Molecular Simulation of Adsorption of Quinoline Homologues on FAU Zeolite
(School of Chemical Engineering and Pharmacy, Key Laboratory for Green Chemical of Ministry of Education, Wuhan Institute of Technology, Wuhan 430070)
Molecular Simulation of Adsorption of Quinoline Homologues on FAU Zeolite
Shen Xizhou; Yan Fang; Li Meiqing; Xiao Yun
(School of Chemical Engineering and Pharmacy, Key Laboratory for Green Chemical of Ministry of Education, Wuhan Institute of Technology, Wuhan 430070)
The Grand Canonical Monte Carlo (GCMC) simulation method was used to investigate the adsorption properties of quinoline homologues (quinoline, 2-methyl quinoline, and 2,4-dimethyl quinoline) on the FAU zeolite. The adsorption heat, adsorption isotherms, and adsorption sites of them were obtained. At the temperature ranging from 673.15 to 873.15 K, the Henry constant of quinoline homologues calculated on the FAU zeolite was applied to simulate their adsorption heat. And its value was more in accordance with the related data reported in the literature. The results showed that their isosteric heat decreased in the following order: 2,4-dimethyl quinoline (118.63 kJ/mol) > 2-methyl quinoline (110.45 kJ/mol) > quinoline (98 kJ/mol), and complied with the order of their adsorbate basicity. The competitive adsorption of three components of quinoline homologues on the FAU zeolite was calculated numerically at a temperature of 773.15 K and a pressure range of 0.1—100 MPa under the Universal force feld. Their adsorption capacity decreased in the following order: quinoline > 2-methyl quinoline >2,4-dimethyl quinoline. The smaller the molecule size of the adsorbate, the greater the saturated adsorption capacity would be. It was found that the quinoline homologues could be adsorbed in the main channels of 12- membered-ring framework of the zeolite. Simultaneously, the infuence of silica/alumina ratio on the adsorption property of quinoline homologues in FAU zeolite was studied. The smaller the silica/alumina ratio, the greater the isosteric heat and adsorption capacity would be.
FAU zeolite; adsorption; molecular simulation; quinoline nitrides; silica alumina ratio
1 Introduction
At present, most domestic and foreign refneries process the FCC feedstock, in which the coker gas oil (CGO) is blended according to a certain proportion to produce light oil. Molecular sieves are widely used as the adsorbent and the catalyst in the refning and chemical processes[1-8]. The FAU-type zeolite is an important catalyst, and the silicon/ aluminum ratio is an important factor affecting the properties of molecular sieves[9-10].
The catalytic cracking reaction is generally a gas-solid catalytic reaction in which the surface adsorption is an important step. The nitrogen compounds in CGO are preferentially adsorbed on acid sites of the cracking catalyst, which can result in the decrease of catalyst activity or even inactivation and can seriously affect the light oil yield from catalytic cracking process[11-15].
Molecular simulation is a simple and effective way to study the nature of adsorption competition. Although some studies regarding the molecular simulation of the adsorption mechanism of basic nitrogen compounds (pyridine, amine, etc.) on the catalyst surface have been reported[16-23], there are few reports about the molecular simulation of the adsorption of quinoline homologues that are also important basic nitrogen compounds in CGO on the FAU zeolite[24-26]. Therefore, it is important to study the mechanism of catalyst poisoning caused by basic nitrogen compounds contained in CGO to carry out the molecular simulation of quinoline homologues adsorption on the surface of molecular sieves.
2 Computational Model and Simulation Method
2.1 Model construction
The Y zeolite has a framework type of FAU with Fd-3 m space group and cell parameters comprisinga=b=c=2.434 5 nm andα=β=γ=90°, and a pore diameter of about 0.74 nm which is composed of 12 tetrahedra of TO4groups. The 2×2×2 unit cells of Y zeolite were used to construct the simulation boxes. The periodic boundary conditions were applied to simulate an infinite system.
2.2 Simulation method
The Grand Canonical Ensemble is the one of μVT, which is composed of an infnite open system by the constantVandT, namely the constants of volume, temperature and chemical potential. The adsorption process is an open system for the fuid in the adsorbent hole. The adsorption process carried out in the molecular sieve is one in which the exchange of materials and energy between the fluid in adsorbent hole and the bulk fluid while the chemical potential is kept equal. Hence, the adsorption behavior of quinoline in the zeolite Y was studied by the Grand Canonical Monte Carlo (GCMC) method.
The Henry constant is presented below:

in whichNis the simulation step size.
