Synthesis of Core-Shell HZSM-5@SBA-15 Composite and Its Performance in the Conversion of Methanol to Aromatics
Wang Lu; Li Jian; Wang Lijuan; Chu Shuang; Yang Lina
(School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
1 Introduction
The most widely used aromatics including benzene, toluene and xylene (BTX) are normally synthesized through the catalytic reforming process with the naphtha used as the feedstock[1]. The availability of naphtha, as one of the important petroleum products, is easily affected by the energy crisis. Also due to the fast deterioration of the global environment researchers are trying to find an alternative way to replace the traditional process[2]. An available way to manufacture aromatics is the conversion of methanol[3].Since methanol can be easily synthesized from coal[4], the conversion of methanol to aromatics (MTA) therefore has grown to be a new focus in the coal chemical industry.
The molecular sieve HZSM-5 is a typical catalyst applied in the MTA process. The alumino-silicate HZSM-5 zeolite contains the MFI-type topology, in which the 5.6 ?×5.3 ? straight channels are connected with the 0.53 nm×0.51 nm sinusoidal pores[5]. It displays high catalytic activity,shape selectivity and hydrothermal stability in the MTA process[6-7]. Although HZSM-5 possesses the required properties of catalyst for MTA, the deactivation caused by coke deposition seriously in fluences its application[8-9].
To address this issue, several methods to modify the HZSM-5 zeolite have been tried. The treatment by acids or alkalis can modulate the surface acidity of HZSM-5[10-11]. But unfortunately, the treatment often destructs the framework of HZSM-5 and even leads to crystalline defects[12]. So the thermal stability and the shape selectivity of HZSM-5 would be significantly reduced[13].The construction of the core-shell materials with ZSM-5 zeolite functioning as the core was also tried in the early time. The ZSM-5 zeolite was covered by amorphous silica through chemical vapor deposition of various silicon alkoxides[14-15]. The structure and properties of core-shell material comprising ZSM-5/amorphous silica were studied systematically. The surface acidity of ZSM-5 zeolite can be modulated by covering with amorphous silica,however, it was also reported that covering a simple silica layer on ZSM-5 would decrease the pore size of the parent ZSM-5[15]. The large molecules can leave the outer surface of ZSM-5/amorphous silica freely; however,the decreased pore size due to the covering of the silica shell is not bene ficial to the internal diffusion of the large molecules. It is well known that ZSM-5 is a typical microporous zeolite, the decrease of its pore size is not fa-
Covering a layer of mesoporous shell on ZSM-5 can improve the inner diffusion of the large molecules[16-17].Recently ZSM-5@meso-SAPO-34 was reported, and an excellent catalytic performance was observed in the MTA process. Since the methanol conversion was equal to approximately 100%[18], the yield of aromatics was higher than that obtained over the pristine ZSM-5 and its physical mixture with SAPO-34. Such results can be attributed to the synergistic effects of ZSM-5 and the mesoporous SAPO-34. In such composite zeolites the diffusion path length was shortened. In spite of such high catalytic activity this catalyst did not show a satisfory cycle length, which possibly may be ascribed to its low surface area and small mesopores measuring about 2―3 nm in diameter.
SBA-15 is a typical ordered mesoporous molecular sieve,which possesses high surface area, uniform mesopores and weak acidity. It may be a good idea to cover it on HZSM-5 zeolite, and in such core-shell structure the acid sites on the external surface of HZSM-5 can decrease because of the covering of HZSM-5 by SBA-15 so that at the same time the diffusion of large molecules such as aromatics and coke precursors can be improved in the ordered mesopores of SBA-15[19]. In this work, HZSM-5@SBA-15 composite was synthesized with the acidic sol-gel coating method. Its catalytic selectivity and cycle length in MTA process were significantly higher as compared to HZSM-5 and HZSM-5@SiO2, the silica shell of which was amorphous.
2 Experimental
2.1 Catalyst preparation
2.1.1 Materials
All chemicals were used directly in our experiments without further purification. Tetraethylorthosilicate (TEOS, AR)and hydrochloric acid (2.0 mol/L) were purchased from the Sinopharm Chemical Reagent Co., Ltd., HZSM-5(Si/Al =27) was provided by the Nankai Catalyst Factory. Pluronic P123 (AR) was obtained from Sigma-Aldrich.
2.1.2 Synthesis of SBA-15
The SBA-15 was prepared by the sol-gel method, and for its detailed synthesis process please refer to the literature[20].
2.1.3 Synthesis of core-shell HZSM-5@SBA-15 and HZSM-5@SiO2
At room temperature 2.0 g of Pluronic P123 was dissolved in a mixture of 49.0 g of water and 12.6 g of 2M HCl under stirring. The solution was heated to 45 °C,into which 4.14 g of TEOS were added under vigorous stirring. After 20 h of stirring, 4.0 g of HZSM-5 precursor were added. Subsequently, the mixture was subjected to the hydrothermal treatment under static conditions for 24 h at 100 °C. The resulting solids were filtered and thoroughly washed with deionized water until the effluent water was neutral, and then the filter cake was dried in an oven for 12 h at 100 °C. At last the P123 template was removed after being subject to calcination at 550 °C for 6 h. In comparison, HZSM-5@SiO2was prepared by the same way as the case of HZSM-5@SBA-15 only without adding P123.
