic activity of the TiO2-40oC-48 h, TiO2-60oC-48 h, and TiO2-80oC-48 h samples could be attributed to the formation of the phase junction between anatase and rutile in these samples, which could effectively inhibit the recombination of photo-generated electron-hole pairs.
The surface photovoltage (SPV) spectroscopy was applied to investigate the behavior of the photogenerated charges in TiO2-T-48 h and TiO2-80oC-t samples, with the results shown in Figure 5. The surface photovoltage method is a well-established technique for the characterization of the carrier separation and transfer behavior with the aid of light[32-33]. Generally, the higher the SPV signal,the higher the separation rate of photoinduced charge carriers would be[34-35].

Figure 4 The photocatalytic activity for the degradation of RhB on (a) TiO2-T-48 h; and (b) TiO2-80 oC-t samples
An apparent SPV response in the UV light range was observed for TiO2-20oC-48 h based on the data of Figure 5a.Obviously, the TiO2-40oC-48 h, TiO2-60oC-48 h, and TiO2-80oC-48 h samples with the mixed phases exhibited stronger SPV response than the TiO2-20oC-48 h sample containing the pure anatase phase. This result implies that the improved separation of photoinduced electron-hole pairs was achieved by the formation of the phase junction in these three samples. Thus, a high photocatalytic activity was reached in the TiO2-T-48 h sample with mixed phases (Figure 4a). Moreover, the TiO2-80oC-48 h sample showed a stronger SPV response, as compared with TiO2-40oC-48 h and TiO2-60oC-48 h samples, although they were dominated by mixed phases. This fact indicates that the phase composition in the phase junction is one of the main factors affecting the separation rate of photoinduced electron-hole pairs.
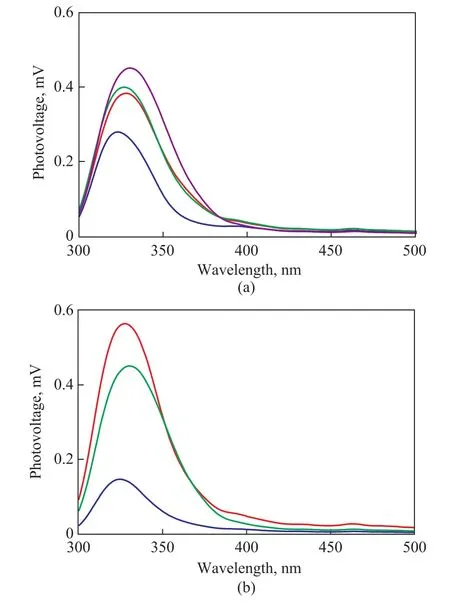
Figure 5 Surface photovoltage (SPV) spectra of (a)TiO2-T-48 h sample; and (b) TiO2-80 oC-t samples
The phenomenon confirming that the formation of the phase junction is beneficial to the improvement of the photocatalytic activity is also observed in the TiO2-80oC-t samples. Compared with the TiO2-80oC-24 h sample with anatase phase, the photocatalytic activity is significantly improved for the TiO2-80oC-47 h and TiO2-80oC-48 h samples with mixed phases. The improvement in the photocatalytic degradation of RhB can be ascribed to the improvement of the charge separation by phase junction(Figure 6), which is verified by Figure 5b. It can be seen that the observed SPV response intensity was significantly strengthened in the TiO2-80oC-47 h and TiO2-80oC-48 h samples with mixed phases. Moreover, it can be further observed that the optimal amount of rutile phase and anatase phase was found to be 18 % and 82 %, as shown in Figure 4b and Figure 5b, respectively.
4 Conclusions
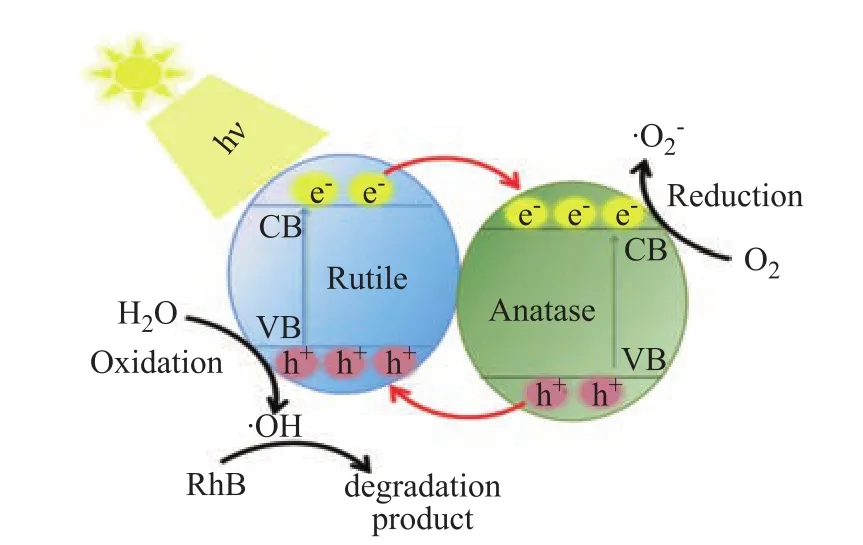
Figure 6 Proposed mechanism of the photocatalytic degradation of RhB on TiO2
A hydrothermal method was used to prepare TiO2nanoparticles with tunable crystalline phase. The influence of curing temperature and curing time before hydrothermal treatment on the crystalline phase, phase composition,morphology, and particle size of TiO2was studied in detail.It is observed that the curing temperature and the curing time have an obvious effect on the crystalline phase and phase composition of TiO2, albeit without obvious effect on the particle size of TiO2. Thus, the crystalline phase and phase composition of TiO2with small anatase particles (~5 nm) and rutile particles (~5 nm) were successfully tuned by changing the curing temperature and the curing time.Moreover, the photocatalytic properties of the synthesized TiO2nanostructures were evaluated in terms of the photodegradation of RhB. TiO2with the anatase and rutile phase junction exhibited the higher photocatalytic activity than the single-phase TiO2. This improvement of the photocatalytic activity might be mainly attributed to the efficient charge separation between anatase and rutile, which was verified by SPV results.