The adsorption heat can be achieved by van’t Hoff’s formula:
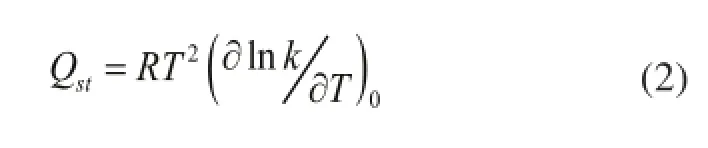
2.3 Parameters of force fi eld
Here, the quinoline and faujasite were selected as the research materials. The Henry constant under different force felds was calculated when the temperature was increased from 623 K to 773 K[27]and 1.1×106steps were performed. The Henry constant is presented below in Figure 1. According to Formula (2), the values of isosteric heat of quinoline in FAU zeolite could be obtained under different force-felds as shown in Figure 1, with the results presented in Table 1.
It can be seen that the difference in the isosteric heat is significant under different force fields as depicted in Table 1. The value of isosteric heat is 97.5 kJ/mol, which agrees well with the literature value[27]measured under the Universal force-field. Hence, the Universal force-feld was selected for investigating the adsorption properties of quinoline homologues on the FAU zeolite.
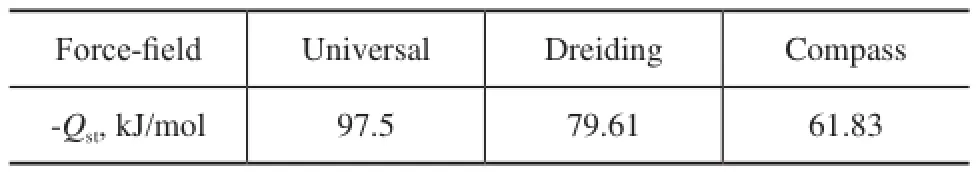
Table 1 The values of isosteric heat of quinoline obtained under different force-fields
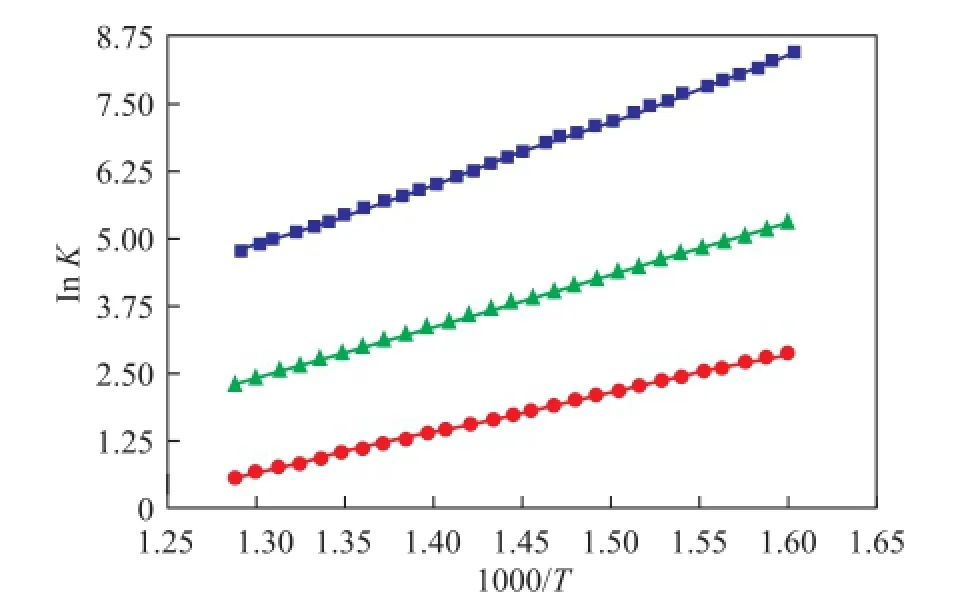
Figure 1 Henry constant of quinoline under different force- fi elds
3 Results and Discussion
3.1 Henry constant and isosteric heat
The relationship between the Henry constant and the temperature of adsorption of quinoline homologues in the FAU zeolite is shown in Figure 2.
The isosteric heat of quinoline homologues on the FAU zeolite can be obtained under Universal force feld by using the ftting data in Figure 2 and Formula (2), with the results shown in Table 2.

Table 2 The isosteric heat of quinoline homologues on FAU zeolite
It can be seen that the isosteric heat of quinoline homologues decreased in the following order: 2,4-dimethyl quinoline (118.63 kJ/mol) > 2-methyl quinoline (110.45 kJ/mol) > quinoline (98 kJ/mol) as depicted in Table 1, which is in agreement with the order of the adsorbate basicity.
