ZSM-5/MAPO Composite Catalyst for Converting Methanol to Ole fins in a Two-Stage Unit with a Dimethyl Ether Pre-Reactor
Wang Lin; Wang Zheng; Jiao Hongqiao; Yong Xiaojing; Luo Chuntao; Liu Dianhua
(1. State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237; 2. State Key Laboratory of High-ef ficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021; 3. Coal to Liquids Chemical Research and Development Center, Shenhua Ningxia Coal Industry Group, Yinchuan 750411)
1 Introduction
The conversion of methanol to ole fins (MTO) has been the focus of many studies over the years because this method can produce the important base chemicals ethylene and propylene from carbon resources other than crude oil, such as natural gas, coal, or biomass[1-4]. SAPO-34 and ZSM-5 are the only catalysts applied in the industry.The former is used in the fluidized bed reactors designed by the Dalian Institute of Chemical Physics[4]and UOP/Hydro[5], and the latter is used in fixed-bed reactors designed by Lurgi[6]and ExxonMobil[7].
In Lurgi’s process, methanol is converted to hydrocarbons by using two fixed-bed reactors and two catalysts.Methanol is placed in an adiabatic dimethyl ether (DME)pre-reactor, where it is converted to DME and water over the Al2O3-type catalysts. The methanol–water–DME stream is routed to the main reactor with steam and recycled olefins. The conversion of methanol and DME over the ZSM-5-type catalysts reaches more than 99%. The design of a two-stage unit with DME pre-reactor not only can reduce the load of a single catalyst and improve the conversion of methanol but can also decrease the amount of heat released by the catalyst during the reaction. In addition, the catalysts exhibit low single-pass yield for light ole fins and high yield for C5+ hydrocarbons. Hence,several ole fin-containing streams can be sent back to the main synthesis loop for conversion of recycled stream to produce additional light ole fins, which requires high energy consumption and increased operating costs. An eco-
The narrow size of zeolite micropores restricts the mass transfer and diffusion of large molecules in the reaction,leading to low target product yield and even rapid deactivation. To overcome the mass transfer problem, scholars have proposed to decrease the diffusion length and the number of bulky molecules[8-10]by synthesizing stable nanosheets, zeolite nanocrystals, hierarchical zeolites, or composite zeolites.
In heterogeneous catalysis, composite zeolites have attracted an increasing attention because of their compensation for lack of a single catalyst. As one of the most wellknown catalysts for the MTO reaction, the ZSM-5 zeolite exhibits high propylene yield and long catalyst life but has low yield of total light ole fins and high yield of C5+hydrocarbons due to their acidity and pore properties.Moreover, molecular sieves with AEI and AFI framework topology (MAPO molecular sieve, such as SAPO-5 and SAPO-18) exhibit excellent performance for catalytic cracking of heavy olefins to light olefins[11-14]. Thus, the combination of ZSM-5 zeolite and MAPO molecular sieve might compensate for the limitations of ZSM-5 and could form an optimal catalyst to satisfy the increasing market demand for ethylene, propylene, and butadiene.
Composite catalysts have been widely investigated[15-23].Bashyam, et al.[17]reported a new class of low-cost(non-precious metal)/(heteroatomic polymer) nanocomposite catalysts for fabricating a PEFC cathode with high oxygen-reduction activity and good performance durability. Razavian, et al.[20]synthesized two types of zeolitic composite systems with binary hierarchical structures comprising ZSM-5 and SAPO-34 molecular sieves;these systems were used as catalytic carriers for propane dehydrogenation reaction to promote the physicochemical properties of ZSM-5 support, increase the propylene yield, and reduce the formation of light compounds.Dagle, et al.[22]evaluated the Pd/ZnO/Al2O3–HZSM-5 catalytic system for direct synthesis of the gasoline-range hydrocarbons from syngas, and the results showed that the bifunctional catalyst comprising PdZn and zeolitic acid sites could provide the required catalytically active sites. Chae, et al.[23]also developed the combination of ZSM-5 and SAPO-34, and the composites exhibited high catalytic performance for the MTO reaction.