[1] Lu Y, Liu G, Zhang J, et al. Fabrication of a monoclinic/hexagonal junction in WO3and its enhanced photocatalytic degradation of Rhodamine B[J]. Chinese Journal of Catalysis, 2016, 37(3): 349–358
[2] Kaur J, Kumar V, Gupta K, et al. A facile strategy for the degradation of recalcitrant textile dyes using highly robust ZnO catalyst[J]. Journal of Chemical Technology &Biotechnology, 2016, 91(8): 2263–2275
[3] Samadi M, Zirak M, Naseri A, et al. Recent progress on doped ZnO nanostructures for visible-light photocatalysis[J]. Thin Solid Films, 2016, 605(4): 2–19
[4] Yang Z, Yan J, Lian J, et al. g-C3N4/TiO2nanocomposites for degradation of ciprofloxacin under visible light irradiation[J]. ChemistrySelect, 2016, 1(18): 5679–5685
[5] Zhang W, Song N, Guan L, et al. Photocatalytic degradation of formaldehyde by nanostructured TiO2composite films[J]. Journal of Experimental Nanoscience,2016, 11(3): 185–196
[6] Sotovázquez L, Cotto M, Ducongé J, et al. Synthesis and photocatalytic activity of TiO2nanowires in the degradation of p-aminobenzoic acid: A comparative study with a commercial catalyst[J]. Journal of Environmental Management, 2016, 167(1): 23–28
[7] Berger T, Sterrer M, Diwald O, et al. Light-induced charge separation in anatase TiO2particles[J]. Journal of Physical Chemistry B, 2005, 109(13): 6061–6068
[8] Xu H, Liu S Q, Zhou S, et al. Morphology and photocatalytic performance of nano-sized TiO2prepared by simple hydrothermal method with different pH values[J].Rare Metals, 2017, 9(72): 1–9
[9] Zhang N, Liu S, Fu X, et al. A simple strategy for fabrication of “Plum-Pudding” type Pd@CeO2semiconductor nanocomposite as a visible-light-driven photocatalyst for selective oxidation[J]. Journal of Physical Chemistry C, 2017, 115(46): 22901–22909
[10] Wang E, Yang W, Cao Y. Unique surface chemical species on indium doped TiO2and their effect on the visible light photocatalytic activity[J]. Journal of Physical Chemistry C,2017, 113(49): 20912–20917
[11] Michalow K A, Vital A, Heel A, et al. Photocatalytic activity of W-doped TiO2nanopowders[J]. Journal of Advanced Oxidation Technologies, 2016, 11(1): 56–64
[12] Li R, Jia Y, Wu J, et al. Photocatalytic degradation and pathway of oxytetracycline in aqueous solution by Fe2O3-TiO2nanopowder[J]. RSC Advances, 2015, 5(51): 40764–40771
[13] Khojasteh H, Salavati-Niasari M, Abbasi A, et al. Synthesis,characterization and photocatalytic activity of PdO/TiO2, and Pd/TiO2, nanocomposites[J]. Journal of Materials Science:Materials in Electronics, 2016, 27(2): 1261–1269
[14] Bleta R, No?l S, Addad A, et al. Mesoporous RuO2/TiO2composites prepared by cyclodextrin-assisted colloidal self-assembly: towards ef ficient catalysts for the hydrogenation of methyl oleate[J]. RSC Advances, 2016,6(18): 14570–14579
[15] Ravishankar T N, Vaz M D O, Khan S, et al. Enhanced photocatalytic hydrogen production from Y2O3/TiO2nano-composites: a comparative study on hydrothermal synthesis with and without an ionic liquid[J]. New Journal of Chemistry, 2016, 40(4): 3578–3587
[16] Zhang J, Wu W, Yan S, et al. Enhanced photocatalytic activity for the degradation of Rhodamine B by TiO2modified with Gd2O3calcined at high temperature[J].Applied Surface Science, 2015, 344(7): 249–256
[17] Zhang J, Yan S, Zhao S, et al. Photocatalytic activity for H2, evolution of TiO2, with tuned surface crystalline phase[J]. Applied Surface Science, 2013, 280(9): 304–311
[18] Xu Q, Ma Y, Zhang J, et al. Enhancing hydrogen production activity and suppressing CO formation from photocatalytic biomass reforming on Pt/TiO2, by optimizing anatase–rutile phase structure[J]. Journal of Catalysis, 2011, 278(2): 329–335