3.2 Adsorption isotherm
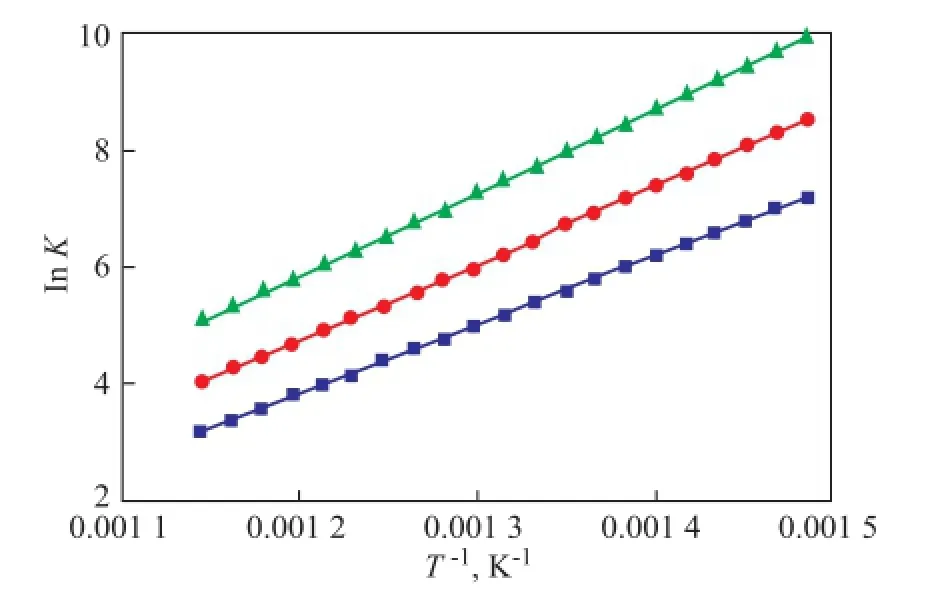
Figure 2 Henry constants of quinoline homologues on FAU zeolite at 673.15—873.15 K
While the Universal force feld was selected, the adsorption isotherms for the quinoline homologues can be calculated respectively at 773.15 K and under 0.01—100 MPa, with the one-component adsorption isotherm shown in Figure 3. It is shown in Figure 3 that the adsorption capacity of quinoline homologues decreased in the following order: quinoline > 2-methyl quinoline > 2,4-dimethyl quinoline. The adsorption capacity of quinoline homologues increased with an increasing pressure. The adsorption capacity has no signifcant increase when the pressure is above 40 MPa.
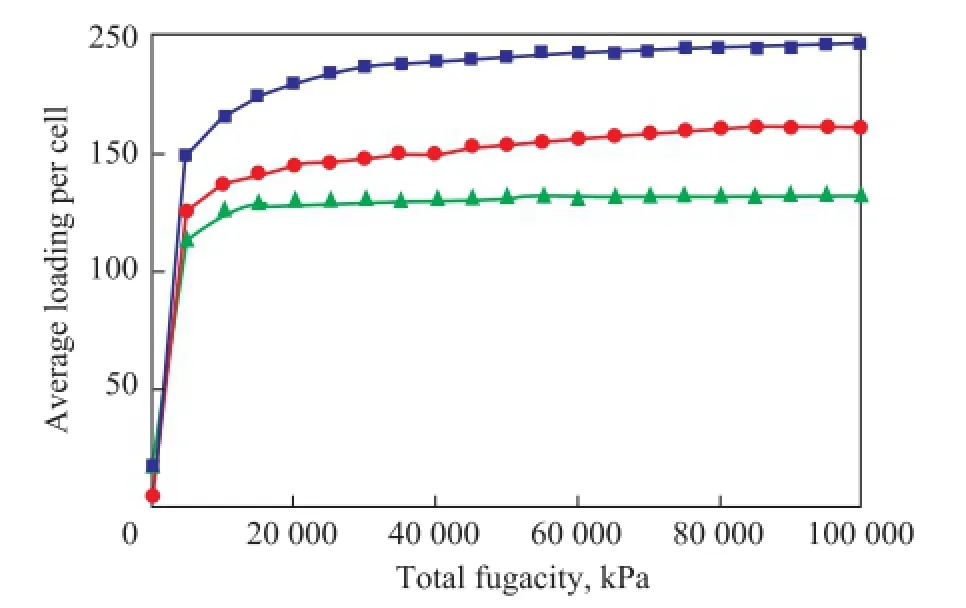
Figure 3 Adsorption isotherms of quinoline homologues on FAU zeolite at 773.15 K
3.3 Adsorption isotherm fi tting
3.3.1 Langmuir isothermal equation
The Langmuir model is given by Formula (3)

whereqis the absolute amount adsorbed in the unit;qmis the maximum adsorption capacity andbis the Langmuir equilibrium constant, which represents the affinity between the adsorbent and the adsorbate;pis the effective pressure, i.e. the fugacity. The data in Figure 3 is fitted according to Formula (3), with the results shown in Table 3.