2.2 Catalyst Characterization
X-ray diffraction (XRD) patterns of the samples were recorded by a Rigaku D/MAX-RB X-ray powder diffractometer equipped with Ni-filtered Cu-Kα radiation(40 kV, 35 mA, with each sample weighing 50 mg).Scanning electron microscopy (SEM) micrographs were recorded on a HITACHI SU-8010 operating at a voltage of 5―15 kV. Transmission electron microscopy (TEM)micrographs were recorded with a JEOL JEM-2100F electron microscope operating at an acceleration voltage of 200 kV. Nitrogen adsorption-desorption measurements of samples were obtained on a Micromeritic ASAP 2045 system. Prior to the measurement, each sample (100 mg)was degassed under vacuum at 300 °C for 10 h at least.The surface area was calculated with the Brunauer-Emmett-Teller (BET) method. The micropore volume and surface area were calculated with the t-plot method. The pore-size distribution was analyzed based on the desorp-tion branch of the isotherm by the Density Functional Theory (DFT) method. The NH3-temperature-programed desorption (NH3-TPD) curves were obtained in the range of 150―600 °C at a temperature increase rate of 20 °C/min on a Micromeritics AutoChem II 2 920 chemisorption analyzer. The Fourier transform infrared (FTIR) spectra of the catalysts after pyridine adsorption were recorded on a FTIR spectrometer (Bruker Vertex 70, Germany) to characterize the surface acid sites. The samples were heated to 100 °C under vacuum for 1 h followed by the adsorption of purified pyridine until saturation. Finally the FTIR cell was evacuated at 150 °C and 350 °C for 30 min for the collection of FTIR spectra at these two temperatures. The amount of coke formed on the deactivated spent catalysts was measured on a thermal gravimetric analyzer (Perkin Elmer TGA, US) in air at a heating rate of 10°C/min from room temperature to 800 °C.
2.3 Catalytic test and products analysis
The MTA reaction was carried out in a continuous- flow fixed-bed reactor with an inner diameter of 16 mm. The catalyst (2.5 g, 40―60 mesh) was loaded in the reactor.The catalyst was pretreated in a nitrogen flow at 410 °C for 1 h, and then methanol was pumped into the reactor.The reaction was conducted under a pressure of 0.1 MPa and at a weight hourly space velocity of 2.7 h-1. The oil phase products were analyzed on an Agilent 7890A gas chromatograph equipped with a FID detector and a capillary column (HP-5). The aqueous phase products were analyzed using an Agilent 6820-GC system equipped with a TCD detector.
3 Results and Discussion
3.1 Structural and textural properties
The XRD patterns of SBA-15, HZSM-5@SiO2, and HZSM-5@SBA-15 are shown in Figure 1. Figure 1a presents the low angle XRD patterns of the samples. For SBA-15 there were three diffraction lines at 2θ=0.9°, 1.6°,and 1.8°, corresponding to (100), (110), and (200) planes,respectively, indicating to the typical long range ordered hexagonal mesopores[20]. The intensity of peaks decreased with the addition of HZSM-5 in SBA-15, which indicated the deterioration in the long range operation of the mesopores. For HZSM-5@SiO2no Bragg reflection peaks were found due to the amorphous structure of SiO2. The large angle XRD patterns of samples are shown in Figure 1b. All the samples showed the characteristic diffraction peaks at 2θ = 8.0°, 9.0°, 14.8°, 24.0°, 29.8°, 45.0°, and 46.0° of an MFI phase zeolite, which indicated the existence of the complete crystalline structure of HZSM-5.Compared with the parent HZSM-5 sample, the intensity of the characteristic diffraction peaks for HZSM-5@SBA-15 and HZSM-5@SiO2both slightly weakened and were basically the same, then it could be concluded that the percentages of the shell materials in these two composites were basically the same as well. According to the Scherrer formula, the average crystal size of HZSM-5 and HZSM-5 in the composites was calculated. The average crystal size of HZSM-5 was 60.6 nm, and the average crystal size of HZSM-5 in HZSM-5@SiO2and HZSM-5@SBA-15 was 56.2 nm and 50.3 nm, respectively. It can be found that the average diameters of HZSM-5 in composite materials decreased slightly, which might be related to the addition of acid during the preparation of composites.Figure 2 shows the SEM micrographs of HZSM-5,HZSM-5@SiO2, and HZSM-5@SBA-15 at different magnification. It can be seen from Figure 2a and Figure 2d that the pristine HZSM-5 exhibits a hexagonal prism shape and the surface of the particles is smooth. The particle size distribution is not so even, some particles smaller than 500 nm marked with the red circles in Figure 2a can also be seen. It can be found from Figure 2e and Figure 2f that both HZSM-5@SiO2and HZSM-5@SBA-15 retained the general shape of the pristine HZSM-5, however, the outer silica shell made the particle expanded. It should be noted that the worm-like particles existed around HZSM-5@SBA-15 composites (Figure 2b), and they were the non-composite-forming SBA-15 particles[21]. Meanwhile in Figure 2c the non-composite-forming silica particles near HZSM-5@SiO2displayed no regular morphology.