[19] Tiwari A, Mondal I, Ghosh S, et al. Fabrication of mixed phase TiO2heterojunction nanorods and their enhanced photoactivities[J]. Physical Chemistry Chemical Physics,2016, 18(22): 15260–15268
[20] El-Sheikh S M, Khedr T M, Zhang G, et al. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light[J].Chemical Engineering Journal, 2016, 310(2): 428–436
[21] Sabry R S, Al-Haidarie Y K, Kudhier M A. Synthesis and photocatalytic activity of TiO2nanoparticles prepared by sol–gel method[J]. Journal of Sol-Gel Science and Technology, 2016, 78(2): 299–306
[22] EL-Mekkawi D M, Labib A A, Mousa H A, et al.Preparation and characterization of nano titanium dioxide photocatalysts via sol–gel method over narrow ranges of varying parameters[J]. Oriental Journal of Chemistry,2017, 33(1): 41–51
[23] Wang Y, Xie Y, Yuan J, et al. Controlled synthesis and photocatalytic activity of TiO2nanoparticles by a novel gel-network precipitation method[J]. Asian Journal of Chemistry, 2013, 25(2): 739–744
[24] Li J H, Hao J H, Wang W. Characterization and photocatalytic activity of TiO2nanoparticles with precipitation method synthesis[J]. Advanced Materials Research, 2013, 750–752(3): 315–318
[25] Kawasaki H, Suda Y, Ohshima T, et al. Preparation of Er2O3and TiO2multilayer films as optical filter using magnetron sputtering deposition[J]. IEEE Transactions on Plasma Science, 2016, 44(12): 3066–3070
[26] Yang B, Mahjourisamani M, Rouleau C M, et al. Low temperature synthesis of hierarchical TiO2nanostructures for high performance perovskite solar cells by pulsed laser deposition[J]. Physical Chemistry Chemical Physics, 2016,18(39): 27067–27072
[27] Zhang Y, Zhang J, Xu Q, et al. Surface phase of TiO2modified with La2O3and its effect on the photocatalytic H2evolution[J]. Materials Research Bulletin, 2014, 53(1):107–115
[28] Gribb A A, Banfield J F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2[J]. American Mineralogist, 1997,82(7/8): 717–728
[29] Zhang J, Yan S, Lu F U, et al. Photocatalytic degradation of Rhodamine B on anatase, rutile, and brookite TiO2[J].Chinese Journal of Catalysis, 2011, 32(6): 983–991
[30] Khan M M, Ansari S A, Pradhan D, et al. Band gap engineered TiO2nanoparticles for visible light induced photoelectrochemical and photocatalytic studies[J]. Journal of Materials Chemistry A, 2014, 2(3): 637–644
[31] Li Zhen, Cong Shan, Xu Yiming. Brookite vs anatase TiO2in the photocatalytic activity for organic degradation in water[J]. ACS Catalysis, 2014, 4(9): 3273–3280
[32] Zhao J, Osterloh F E. Photochemical charge separation in nanocrystal photocatalyst films: Insights from surface photovoltage spectroscopy[J]. Journal of Physical Chemistry Letters, 2014, 5(5): 782–786
[33] Gurudayal, Chee P M, Boix P P, et al. Core-shell hematite nanorods: a simple method to improve the charge transfer in the photoanode for photoelectrochemical water splitting[J]. ACS Applied Materials & Interfaces, 2015,7(12): 6852–6875
[34] Zhong J, Li J, Wang T, et al. Improved solar-driven photocatalytic performance of Ag3PO4/ZnO composites benefiting from enhanced charge separation with a typical Z-scheme mechanism[J]. Applied Physics A, 2016, 122(1): 1–6
[35] Yang Q, Zhong J, Li J, et al. Photo-induced charge separation properties of NiO/Bi2O3heterojuctions with efficient simulated solar-driven photocatalytic performance[J]. Current Applied Physics, 2017, 17(4):484–487
- 中國煉油與石油化工的其它文章
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
- Study on Mechanical Performance and Wear Resistance of Halloysite Nanotubes/PTFE Nanocomposites Prepared by Employing Solution Mixing Method
- Study on a New Type of Lubricating Oil for Miniature Bearing Operating at Ultra-low Temperature
- Lubricating Performance of Rapeseed Oil Under Electromagnetic Field
- Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption