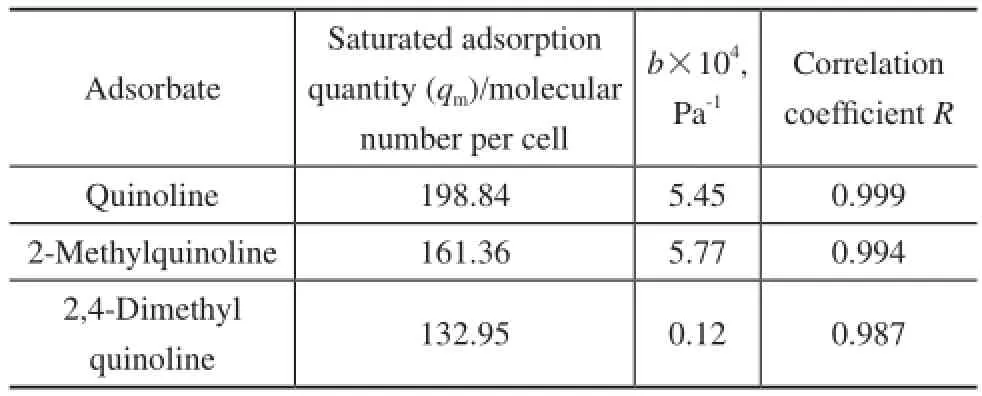
Table 3 The fit Langmuir coefficients for quinoline homologues on FAU zeolite
It is indicated that the Langmuir model fts the data well at 773K as evidenced by the data listed in Table 3. The saturated capacity for adsorption of quinoline homologues on the FAU zeolite decreased in the following order: quinoline > 2-methyl quinoline > 2,4-dimethyl quinoline. Therefore, the smaller the molecular size, the larger the saturated adsorption capacity. Thus, we can conclude that the saturated adsorption capacity correlates well with the molecular size.
3.4 Competitive adsorption of three quinoline homologues on the FAU zeolite
3.4.1 Adsorption isotherms
Adsorption isotherms of three quinoline homologues, the molar ratio of which is 1:1:1, on the FAU zeolite can be obtained at 773.15 K under Universal force feld when the pressure of the system varies from 0.01 kPa to 420 kPa, with the results shown in Figure 4.
It can be seen that the competitive adsorption capacity of three quinoline homologues on the FAU zeolite decreased in the following order: quinoline > 2-methyl quinoline > 2,4-dimethyl quinoline. Therefore, the smaller the molecule size is, the greater the adsorption capacity would be. The pore diameter of the FAU zeolite is about 0.74 nm, and the diameter of hexagonal prism, β cage and supercage is 0.26 nm, 0.66 nm and 1.2 nm, respectively, while the molecular diameter of quinoline, 2-methyl quinoline and 2,4-dimethyl quinoline is about 0.73 nm, 0.78 nm and 0.83 nm, respectively.
Quinoline has a molecular size of 0.73 nm that is commensurate with the size of the channel of FAU zeolite. So quinoline can be adsorbed on acid sites in the channels of FAU zeolite easily. In contrast, 2-methyl quinoline and 2,4-dimethyl quinoline have a bigger size than the diam-eter of the channels of FAU zeolite. Then 2,4-dimethyl quinoline would enter the channels of FAU zeolite with diffculty because of the steric hindrance effect. At present, the molecular size of quinoline homologues plays a dominant role in their competitive adsorption capacity.
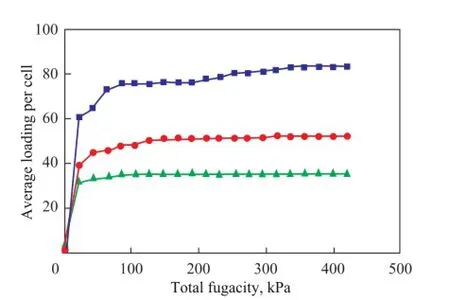
Figure 4 Adsorption isotherms of quinoline nitrides on FAU zeolite
3.4.2 Adsorption sites
It can be seen that the competitive adsorption of quinoline homologues occupied the main channel of the twelvemembered-ring pores primarily when the quinoline homologues were adsorbed in FAU zeolite simultaneously as shown in Figure 5. Then 2,4-dimethyl quinoline could only occupy the main channels of the twelve-memberedring pores because of their relatively larger diameter. Therefore 2,4-dimethyl quinoline molecules could be adsorbed in the biggest supercages of FAU zeolite, whereas quinoline and 2-methyl quinoline could occupy the smaller four-membered-ring channels and six-membered-ring channels in a random distribution.