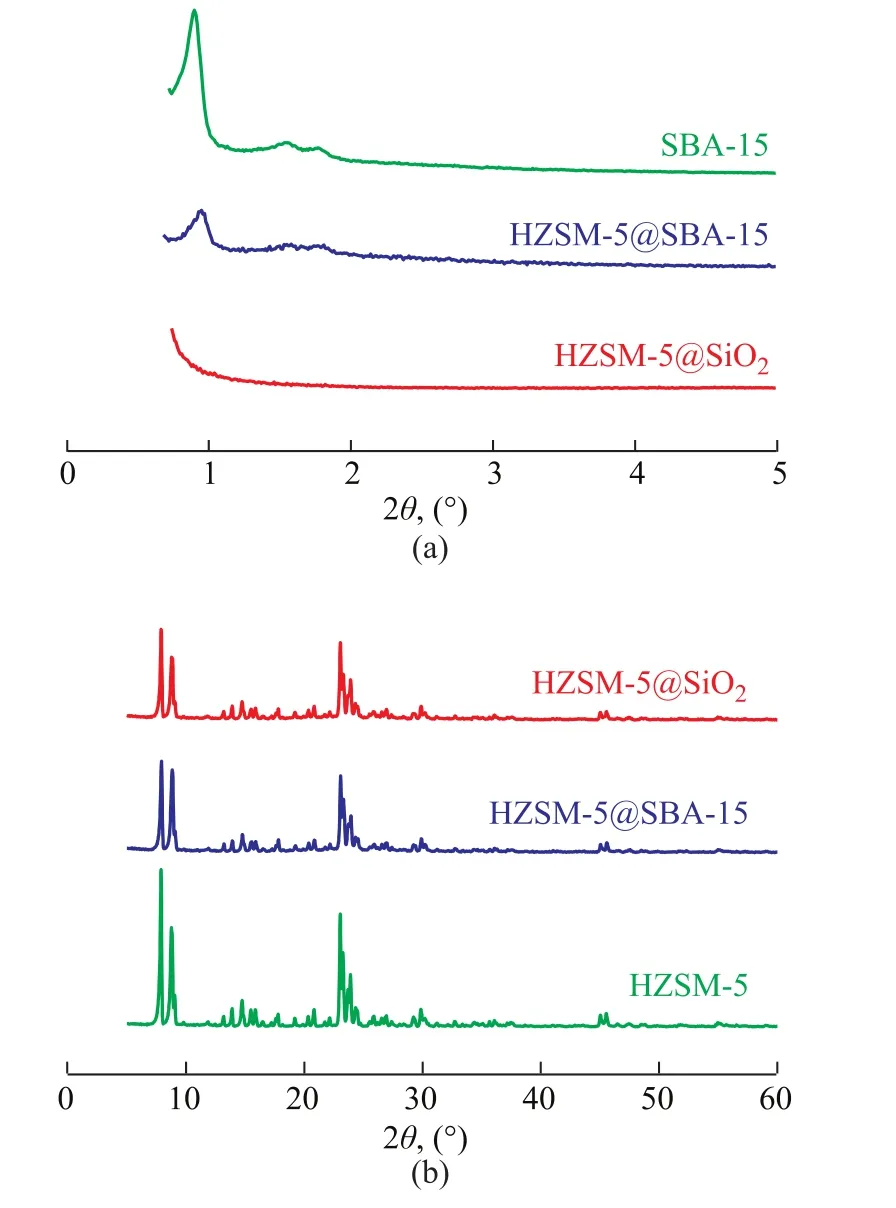
Figure 1 Small angle (a) and large angle (b) XRD patterns of SBA-15, HZSM-5@SiO2 and HZSM-5@SBA-15
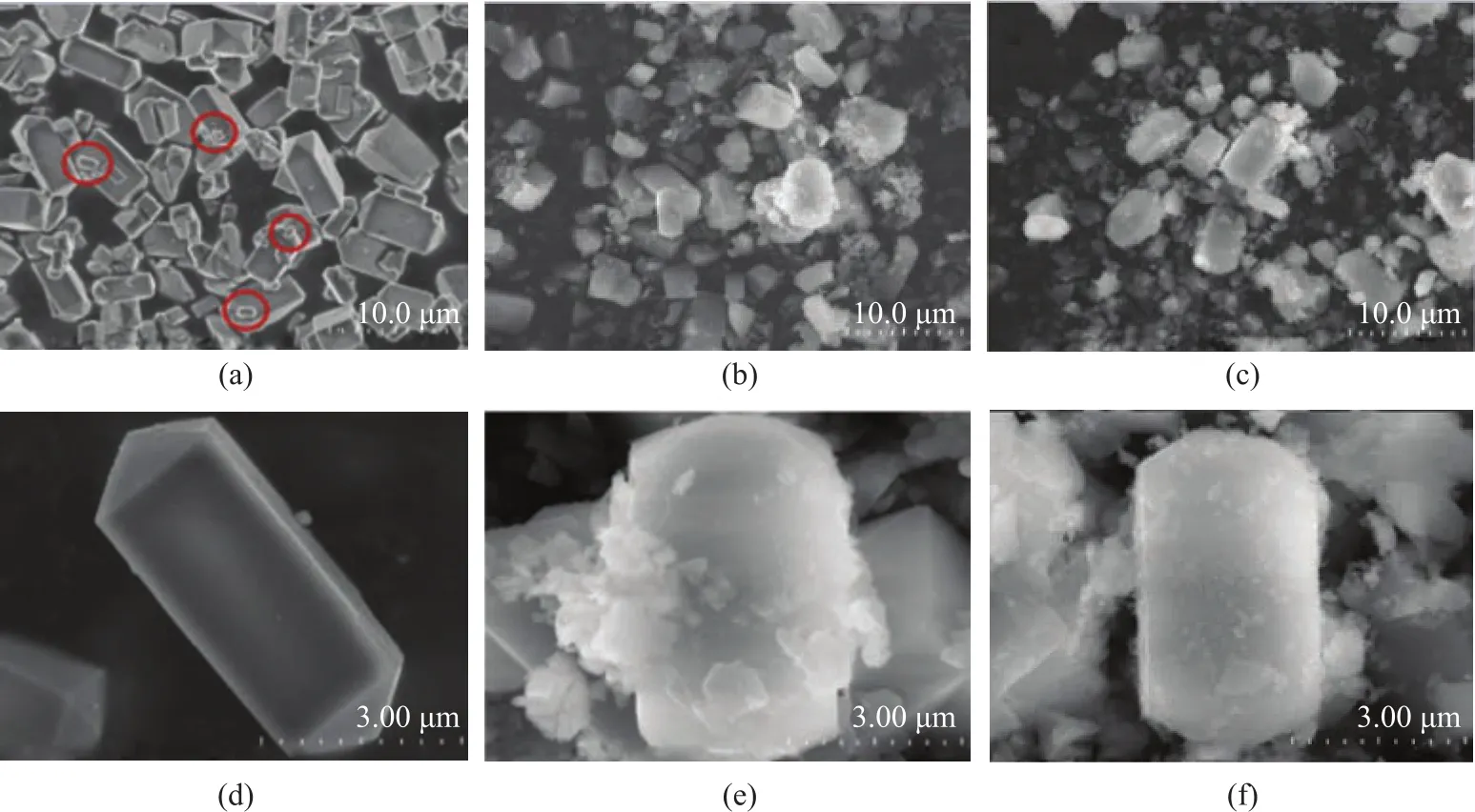
Figure 2 SEM of HZSM-5(a, d), HZSM-5@SBA-15 (b, e) and HZSM-5@SiO2 (c, f) at different magnification
In order to further clarify the pore structure, TEM of HZSM-5@SBA-15 is presented in Figure 3 at different magnification. The particles in Figure 3a were further magnified in Figure 3b, with the micro-mesoporous coreshell composite using HZSM-5 as the core and SBA-15 as the shell being presented in Figure 3b. In order to observe the mesoporous structure of the shell in detail, the typical regions were magnified and circled with rectangular border in the figure. The hexagonal arranged uniform mesopores of the shell are the same as expected indeed.The N2adsorption–desorption isotherms of the samples are displayed in Figure 4 and their textural properties are summarized in Table 1. In Figure 4a, HZSM-5 exhibits a type I isotherm, indicating to a characteristic micropore framework. SBA-15 displays the type IV adsorption and desorption isotherms which are related to capillary condensation. The distinct hysteresis loop of type H1 in the p/p0range of 0.4―1.0 can illustrate the straight cylindrical mesoporous structure in SBA-15[22]. As for HZSM-5@SBA-15, the isotherm still belongs to type IV with H1 hysteresis loop, however, the slope of the isotherm is not so high as SBA-15 owing to the decrease of the order degree after its combination with HZSM-5. Compared with HZSM-5@SBA-15, HZSM-5@SiO2also has the isotherm of type IV, nevertheless the hysteresis loop is not so obvious, and it can be classified as type H3, while the corresponding pore structure is composed of the slit mesopores or accumulated mesopores in the sample.

Figure 3 TEM of HZSM-5@SBA-15 at different magnification
The pore size distribution of the samples is listed in Figure 4b. The pore size distribution of HZSM-5 is mainly at 0.58 nm, and both HZSM-5@SBA-15 and HZSM-5@SiO2present one peak at around 0.58 nm which is attributed to the micropores of HZSM-5 along with several peaks ranging from 1.5 nm to 2.5 nm. These enlarged pores may be caused by the acid taking part in the synthesis process. As for HZSM-5@SBA-15, a distribution peak at around 5.7 nm was observed, which corresponded to the mesopores of SBA-15. In Table 1, the specific surface area of HZSM-5@SBA-15 is obviously higher than that of HZSM-5 and HZSM-5@SiO2. In these two composites HZSM-5 is still the main component so that the micropore surface areas are higher than the external surface area.Both Vmicroand Smicroof HZSM-5@SBA-15 are larger than HZSM-5@SiO2and HZSM-5, which can be attributed to the formation of some micropores in the pore wall because of the participation of the template P123[23].

Table 1 Pore structure parameters of different samples
3.2 Acidic properties
The NH3-TPD patterns are illustrated in Figure 5. The results for analysis of all catalysts showed two desorption peaks, denoting a low-temperature peak centered around 220 °C and a high-temperature peak located around 450 °C,which were mainly attributed to the weak and strong acid sites, respectively[24]. Compared with the pristine HZSM-5,the intensity of desorption peaks for both HZSM-5@SBA-15 and HZSM-5@SiO2decreased, and such decrease was more apparent for the low temperature peak, which proved the function of the shell capable of reducing the acidity especially the weak acid amounts. The high-temperature peaks of them showed a little shift toward the lower temperature, suggesting a decrease of the strong acid strength.The acid data of samples are listed in Table 2, showing that the amount of weak acid for the core-shell composite decreased by about one half and the strong acid amount decreased by one third. The total acid amount of HZSM-5@SBA-15 is higher than that of HZSM-5@SiO2.The Py-FTIR spectrometry was performed to make a study on the nature of acid sites. As shown in Figure 6, three bands at around 1 450 cm-1, 1 546 cm-1, and 1 490 cm-1corresponded to adsorption of the pyridine on Lewis acid sites, Br?nsted acid sites and the co-adsorption on Lewis and Br?nsted acid sites, respectively[25]. Results at 150 °C and 350 °C both indicated that the amounts of different kinds of acids decreased in the following order:HZSM-5> HZSM-5@SBA-15>HZSM-5@SiO2. The detailed data are depicted in Table 2, denoting that the acid sites in samples were mainly composed of Lewis acid sites, and the amount of this kind of acid decreased not so obviously as the Br?nsted acid sites.