In this study, we combined the ZSM-5 zeolite and the molecular sieve with AEI and AFI framework topology to produce a desired catalyst with high selectivity to olefins, as a result of the adjusted acid properties of ZSM-5 and the cracking properties of MAPO molecular sieve.In order to improve the catalytic activity and stability for MTP, the composite zeolite was also loaded with 3%of P. Moreover, the catalytic property of the catalysts was evaluated in a two-stage unit provided with a DME pre-reactor operating under conditions similar to those in an industrial fixed-bed unit.
2 Experimental
2.1 Preparation of catalysts
MAPO molecular sieve was synthesized according to the method described in literature[23]. The molar composition of the synthesis mixture was composed of 1.0Al2O3:1.3P2O5: 1.2TEA : 300H2O.
The template-free commercial HZSM-5 powder (SiO2/Al2O3= 120, supplied by the Catalyst Plant of Nankai University, China) was added to the synthetic gel of MAPO molecular sieve (with the mass ratio of ZSM-5 and the synthetic gel of MAPO equating to 1:10) to prepare the ZSM-5/MAPO composite zeolite (with the mass ratio of ZSM-5/MAPO being equal to 90:10).
2.2 Characterization
The X-ray diffraction (XRD) patterns of the samples were recorded on a Rigaku D/MAX 2200PC diffractometer using Cu Kα radiation at an accelerating voltage of 40 kV and a current of 40 mA with a scanning rate of 4°/min.The scanning electron microscopy (SEM) images of the samples were obtained using a KYKY-2800B microscope for analyzing the crystal morphology. The IR spectra were recorded on a Tensor 27 FTIR spectrometer (Brooke,Germany) with a resolution of 4 cm?1. The acid amount and acid strength of the samples were determined by temperature-programmed desorption of ammonia (NH3–TPD,Quantachrome, USA) with an on-line thermal conductivity detector. The BET specific surface area and pore struc-ture of the samples were determined by nitrogen adsorption–desorption analysis at liquid nitrogen temperature(77 K) by using an automatic Micromeritics ASAP 2420 apparatus.
2.3 Catalyst activity test
The MTO reaction was conducted in an automatic fixedbed microreactor equipped with two reaction tubes (L =500 mm, Di=19 mm) operating at 480 °C and under atmospheric pressure. The first reaction tube was filled with 2 g of the Al2O3-type catalysts and used as a pre-reactor for converting methanol to DME at a constant reaction temperature of 300 °C. The equilibrium between MeOH and DME was rapidly obtained in this reactor. The second reaction tube, namely the MTO reactor, converted the mixture of methanol and DME to mainly light ole fins, along with some paraf fins and aromatics, at a constant reaction temperature of 480 °C. The weight hourly space velocity(WHSV) of methanol was 1 h?1, the catalyst loading was 2 g, and the introduced water/methanol molar ratio was 0.7. The total products formed thereby were analyzed using an on-line gas chromatograph equipped with a flame ionization detector and an HP PLOT-Q capillary column(30 m × 0.32 mm × 0.5 μm). The conversion and product selectivity can be calculated as follows:

where Con. and xirepresent the methanol conversion and product selectivity, respectively. I0(mol) is the initial amount of methanol, I (mol) is the amount of methanol in the mixture of the reactor ef fluents, and Ii(mol) is the amount of methanol transformed into component i.
3 Results and Discussion
3.1 XRD analysis
The phase transformation was investigated by XRD and IR analyses. Figure 1 shows the XRD patterns of the samples. The XRD patterns of ZSM-5/MAPO composite catalyst showed that the composite catalyst exclusively consisted of the characteristic diffraction peaks for MFI,AEI and AFI. This finding indicated that the composite zeolites were made up of ZSM-5, AlPO-18 or SAPO-18,AlPO-5 or SAPO-5, which were consistent with previously published results[24-25].
And silicon may come from the framework of ZSM-5 zeolite. Hence, further increase in the heating time could lead to a decreased formation of AFI molecular sieve and an enhanced formation of AEI molecular sieve[26]. In contrast to those of MAPO molecular sieve, the XRD patterns of the composites exhibited the weak intensity peaks,which were characteristic of the AFI molecular sieve, and the high-intensity peaks, which were characteristics of the AEI molecular sieve. This finding might explain that the addition of ZSM-5 was conducive to the formation of AEI molecular sieve. The mass of AFI molecular sieve,AEI molecular sieve, and ZSM-5 zeolite incorporated in the composites was 4%, 6%, and 90%, respectively, and could be calculated according to Eqs. (3) and (4). After the composites were modified by NH4H2PO4, the XRD patterns of the samples showed no obvious changes. No peak of impurities was detected, and the intensity of the diffraction peaks decreased. This phenomenon is mainly attributed to the framework defects caused by dealumination during P modification[27].