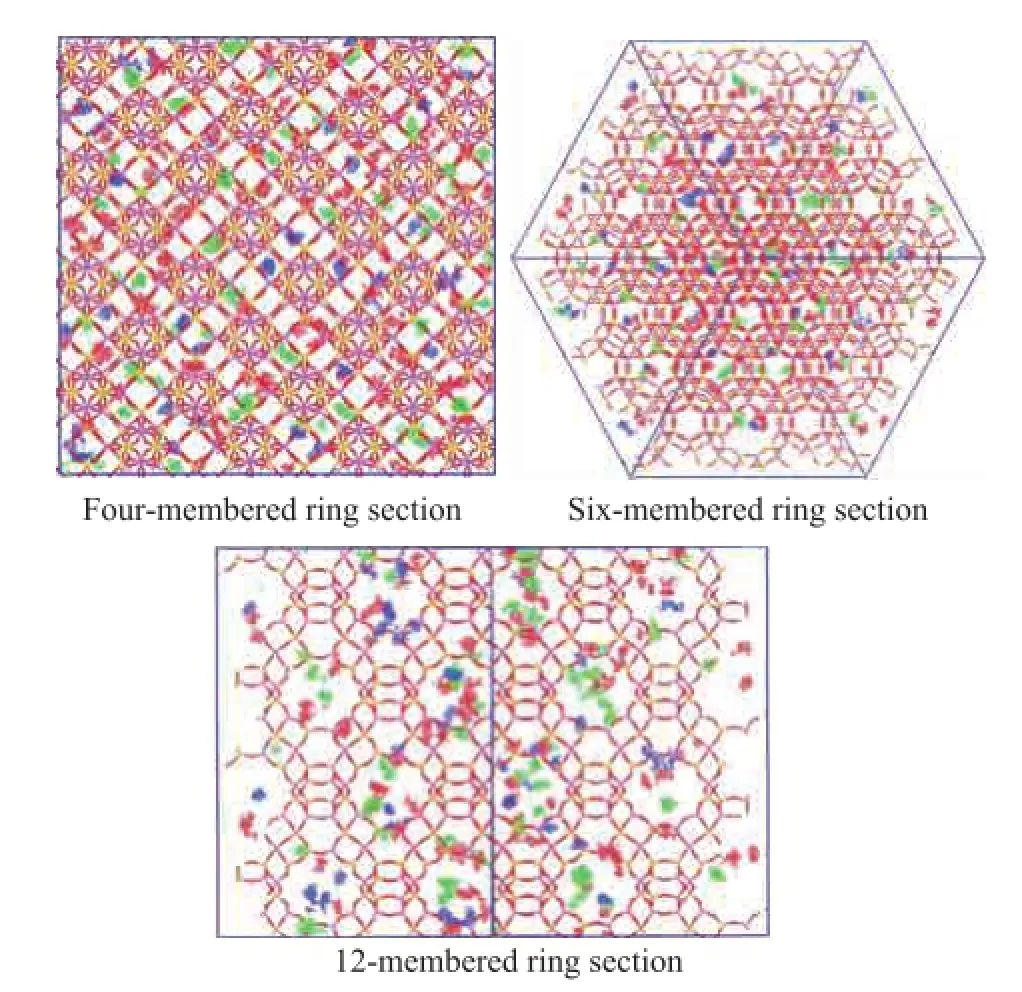
Figure 5 Adsorption sites of quinoline homologues on FAU zeolite
4 Silica/Alumina Ratio
The formula for FAU zeolite is as follows:
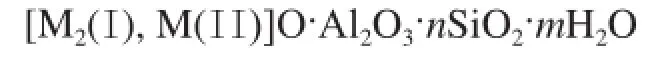
in whichnis the value of silica/alumina ratio.
The silica/alumina ratio is an important factor which affects the properties of molecular sieves. The adsorption heat and capacity for adsorption of quinoline homologues on FAU zeolites with a silica/alumina ratio of 1:1 can be obtained when the temperature increases from 573 K to 873 K under a pressure ranging from 0 to100 MPa after the simulation of 4×105steps.
4.1 Influence of silica/alumina ratio on adsorption heat
The adsorption heat of quinoline homologues on FAU zeolite with different silica/alumina ratio at 573.15 K to 873.15 K is calculated, with the results shown in Table 4.