Figure 4 N2 adsorption and desorption isotherms (a) and pore size distribution (b) of HZSM-5, HZSM-5@SiO2, and HZSM-5@SBA-15
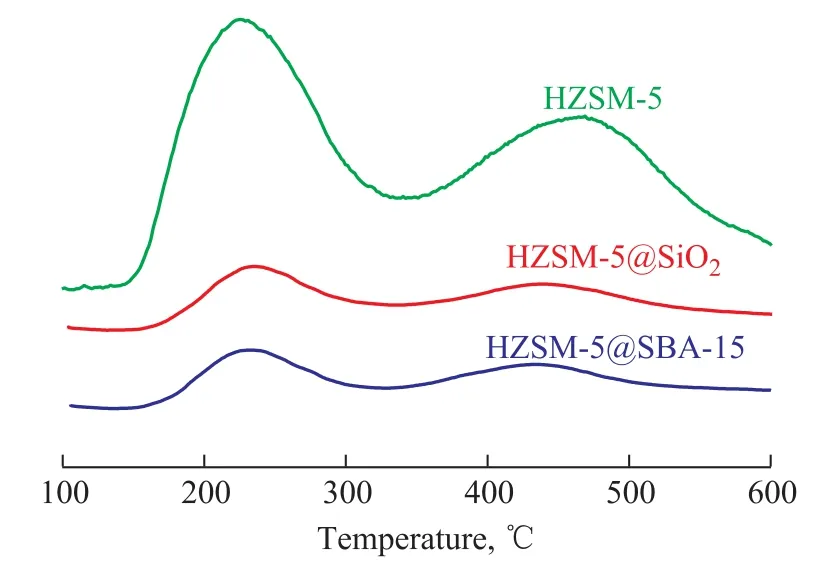
Figure 5 NH3-TPD pro files of HZSM-5, HZSM-5@SiO2 and HZSM-5@SBA-15

Figure 6 Py-FTIR spectra of HZSM-5, HZSM-5@SiO2 and HZSM-5@SBA-15
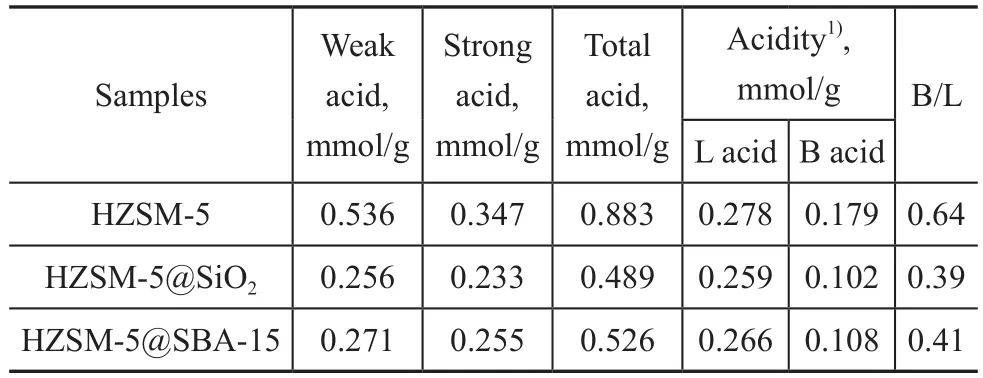
Table 2 NH3-TPD and Py-FTIR analyses of the samples
3.3 Catalytic performance
The catalytic performance of the HZSM-5@SBA-15 catalyst in MTA process was tested and compared with HZSM-5@SiO2and HZSM-5. The methanol conversion,oil yield and BTX selectivity were correlated to the time on stream in Figure 7. Meanwhile, the detailed results of the product distribution achieved by MTA reaction after 16 h on stream are listed in Table 3.
As shown in Figure 7a, all the samples exhibited a high initial activity, with a high methanol conversion (almost 100%) at the initial stage, while the catalytic activity of HZSM-5 was deactivated very quickly. In Figure 7b the oil yield of HZSM-5@SBA-15 was much higher than that of HZSM-5@SiO2and HZSM-5. The B acid sites can catalyze the formation of the dimethyl ether, but it can also play a catalytic role for the formation of the coke deposition, and hence it is necessary to adjust the amount of B acid[26]. The reduced B acid amount of HZSM-5@SBA-15 and HZSM-5@SiO2could decrease the probability of further formation of coke deposition on the catalyst, and thereby the catalyst cycle length was significantly prolonged as compared with HZSM-5[27]. As for HZSM-5@SBA-15 and HZSM-5@SiO2, the B acid amount showed a little difference, but the cycle length of HZSM-5@SBA-15 was much longer than HZSM-5@SiO2. The ordered mesoporous SBA-15 shell materials has the diffusion advantage for coke precursor and aromatics, and it can play a key role in increasing the cycle length of the catalyst.
In Figure 7c, it should be noted that HZSM-5@SBA-15 has obviously higher BTX selectivity than HZSM-5@SiO2. The BTX selectivity is usually affected by the acid properties and the pore structure of the catalyst, and it is reported that with the increase of ratio of B/L acids, the BTX selectivity also increases[28]. However, in this work the test result did not match this law, and it could be only attributed to the good dispersion of the molecules in the ordered hexagonal mesopores having larger pore size as compared to the amorphous silica.