where w is the component content in the composites; F is the integral intensity of the highest characteristic peaks for various samples; K is the ratio of the highest characteristic peaks for samples and Al2O3when they are mixed at a mass ratio of 1:1; and a and b represent the a and b phases, respectively.
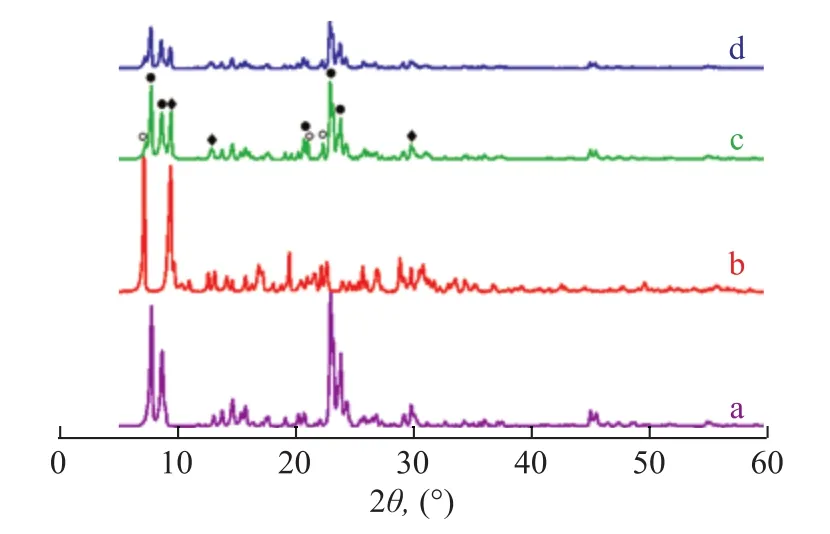
Figure 1 X-ray diffraction patterns of samples a—ZSM-5; b—MAPO; c—ZSM-5/MAPO; d—3%P-ZSM-5/MAPO
3.2 SEM analysis
The SEM images (Figure 2) indicated that the commercial ZSM-5 catalyst was composed of fine ZSM-5 particles,with a crystal size of 1–3 μm. The MAPO molecular sieve exhibited clavate, square prism, and hexagonal-prism-like morphologies, which were characteristic of the AFI and AEI molecular sieves[28-29]. The morphology of ZSM-5 was not significantly altered. However, the particle size increased, and the morphology of the MAPO molecular sieve significantly changed from clavate and hexagonal prism to square prism in the ZSM-5/MAPO composite catalyst. This observation was in agreement with the XRD results of the composites showing weak peaks characteristic of the AFI molecular sieve and intense peaks characteristic of the AEI molecular sieve.

Figure 2 SEM images of ZSM-5 (a), MAPO (b), and ZSM-5/MAPO (c)
3.3 IR analysis
Figure 3 shows the IR spectra of ZSM-5, MAPO molecular sieve, and the composites. In addition to the most characteristic IR bands of ZSM-5 zeolite, the AFI and AEI molecular sieves, the composites exhibited two new IR bands at 732 cm?1and 786 cm?1. The former was attributed to the change in the oxygen symmetric stretching vibration of phosphoric (aluminum) bonds in the AFI molecular sieve, and the latter is attributed to the shift in the wavenumber from 795 cm?1to 786 cm?1[30]. This finding was also consistent with the XRD results.

Figure 3 IR spectra of ZSM-5 (a), MAPO (b) and ZSM-5/MAPO (c)
3.4 NH3-TPD analysis
Figure 4 illustrates the temperature-programmed desorption of ammonia (NH3-TPD) of the samples. All samples exhibited a typical NH3-TPD spectrum with two temperature peaks within 150―350 °C and 350―550 °C, corresponding to weak and strong acid sites, respectively. The peak areas represented the amount of NH3eluted from the weak and strong acid sites or the number of weak and strong acid sites, respectively. The acid strength and amount of acid sites for ZSM-5 exceeded those of other samples. However, to our surprise, the MAPO molecular sieve also showed some acidity possibly due to the modification of the MAPO molecular sieve by other atoms in the raw materials. This finding was consistent with the outcome of catalytic reaction .