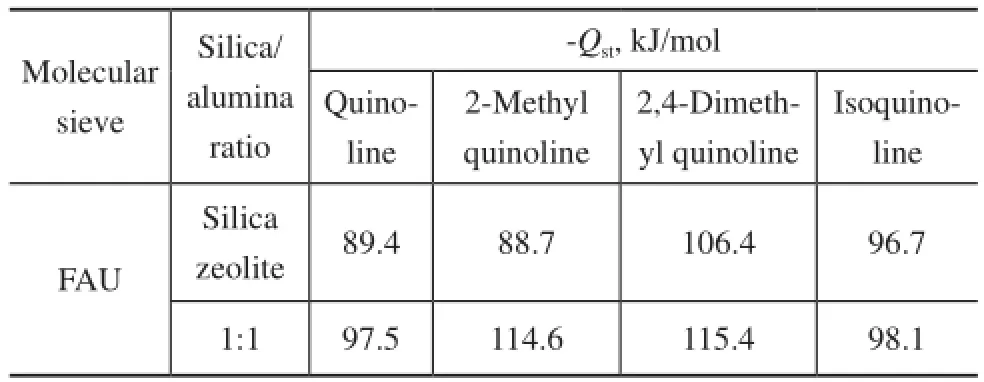
Table 4 Adsorption heat of quinoline homologues on FAU zeolite with different silica/alumina ratios
It can be concluded from Table 4 that the smaller the silica/alumina ratio, the greater the isosteric heat formed on the molecular sieves with the same crystal cell. The reason might be that silicon atoms have much less activity than that of aluminum atoms, and the aluminum atoms can form stronger protonic acid in zeolites.
4.2 Influence of silica/alumina ratio on adsorption capacity
The capacity for adsorption of quinoline homologues on the FAU zeolite with different silica/alumina ratios at 573.15 K under a pressure ranging from 0 to 100 MPa ispresented in Figure 6—8.
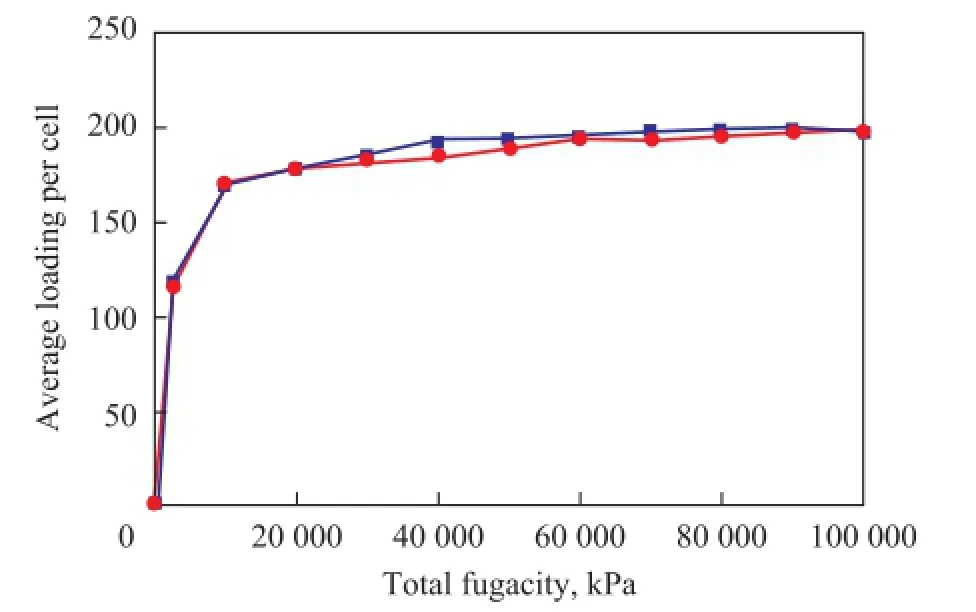
Figure 6 Adsorption isotherms of quinoline on FAU zeolite with different silica/alumina ratio
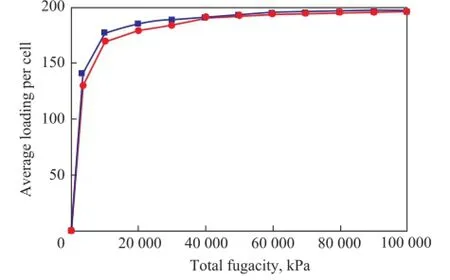
Figure 7 Adsorption isotherms of isoquinoline on FAU zeolite with different silica/alumina ratios
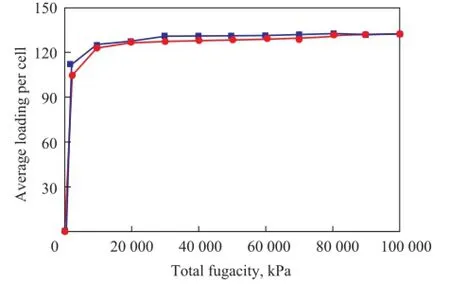
Figure 8 Adsorption isotherms of 2-methyl quinoline on FAU zeolite with different/silica alumina ratios
It can be concluded that the influence of silica/alumina ratio on the adsorption heat and the capacity for adsorption of quinoline homologues in FAU zeolite are consistent with the results presented in Figures 6—8. During the initial adsorption stage, the bigger the silica/alumina ratio was, the smaller the adsorption capacity would be. The difference between them gradually tapers off with the increase in the pressure. At a pressure of 100 MPa, the adsorption capacity is basically identical. It has been shown that aluminum atoms can form stronger protonic acid under smaller pressure. However, the quinoline homologues have stronger alkalinity than other compounds. Thus, the smaller the silica/alumina ratio was, the greater the isosteric capacity would be. The saturated adsorption is basically identical with the case of operation under increased pressure.