After the introduction of amorphous silica and SBA-15 shells outside the HZSM-5 zeolite, the yield of C9+ hydrocarbons decreased remarkably from 64% to 36% and 34%,respectively, and the BTX yield increased from 31% to 51%and 56%, respectively, as shown in Table 3. In general, C9+hydrocarbons are the products derived from the light ole fins and BTX, which can easily formed over the external acid sites[19]. The covering of the external acid sites of HZSM-5 with silica shell may modify the acidic properties, and this reaction can be effectively restrained. Therefore, the percentages of BTX and C2-C5hydrocarbons in the products were both improved on the core-shell composites. Since HZSM-5@SBA-15 had the ordered mesoporous shell, the BTX products could diffuse more freely in HZSM-5@SBA-15 than in HZSM-5@SiO2,and could leave the catalyst quickly,and consequently the further alkylation of BTX was controlled better. Then it is not surprising that HZSM-5@SBA-15 has the highest BTX selectivity.
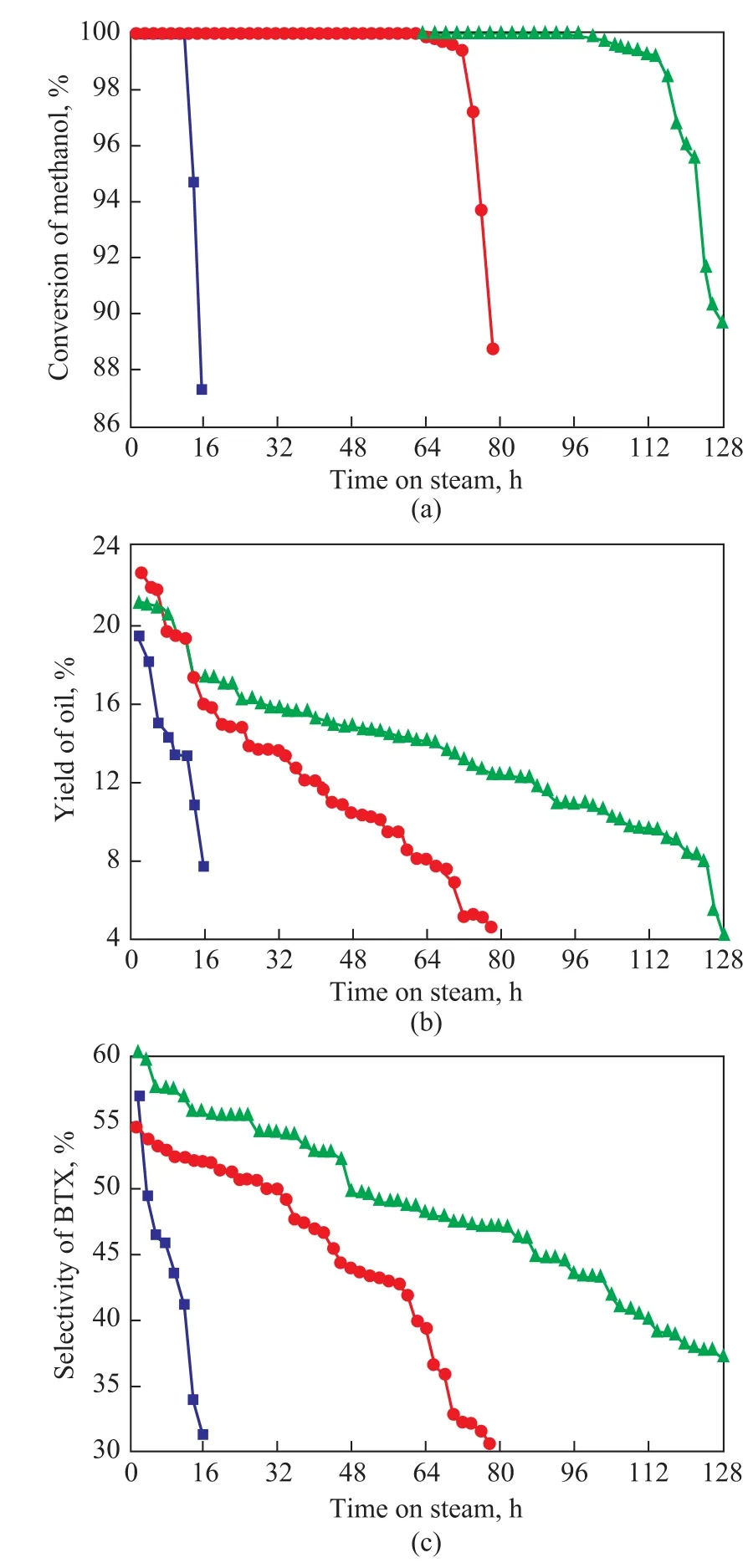
Figure 7 Methanol conversion (a), yield of oil (b) and selectivity towards BTX (c) in MTA reaction
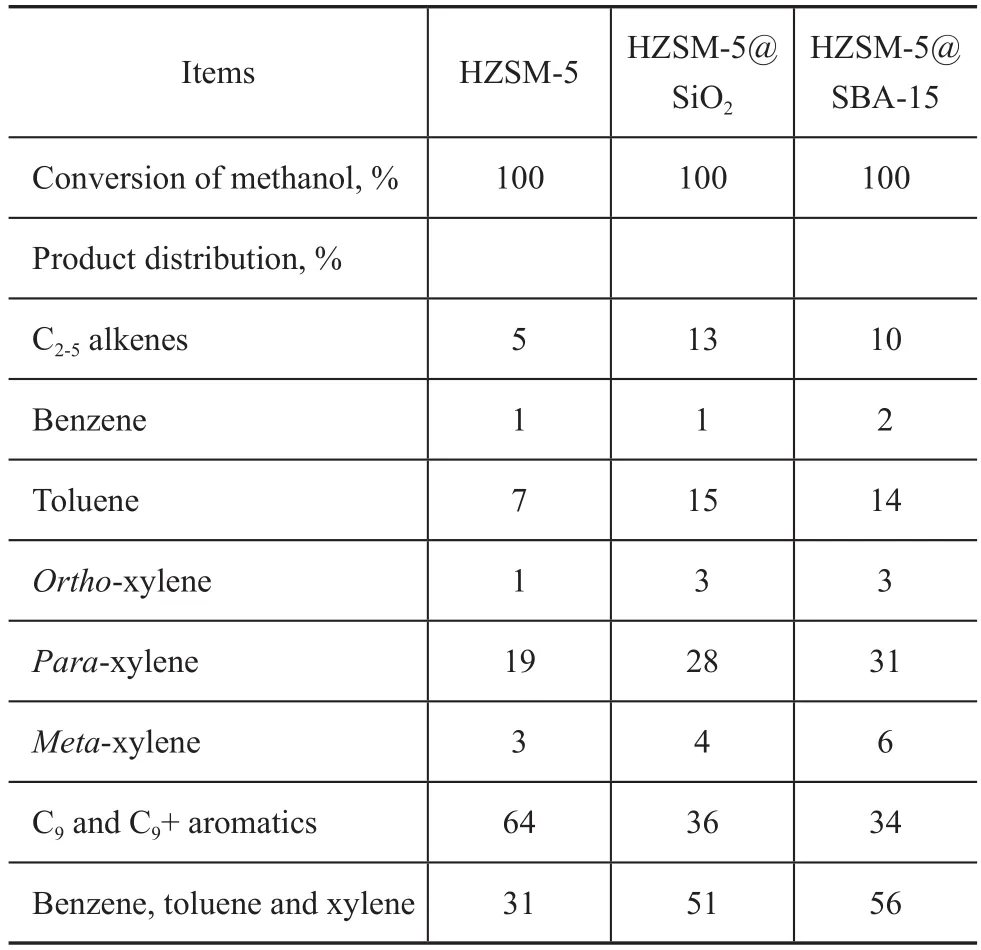
Table 3 Product distribution over HZSM-5, HZSM-5@SiO2 and HZSM-5@SBA-15 in MTA reaction
The used and deactivated catalysts under the conditions depicted in Table 3 were characterized with TGA in air atmosphere to verify the catalyst cycle length results, with the weight loss curves shown in Figure 8. In general, the weight loss before 300 °C was caused by the adsorbed water, and the weight loss