The number of strong acid sites and weak acid sites in the composites obviously decreased compared with those in ZSM-5. The high- and low-temperature peaks shifted from 430 °C to 410 °C and from 260 °C to 205 °C,respectively (Table 1). The amount of the total acid sites significantly decreased after the introduction of the MAPO molecular sieve. Moreover, the intensity and temperature of the peaks continued to decrease and shifted toward low temperatures. Hence, the concentration and strength of strong acid sites were decreased by the introduction of P, as evidenced by the high-temperature peak.In generally, the P species, such as polyphosphates, shortchain polyphosphates, and pyrophosphates, could form in the channels of molecular sieves after P modification.Most of these species could interact with both acidic and nonacidic hydroxyl groups, especially at the strong acid sites, leading to partial collapse of the molecular sieve framework[31-32]. Furthermore, weak acid sites are considered to be capable of promoting the generation of DME from methanol, and meanwhile, the strong acid sites can easily lead to side reactions, which can produce heavy hydrocarbons that can negatively affect the stability of the catalyst[33].
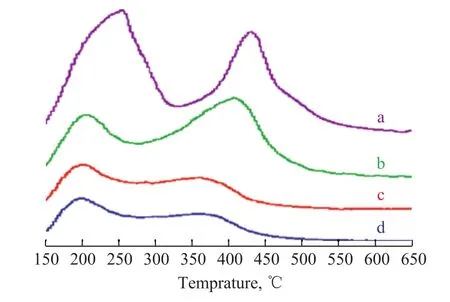
Figure 4 NH3-TPD of ZSM-5 (a), ZSM-5/MAPO (b),MAPO (c) and 3%P-ZSM-5/MAPO (d)
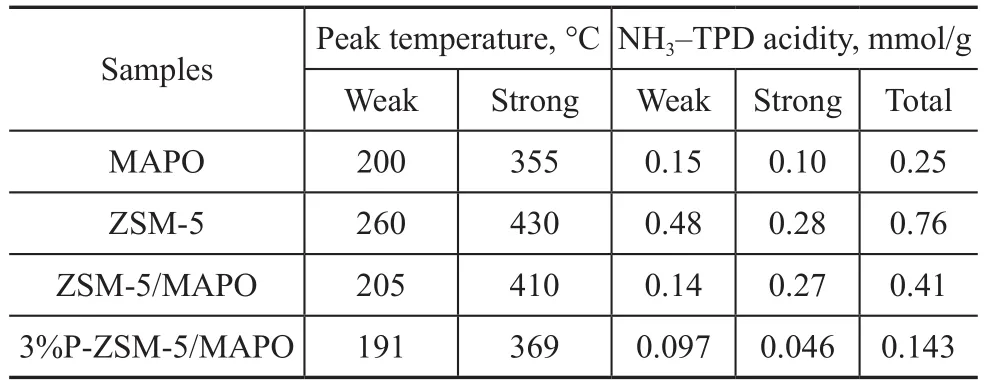
Table 1 NH3-TPD results of the catalysts
3.5 Textural property analysis
The textural properties of the catalyst samples are listed in Table 2. The composites showed higher specific surface area (BET and Langmuir surface area), total pore volume (VT), and average pore diameter (DAA) but lower micropore volume (VM) as compared with ZSM-5. This finding could be attributed to the destruction or overlay of some micropores in ZSM-5 during synthesis.Moreover, the average pore size significantly increased after P modification because of the production of mesopores after washing out of the amorphous species in the crystals and/or the dealumination during P modification.The generation of large pores would be bene ficial to the mass transfer.