5 Conclusions
(1) Universal force-feld was more favorable for calculating the isosteric heat of quinoline in the FAU zeolite the Si/Al ratio of which was 1.1 than that calculated by the Compass and Dreiding force-feld methods. The value of isosteric heat of quinoline was 97.5 kJ/mol which was in accordance with the literature value obtained under the Universal force-feld.
(2) It was shown that the isosteric heat of quinoline homologues decreased in the following order: 2,4-dimethyl quinoline > 2-methyl quinoline > quinoline, which complied with the order of quinoline homologues in terms of their adsorbate basicity
(3) The Langmuir model can be utilized to ft the adsorption isotherms of quinoline homologues on FAU zeolite under the Universal force feld.
(4) The competitive adsorption capacity of three quinoline homologues on the FAU zeolite decreased in the following order: quinoline > 2-methyl quinoline > 2,4-dimethyl quinoline. The saturated adsorption capacity is related to the molecular size of quinoline homologues. The smaller the molecule size was, the greater the adsorption capacity would be.
(5) The quinoline homologues occupied the main channels of twelve-membered-ring pore system primarily. Quinoline and 2-methyl quinoline occupied the fourmembered-ring channels and six-membered-ring channels in a small quantity and at random distribution, while 2,4-dimethyl quinoline only occupied the main twelvemembered-ring channels.
(6) The adsorption heat and adsorption capacity of quinoline homologues on the FAU zeolite increased with thedecrease of silica/alumina ratio.
Reference
[1] Sang Y, Jiao Q Z, Li H S, et al. HZSM-5/MCM-41 composite molecular sieves for the catalytic cracking of endothermic hydrocarbon fuel: nano-ZSM-5 zeolites as the source[J]. Journal of Nanoparticle Research, 2014, 16(12): 2755-2765
[2] Hong X, Tang K. Modification and nitrogen adsorption properties of zeolite NaY [J]. Journal of Fuel Chemistry and Technology, 2015, 43(2): 214-220 (in Chinese)
[3] De Baerdemaeker T, Yilmaz B, Muller U, et al. Catalytic applications of OSDA-free Beta zeolite[J]. Journal of Catalysis, 2013, 308: 73-81
[4] Xu X Y, Sun Y, Shen J, et al. Adsorption behavior of basic nitrides in model oil on HY and USY molecular sieves [J]. Chemical Industry and Engineering Progress, 2014, 33(4): 1035-1040 (in Chinese)
[5] Bastiani R, Lam Y L, Henriques C A, et al. Application of ferrite zeolite in high-olefn catalytic cracking[J]. Fuel, 2013, 107: 680-687
[6] Lai J L, Song L J, Sun Z L. A frequency-response study on sorption of thiophene and benzene on NiY zeolite [J]. China Petroleum Processing and Petrochemical Technology, 2011, 13(2): 24-28
[7] Tang L, Shen J. USY zeolite adsorption properties of basic nitrogen in CGO and its application in dentrification [J]. Specialty Petrochemicals, 2014, 31(4): 18-21(in Chinese)
[8] Liu Yibin, Li Yuzhen, Ding Xue. Adsorption Simulation of Basic Nitrogen Compounds in ZSM-5 and USY Zeolites by Grand Canonical Monte Carlo Method [A]. Advanced Materials Research, 2015, 1096:189-193
[9] Jiang H, Sun W, Wang P. Molecular simulation of adsorption properties of propane on NanZSM-5 zeolites with different Si/Al ratios [J]. Yunnan Chemical Industry, 2011, 38(6): 1-5 (in Chinese)
[10] Sethia G, PillaiR S, Dangi G P, et al. Sorption of methane, nitrogen, oxygen, and argon in ZSM-5 with different SiO2/ Al2O3ratios: Grand Canonical Monte Carlo simulation and volumetric measurements [J]. Industrial & Engineering Chemistry Research, 2010, 49(5): 2353-2362
[11] Chen X B, Sun J P, Shen B Y, et al. Effect of basic nitrogen compounds of USY and ZSM-5-type catalytic cracking catalysts and catalytic properties[J]. Journal of China University of Petroleum (Edition of Natural Sciences), 2012, 36(5): 164-168, 174(in Chinese)
[12] Li Z K, Wang G, Liu Y D, et al. Study on reaction performance and competitive adsorption effect during coker gas oil catalytic cracking[J]. Fuel Process Technol, 2013, 115: 1-10
[13] Li Z K, Wang G, Shi Q, et al. Retardation effect of basic nitrogen compounds on hydrocarbons catalytic cracking in coker gas oil and their structural identifcation[J]. Ind Eng Chem Res, 2011, 50(7): 4123-4132
[14] Shen B X, Chen X B, Sun J P, et al. FCC catalyst poisoning mechanism of nitrogen-containing compounds and their countermeasures[J]. Petrochemical Technology, 2013, 44(4): 457-462
[15] Wang G, Liu Y D, Wang X Q, et al. Studies on the catalytic cracking performance of coker gas oil[J]. Energy Fuels, 2009, 23(4): 1942–1949
[16] Wang B, Zhang Y, Zuo M, et al. Kinetics of pyridine desorption from acid sites on HY zeolite and characterization of its acid strength[J]. Petrochemical Technology, 2014, 43(3): 264-268.(in Chinese)
[17] Liu Y J, Sun X Y, Long Y Z, et al. H-STI zeolite adsorption molecular simulation of ammonia molecules[J]. Journal of Jilin Institute of Chemical Technology, 2010, 27 (1): 12-14(in Chinese)
[18] Shen X Z, Li M Q, Zhou H, et al. Molecular simulation of adsorption of amine on FAU zeolite nitride[J]. Computers and Applied Chemistry, 2011, 28(1): 1-4(in Chinese)
[19] Zhang J F, Burke N, Yang Y X. Molecular simulation of propane adsorption in FAU zeolites[J]. Journal of Physical Chemistry C, 2012, 116(17): 9666-9674
[20] Ding X, Liu Y B, Yang C H, et al. Molecular simulation and thermodynamic analysis of FCC dry gas adsorption in ZSM-5 zeolite[J]. Petroleum Processing and Petrochemicals, 2015, 46(9): 58-64 (in Chinese)
[21] Ding X, Liu Y B, Yang C H, et al. Molecular simulations of FCC dry gas components adsorption in zeolite Y[J]. China Petroleum Processing and Petrochemical Technology, 2016, 18 (1): 99-107
[22] Zhang J F, Burke N, Zhang S C, et al. Thermodynamic analysis of molecular simulations CO2and CH4adsorption in FAU zeolites[J]. Chemical Engineering Science, 2014, 113: 54-61
[23] Sun X Y, Li J W, Li Y X, et al. Adsorption of benzeneand propylene in zeolite ZSM-5: Grand Canonical Monte Carlo simulations[J]. Chem Res Chin Univ, 2009, 25(3): 377-382
[24] Wang Y F, Bu C J, Chi Z M, et al. Adsorption of quinoline on zeolite Al-MCM-41[J]. Journal of Chemical Industry and Engineering (China), 2015, 9: 3597-3604 (in Chinese)
[25] Santarossa G, Iannuzzi M, Vargas A, et al. Adsorption of naphthalene and quinoline on Pt, Pd and Rh: A DFT study [J].Chem Phys Chem, 2008, 9(3): 401-413
[26] Yu D Y, Xu H, Que G H, et al. Study on conversion of basic nitrogen compound quinoline in FCC [J]. Journal of Fuel Chemistry and Technology, 2004, 32(1): 43-47(in Chinese)
[27] Tkhoang K S, Romanovskiy B V, Topchieva K V, et al. Adsorptive capacity and catalytic activity of zeolites. II. Heats of adsorption of several hydrocarbons and nitrogencontaining compounds on type-Y zeolite[J]. Journal of Catalysis,1968, 10(2): 209-211
Received date: 2016-09-18; Accepted date: 2016-10-24.
Prof. Shen Xizhou, Telephone:+86-13886050956; E-mail: xzhoush@163.com
- 中國煉油與石油化工的其它文章
- Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
- Optimization and Control of Extractive Distillation with Heat Integration for Separating Benzene/Cyclohexane Mixtures
- Study on the Effect of Surface Modi fi cation on the Properties of Bentonite Greases
- Improvement of Magnetic Field on Tribological Properties of Lubricating Oils with Zinc Butyloctyldithiophosphate
- Study on Tribology Performance of Different Lubricant Additives under Atmospheric Pressure and High Vacuum Conditions
- Investigation on Microcrystalline Wax Doped Asphalts by Temperature Control Mode of Atomic Force Microscopy