between 300 °C and 750 °C was assigned to the decomposition of coke[29]. It is evident that the weight loss of the deactivated HZSM-5 was the highest, about 7.2%. In comparison, the weight losses of HZSM-5@SBA-15 and HZSM-5@SiO2were around 4.4% and 5.0%, respectively, and they were much lower than the rate observed on the HZSM-5 zeolite. HZSM-5@SBA-15 had a higher coke resistance than HZSM-5@SiO2. Such excellent coke resistance of HZSM-5@SBA-15 would bene fit from the modulated external acid sites of HZSM-5 and the ordered mesopores of SBA-15.
4 Conclusions

Figure 8 TGA pro files of the samples
The core–shell structured HZSM-5@SBA-15 with micro-mesopores was successfully synthesized via the hydrothermal method using Pluronic P123 as the template.The ordered mesoporous shell could adjust the external acid properties and improve the products diあusion to a greater extent, which enhanced the catalytic selectivity and cycle length in the MTA process. The conversion of the methanol was almost 100%, and the BTX selectivity could reach 56% and the catalyst cycle length was about 128 h. Such results were much better than HZSM-5@SiO2provided with the amorphous silica shell.
Acknowledgment:The work was supported by the Program for Liaoning Excellent Talents in Universities,abbreviated as “LNET” (LJQ2015062), the Program for Science and Technology Agency of Liaoning Province (20170540585), the General Scientific Research Project of Liaoning Provincial Department of Education (L2015296, L2016018) and the Science and Technology Planning Project of Fushun(FSKJHT201376).