Table 2 Textural properties of the catalysts
3.6 Catalytic performance
The composites are the key factor in the MTO process because they not only can significantly in fluence the product distribution but can also enhance the selectivity to ole fins[34]. As shown in Figure 5, all catalysts, except the MAPO molecular sieve, exhibited complete methanol conversion, but their product yields significantly differed. ZSM-5 exhibited high selectivity to C2-4(21.8%)and C5+ (21.4%) because of its relatively large pore size and strong acidity, resulting in the occurrence of several side reactions. The MAPO molecular sieve with AEI and AFI framework topology exhibited low selectivity to C5+(4.0%) and partial methanol conversion because of its catalytic property for cracking macromolecules and weak acidity, respectively. Compared with ZSM-5 and MAPO molecular sieve, the ZSM-5/MAPO composite catalyst exhibited higher activity and selectivity to C2-4=(84.0%),C2=(36.3%), and C3=(36.2%) along with a complete methanol conversion. Furthermore, the 3%P-ZSM-5/MAPO composite catalyst showed a further increase in the selectivity to C3=(52.1%) and C2-4=(83.1%) and in the propylene to ethylene ratio (P/E, 5.8) along with a complete methanol conversion; hence, this composite was more suitable for the MTP process than other samples tested.
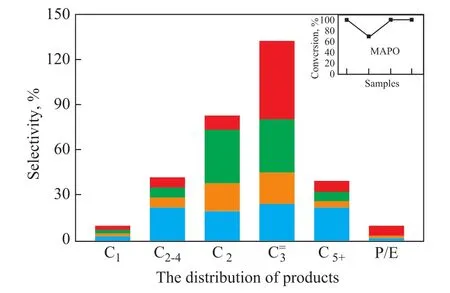
Figure 5 MTO activity of the catalysts(Reaction conditions: T = 480 °C, and WHSV = 1 h?1, with the results obtained after 5 h of reaction.)
The non-desired products in the MTO reaction were produced by side reactions on the acid sites, and such reactions could occur due to the strong acidity of catalysts.In addition, the MAPO molecular sieve could crack C5+to light olefins, which in turn would enhance the mass transfer and decrease the acid sites, thereby suppressing the side reactions and leading to a high selectivity to light ole fins.
The C5+ components and alkanes in the MTO reaction mainly originate from the side reactions because of unsuitable concentration and strength of acid sites. Polymethylbenzenes, which are hydrocarbon-pool intermediates dissociating light alkenes[35-36], can also be converted to polycyclic aromatics on stream and finally to coke[37-38].Thus, a suitable amount of active hydrocarbon pool species and a low amount of aromatics are required for the MTP reaction. The selectivity of the ZSM-5 catalyst to C5+ hydrocarbons was very high (Figure 5), which could promote the coke formation and accelerate the catalyst deactivation.
The concentration and strength of acid sites decreased in the MAPO molecular sieve, suppressing the hydride transfer and cyclization, which could eventually lead to the formation of aromatics. The MAPO molecular sieve could also crack C5+ components into light products. So we can obtain a low C5+ amount and high selectivity to ole fins, which are crucial for minimal coke formation and high catalyst stability in the MTO reaction.
The appropriate concentration and strength of acid sites,which are crucial for the selectivity of propylene and the stability of catalysts in the MTP reaction, can be achieved by modification with P[39].
4 Conclusions
A ZSM-5/MAPO composite catalyst was synthesized through a conventional hydrothermal method, followed by P modification. The conversion of methanol to ole fins over the composites was investigated in a fixed-bed process with a two-stage unit similar to an industrial reactor.The first reaction tube filled with the Al2O3-type catalyst was a pre-reactor for converting methanol into DME and water. The second reaction tube was a MTO reactor for converting a mixture of methanol and DME to hydrocarbons over the composite catalyst. The composites exhibited high cooperative property: firstly, the reduction of the acidity over ZSM-5 effectively restrained side reactions because of the MAPO molecular sieve covering;and secondly, the C5+ components were cracked further by MAPO molecular sieve. The mass transfer was also enhanced due to the generation of small molecules. Thus,the ZSM-5/MAPO composite catalyst exhibited high selectivity to C2-4=(84.0%) and low selectivity to C5+(6.3%). The characterization results demonstrated that the composites led to the partial elimination of the strong acid sites on the ZSM-5 catalyst, thereby enhancing the shape selectivity for ole fins. The catalytic selectivity and stability in terms of propylene production can be further improved by modification with P.
Acknowledgement:This work was financially supported by the National International Cooperation S & T Project of China (No.2015DFA40660).
[1] Butter S A. Production of chemicals from methanol: I. Low molecular weight olefins[J]. Journal of Catalysis, 1980,61(1): 155–164
[2] Chang C D. Methanol conversion to light olefins[J].Catalysis Reviews, 1984, 26(3/4): 323–345
[3] Stocker M. Methanol-to-hydrocarbons: catalytic materials and their behavior[J]. Microporous and Mesoporous Materials, 1999, 29(1/2): 3–48
[4] Tian P, Wei Y, Ye M, et al. Methanol to olefins (MTO):From fundamentals to commercialization[J]. ACS Catalysis, 2015, 5(3): 1922–1938
[5] Chen J Q, Bozzano A, Glover B, et al. Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process[J]. Catalysis Today, 2005,106(1/4): 103–107
[6] Koempel H, Liebner W. Lurgi’s methanol to propylene(MTP?) report on a successful commercialisation[J].Studies in Surface Science & Catalysis, 2007, 167: 261–267
[7] Fleisch T H, Sills R A. Large-scale gas conversion through oxygenates: Beyond GTL-FT[J]. Studies in Surface Science & Catalysis, 2004, 147(4): 31–36
[8] Na K, Jo C, Kim J, et al. Directing zeolite structures into hierarchically nanoporous architectures[J]. Science, 2011,333(6040): 328
[9] Ng E P, Chateigner D, Bein T, et al. Capturing ultrasmall EMT zeolite from template-free systems.[J]. Science,2012, 335(6064): 70–73
[10] Zhang X, Liu D, Xu D, et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching[J]. Science,2012, 336(6089): 1684–1687
[11] Kazusa Terasaka H I A X. Control of morphology and acidity of SAPO-5 for the methanol-to- olefins (MTO)reaction[J]. Journal of Advanced Chemical Engineering,2015, 5(4): 138–144
[12] Nazari M, Behbahani R M, Moradi G, et al. A facile synthesis route for modifying the catalytic performance of SAPO-18 in MTO process[J]. Journal of Porous Materials,2016, 23(4): 1037–1046
[13] Gayubo A G, Aguayo A T, Alonso A, et al. Reaction scheme and kinetic modelling for the MTO process over a SAPO-18 catalyst[J]. Catalysis Today, 2005, 106(1/4):112–117
[14] Chen J, Thomas J M. MAPO-18 (M= Mg, Zn, Co): A new family of catalysts for the conversion of methanol to light olefins[J]. Journal of The Chemical Society, Chemical Communications, 1994(5): 603–604
[15] Andersen N I, Serov A, Atanassov P. Metal oxides/CNT nano-composite catalysts for oxygen reduction/oxygen evolution in alkaline media[J]. Applied Catalysis B-environmental, 2015, 163: 623–627
[16] Faungnawakij K, Tanaka Y, Shimoda N, et al. Hydrogen production from dimethyl ether steam reforming over composite catalysts of copper ferrite spinel and alumina[J]. Applied Catalysis B-Environmental, 2007,74(1): 144–151
[17] Bashyam R, Zelenay P. A class of non-precious metal composite catalysts for fuel cells[J]. Nature, 2006,443(7107): 63–66
[18] Wang W, Serp P, Kalck P. Photocatalytic degradation of phenol on MWNT and titania composite catalysts prepared by a modified sol–gel method[J]. Applied Catalysis B-Environmental, 2005, 56(4): 305–312
[19] Liu W, Flytzanistephanopoulos M. Total oxidation of carbon-monoxide and methane over transition metal fluorite oxide composite catalysts[J]. Journal of Catalysis,1995, 153(2): 304–316
[20] Razavian M, Fatemi S. Synthesis and application of ZSM-5/SAPO-34 and SAPO-34/ZSM-5 composite systems for propylene yield enhancement in propane dehydrogenation process[J]. Microporous and Mesoporous Materials, 2015,201: 176–189
[21] Li X, Xia T, Xu C, et al. Synthesis and photoactivity of nanostructured CdS–TiO2composite catalysts[J]. Catalysis Today, 2014, 225: 64–73
[22] Dagle R A, Lizarazoadarme J, Dagle V L, et al. Syngas conversion to gasoline-range hydrocarbons over Pd/ZnO/Al2O3and ZSM-5 composite catalyst system[J]. Fuel Processing Technology, 2014, 123: 65–74
[23] Chae H, Song Y, Jeong K, et al. Physicochemical characteristics of ZSM-5/SAPO-34 composite catalyst for MTO reaction[J]. Journal of Physics and Chemistry of Solids, 2010, 71(4): 600–603
[24] Wendelbo R, Akporiaye D, Andersen A, et al. Synthesis,characterization and catalytic testing of SAPO-18,MgAPO-18, and ZnAPO-18 in the MTO reaction[J].Applied Catalysis A-General, 1996, 142(2): 1197–1207
[25] Girnus I, Jancke K, Vetter R, et al. Large AlPO4-5 crystals by microwave heating[J]. Zeolites, 1995, 15(1): 33–39
[26] Huang Y, Demko B A, Kirby C W. Investigation of the evolution of intermediate phases of AlPO4-18 molecular sieve synthesis[J]. Chemistry of Materials, 2003, 15(12):2437–2444
[27] Zhao G, Teng J, Xie Z, et al. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene[J]. Journal of Catalysis, 2007, 248(1):29–37
[28] Carreon M L, Li S, Carreon M A. AlPO-18 membranes for CO2/CH4separation[J]. Chemical Communications, 2012,48(17): 2310–2312
[29] Naydenov V, Tosheva L, Antzutkin O N, et al. Meso/macroporous AlPO-5 spherical macrostructures tailored by resin templating[J]. Microporous and Mesoporous Materials, 2005, 78(2): 181–188
[30] Zhang Zhe Z B. Preparation and catalytic cracking activity of ZSM-5(core)/AlPO-4-5(shell) binary structure zeolite[J]. Chinese Journal of Catalysis, 2003, 24(11):856–860
[31] Lischke G, Eckelt R, Jerschkewitz H G, et al. Spectroscopic and physicochemical characterization of P-modified H-ZSM- 5[J]. Journal of Catalysis, 1991, 132(1): 229–243
[32] Xue N, Chen X, Lei N, et al. Understanding the enhancement of catalytic performance for ole fin cracking:Hydrothermally stable acids in P/HZSM-5[J]. Journal of Catalysis, 2007, 248(1): 20–28
[33] Ono. Transformation of lower alkanes into aromatic hydrocarbons over ZSM-5 zeolites[J]. Journal of Catalysis,1992, 34(3): 179–226
[34] Keil F J, Hinderer J, Garayhi A R. Diffusion and reaction in ZSM-5 and composite catalysts for the methanol-toole fins process[J]. Catalysis Today, 1999, 50(3): 637–650.[35] Olsbye U, Bjorgen M, Svelle S, et al. Mechanistic insight into the methanol-to-hydrocarbons reaction[J]. Catalysis Today, 2005, 106(1): 108–111
[36] Hunger M, Wang W. Formation of cyclic compounds and carbenium ions by conversion of methanol on weakly dealuminated zeolite H-ZSM-5 investigated via a novel in situ CF MAS NMR/UV-Vis technique[J]. Chemical Communications, 2004, 10(5): 584–585
[37] Bjorgen M, Olsbye U, Petersen D, et al. The methanolto-hydrocarbons reaction: insight into the reaction mechanism from [12C]benzene and [13C]methanol coreactions over zeolite H-beta[J]. Journal of Catalysis,2004, 221(1): 1–10.
[38] Marcus D M, Song W, Ng L L, et al. Aromatic hydrocarbon formation in HSAPO-18 catalysts: Cage topology and acid site density[J]. Langmuir, 2002, 18(22) :8386–8391
[39] Liu J, Zhang C, Shen Z, et al. Methanol to propylene:Effect of phosphorus on a high silica HZSM-5 catalyst[J].Catalysis Communications, 2009, 10(11): 1506–1509
- 中國煉油與石油化工的其它文章
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Bitumen Recovery from Indonesian Oil Sands Using ASP (Alkali, Surfactant and Polymer) Agent
- Study on Mechanical Performance and Wear Resistance of Halloysite Nanotubes/PTFE Nanocomposites Prepared by Employing Solution Mixing Method
- Study on a New Type of Lubricating Oil for Miniature Bearing Operating at Ultra-low Temperature
- Lubricating Performance of Rapeseed Oil Under Electromagnetic Field
- Preparation and Modification of Micro-Mesoporous Carbon Materials for Toluene Adsorption