[1] Mahmoudi J, Lotfollahi M N. Extraction of benzene from a narrow cut of naphtha via liquid-liquid extraction using pure-sulfolane and 2-propanol-sulfolane-mixed solvents[J].Korean Journal of Chemical Engineering, 2010, 27(1):214–217
[2] Wang F, Xiao W Y, Xiao G M. Atomic layer deposition of zinc oxide on HZSM-5 template and its methanol aromatization performance[J]. Catalysis Letters, 2015, 145(3):860–867
[3] Kang S H, Bae J W, Prasad P, et al. In fluence of Ga addition on the methanol synthesis activity of Cu/ZnO catalyst in the presence and absence of alumina[J]. Journal of Industrial & Engineering Chemistry, 2009, 15(3): 665–669
[4] Kamarudin S K, Shamsul N S, Ghani J A, et al. Production of methanol from biomass waste via pyrolysis[J]. Bioresource Technology, 2013, 129(2): 463–468
[5] Chester A W, Derouane E G. Zeolite Characterization and Catalysis[M]. Springer, 2009, 47(1): 241–50
[6] Zhang G Q, Bai T, Chen T F, et al. Conversion of methanol to light aromatics on Zn-modified nano-HZSM-5 zeolite catalysts[J]. Industrial & Engineering Chemistry Research,2014, 53(39): 14932–14940
[7] Niu X, Gao J, Wang K, et al. In fluence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics[J]. Fuel Processing Technology, 2017, 157: 99–107
[8] Min Q, Wu R, Zhao L, et al. Size-selective proteolysis on mesoporous silica-based trypsin nanoreactor for low-MW proteome analysis[J]. Chemical Communications, 2010,46(33): 6144–6146
[9] Melánová K, Cvejn D, Bure? F, et al. Organization and intramolecular charge-transfer enhancement in tripodal tris[(pyridine-4-yl)phenyl]amine push-pull molecules by intercalation into layered materials bearing acidic functionalities[J]. Dalton Transactions, 2014, 43(27): 10462–10470
[10] Sun H, Peng P, Wang Y, et al. Preparation, scale-up and application of meso-ZSM-5 zeolite by sequential desilication-dealumination[J]. Journal of Porous Materials, 2017,391: 1–13
[11] Zhao L, Xu C, Gao S, et al. Effects of concentration on the alkali-treatment of ZSM-5 zeolite: a study on dividing points[J]. Journal of Materials Science, 2010, 45(19):5406–5411
[12] Ni Y, Sun A, Wu X, et al. The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol[J]. Microporous and Mesoporous Materials, 2011, 143(2/3): 435–442
[13] Kumar S, Sinha A K, Hegde S G, et al. In fluence of mild dealumination on physicochemical, acidic and catalytic properties of H-ZSM-5[J]. Journal of Molecular Catalysis A: Chemical, 2000, 154(1/2): 115–120
[14] Hibino T, Niwa M, Murakami Y. Shape-selectivity over HZSM-5 modified by chemical vapor deposition of silicon alkoxide[J]. Journal of Catalysis, 1991, 128(2): 551–558
[15] Kim J H, Ishida A, Okajima M, et al. Modification of HZSM-5 by CVD of various silicon compounds and generation of para-selectivity[J]. Journal of Catalysis, 1996,161(1): 387–392
[16] Zhang Q, Li C, Xu S, et al. Synthesis of a ZSM-5(core)/SAPO-5(shell) composite and its application in FCC[J].Journal of Porous Materials, 2013, 20(1): 171–176
[17] Li L, Cui X, Li J, et al. Synthesis of SAPO-34/ZSM-5 composite and its catalytic performance in the conversion of methanol to hydrocarbons[J]. Journal of the Brazilian Chemical Society, 2015, 17(1): 1–5
[18] Zhang L, Jiang Z X, Yu Y, et al. Synthesis of core-shell ZSM-5@meso-SAPO-34 composite and its application in methanol to aromatics[J]. RSC Advances, 2015, 5(69):55825–55831
[19] Chu S, Guo X, Li J, et al. Synthesis of Ga2O3/HZSM-5@cubic ordered mesoporous SiO2with template Pluronic F127 to improve its catalytic performance in the aromatization of methanol[J]. Journal of Porous Materials, 2017,24(4): 1069–1078
[20] Zhao D, Feng J, Huo Q, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores[J]. Science, 1998, 279(5350): 548–552
[21] Cruz-Rodríguez K, García-Alamilla R, Ramos-Galván C E, et al. Zirconium and phosphorus modified SBA-15:texture, enhanced acidity and methanol dehydration activity[J]. Reaction Kinetics, Mechanisms and Catalysis, 2017,120(1): 371–384
[22] Margolese D, Melero J A, Christiansen S C, et al. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups[J]. Chemistry of Materials, 2000,12(8):2448–2459
[23] Okamoto M, Fukukita Y, Haga M, et al. Effect of addition of alkali metal salts in hydrothermal synthesis and post heat-treatment on porosity of SBA-15[J]. Journal of Porous Materials, 2009, 16(2):135–140
[24] Liu N, Zhu X, Hua S, et al. A facile strategy for preparation of phosphorus modified HZSM-5 shape-selective catalysts and its performance in disproportionation of toluene[J].Catalysis Communications, 2016, 77: 60–64
[25] Wu X, Alkhawaldeh A, Anthony R G. Investigation on acidity of zeolites bound with silica and alumina[J]. Studies in Surface Science & Catalysis, 2000, 143: 217–225
[26] Fei W, Wei Y X, Guo M X. Atomic layer deposition of zinc oxide on HZSM-5 template and its methanol aromatization performance[J]. Catalysis Letters, 2015, 145(3):860–867
[27] Zaidi H A, Pant K. K. An oxalic acid-treated ZnO/CuO/HZSM-5 catalyst with high resistance to coke formation for the conversion of methanol to hydrocarbons[J]. International Journal of Green Energy, 2014, 11(4): 376–388
[28] Razavian M, Fatemi S. Synthesis and application of ZSM-5/SAPO-34 and SAPO-34/ZSM-5 composite systems for propylene yield enhancement in propane dehydrogenation process[J]. Microporous & Mesoporous Materials, 2015,201: 176–189
[29] Xin Y, Qi P, Duan X, et al. Enhanced performance of Zn-Sn/HZSM-5 catalyst for the conversion of methanol to aromatics[J]. Catalysis Letters, 2013, 143(8): 798–806
- 中國煉油與石油化工的其它文章
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
- Study on Mechanical Performance and Wear Resistance of Halloysite Nanotubes/PTFE Nanocomposites Prepared by Employing Solution Mixing Method
- Study on a New Type of Lubricating Oil for Miniature Bearing Operating at Ultra-low Temperature
- Lubricating Performance of Rapeseed Oil Under Electromagnetic Field
- Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption

