miR-30c promotes Schwann cell remyelination following peripheral nerve injury
Sheng Yi, Qi-hui wang, Li-li Zhao, Jing Qin, Ya-xian wang, Bin Yu, Song-lin Zhou
Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-innovation Center of Neuroregeneration, Nantong University,Nantong, Jiangsu Province, China
How to cite this article: Yi S, Wang QH, Zhao LL, Qin J, Wang YX, Yu B, Zhou SL (2017) miR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen Res 12(10):1708-1715.
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province, China, No. BK20150409; the Natural Science Foundation of Jiangsu Higher Education Institutions of China, No. 15KJB180013; the Natural Science Foundation of Nantong of Jiangsu Province, No. MS12015043; Postdoctoral Science Foundation of China, No. 2016M600435; Postdoctoral Science Foundation of Jiangsu Province of China, No. 1601056A; and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
miR-30c promotes Schwann cell remyelination following peripheral nerve injury
Sheng Yi, Qi-hui wang, Li-li Zhao, Jing Qin, Ya-xian wang, Bin Yu, Song-lin Zhou*
Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-innovation Center of Neuroregeneration, Nantong University,Nantong, Jiangsu Province, China
How to cite this article: Yi S, Wang QH, Zhao LL, Qin J, Wang YX, Yu B, Zhou SL (2017) miR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen Res 12(10):1708-1715.
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province, China, No. BK20150409; the Natural Science Foundation of Jiangsu Higher Education Institutions of China, No. 15KJB180013; the Natural Science Foundation of Nantong of Jiangsu Province, No. MS12015043; Postdoctoral Science Foundation of China, No. 2016M600435; Postdoctoral Science Foundation of Jiangsu Province of China, No. 1601056A; and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Differential expression of miRNAs occurs in injured proximal nerve stumps and includes miRNAs that are firstly down-regulated and then gradually up-regulated following nerve injury.ese miRNAs might be related to a Schwann cell phenotypic switch. miR-30c, as a member of this group, was further investigated in the current study. Sprague-Dawley rats underwent sciatic nerve transection and proximal nerve stumps were collected at 1, 4, 7, 14, 21, and 28 days post injury for analysis. Following sciatic nerve injury, miR-30c was down-regulated, reaching a minimum on day 4, and was then upregulated to normal levels. Schwann cells were isolated from neonatal rat sciatic nerve stumps, then transfected with miR-30c agomir and co-cultured in vitro with dorsal root ganglia.e enhanced expression of miR-30c robustly increased the amount of myelin-associated protein in the co-cultured dorsal root ganglia and Schwann cells. We then modeled sciatic nerve crush injury in vivo in Sprague-Dawley rats and tested the effect of perineural injection of miR-30c agomir on myelin sheath regeneration. Fourteen days aer surgery, sciatic nerve stumps were harvested and subjected to immunohistochemistry,western blot analysis, and transmission electron microscopy.e direct injection of miR-30c stimulated the formation of myelin sheath,thus contributing to peripheral nerve regeneration. Overall, our findings indicate that miR-30c can promote Schwann cell myelination following peripheral nerve injury.e functional study of miR-30c will benefit the discovery of new therapeutic targets and the development of new treatment strategies for peripheral nerve regeneration.
nerve regeneration; peripheral nerve regeneration; peripheral nerve injury; sciatic nerve; miRNAs; miR-30c; dedifferentiation;Schwann cells; myelination; in vivo; in vitro; neural regeneration
Introduction
Injured peripheral nerves retain certain regenerative abilities(Gu et al., 2010; Liu et al., 2011), which is in contrast to the very limited capacity of central nervous system neurons to regenerate. However, full recovery of peripheral nerves aer severe injury is impossible (Geuna et al., 2013). Successful regeneration of injured nerves requires the intrinsic regenerative capability of the neurons and a suitable microenvironment (Chen et al., 2007; Barrette et al., 2008).
Schwann cells are the main glial cells in the peripheral nervous system and are involved in the formation of Bunger bands, the secretion of growth factors, cytokines, and neuro-trophic factors.ey also orchestrate the remyelination of axons; therefore, they establish a permissive local microenvironment for nerve regeneration (Chen et al., 2005; Webber et al., 2010; Wang et al., 2013). It is worth noting that the cellular states of Schwann cells are different in the distinct phases following peripheral nerve injury. In the acute phase post injury, Schwann cells dedifferentiate into stemlike cells, proliferate and migrate to guide axonal regrowth(Freidin et al., 2009; Quintes et al., 2016). Aer the initiation of axonal elongation, dedifferentiated Schwann cells undergo redifferentiation and remyelination, further participating in the regeneration of myelin sheath (Sundem et al., 2016;Tian et al., 2016).e phenotypic changes of Schwann cells are regulated by a variety of coding and non-coding genes(Xie and Eggan, 2016).
MicroRNAs (miRNAs) are short non-coding RNAs that negatively regulate their target genes at the post-transcriptional level by directly binding to the 3′-untranslated region of complementary mRNAs (Ambros, 2004; Bartel, 2015). A large number of miRNAs are differentially expressed following peripheral nerve injury and they play important roles in modulating the phenotype of Schwann cells (Bremer et al.,2010; Yu et al., 2010). Based on previously obtained miRNA microarray data, we identified 77 differentially expressed miRNAs in injured proximal nerve stumps. According to their expression patterns, these miRNAs were clustered into four patterns: patterns 6, 26, 76, and 9 (Yu et al., 2012a).In pattern 9, miRNAs were down-regulated in the acute phase post injury and were then up-regulated at later time points.is temporal expression pattern was similar to the timeline of Schwann cell redifferentiation indicating that dysregulated miRNAs in this pattern might be involved in the redifferentiation of Schwann cells and the myelination process. Involvement of miRNAs in nerve myelination was recently shown by independent studies of Dicer1-deficient Schwann cells (Pereira et al., 2010; Verrier et al., 2010; Yun et al., 2010; Viader et al., 2011).
We focused here on miR-30c, the miRNA with the most significantly changed expression in pattern 9. We studied its involvement in peripheral nerve injury and regeneration,so as to search for a new treatment strategy for peripheral nerve injury.
Materials and Methods
Ethics statement
All the experimental procedures involving animals were ethically approved by the Administration Committee of Experimental Animals, Jiangsu Province, China (No. 20150304-003) and were used in accordance with Institutional Animal Care guidelines of Nantong University.
Animals
All experimental animals were obtained from the Experimental Animal Center of Nantong University (No. SCXK[Su] 2014-0001 and SYXK [Su] 2012-0031) and were housed in large temperature- and humidity-controlled cages with sawdust bedding. Animals had free access to tap water and food ad libitum. We used 54 adult, male Sprague-Dawley rats weighing 180–200 g; 42 rats were used for the sciatic nerve transection model, and 12 were used for the in vivo experiment. Schwann cells were obtained from 30 neonatal 1-day-old Sprague-Dawley rats of either sex. Dorsal root ganglia (DRGs) were isolated from embryonic 15 day Sprague-Dawley rats of either sex.
Sciatic nerve transection
Thirty-six Sprague-Dawley rats were subjected to surgical transection of sciatic nerves. Briefly, each rat was anaesthetized by injection of a mixture of 85 mg/kg trichloroacetaldehyde monohydrate (RichJoint, Shanghai, China), 42 mg/kg magnesium sulfate (Xilong Scientific, Guangzhou,China), and 17 mg/kg sodium pentobarbital (Sigma-Aldrich,St. Louis, MO, USA).e sciatic nerve was exposed and li-ed through a 30 mm-long incision on the lateral side of the mid-thigh of the left hind limb. A 10 mm-long nerve segment was excised at the site just proximal to the division of tibial and common peroneal nerves. Six rats were sacrificed by decapitation at each time-point of 1, 4, 7, 14, 21, and 28 days post injury, and 5 mm-long proximal nerve stumps were collected. Sham-operated rats (with sciatic nerves exposed but uninjured) served as controls and were designated as 0 days post-sciatic nerve injury.
RNA extraction, microarray and bioinformatic analysis
Total RNA was extracted from proximal nerve stumps using a mirVanaTM miRNA isolation kit (Ambion, Austin, TX,USA) or TrizolTM reagent (Life Technologies, Carlsbad,CA, USA) for subsequent miRNA and mRNA microarray analyses.e quality of isolated RNA samples was evaluated using an Agilent Bioanalyzer 2100 (Agilent technologies,Santa Clara, CA, USA) and then quantified using a Nano-Drop ND-1000 spectrophotometer (Infinigen Biotechnology, City of Industry, CA, USA). Genome-wide miRNA and mRNA expression patterns were determined and analyzed by microarray as previously described (Zhou, et al., 2014).Potential mRNA targets of miRNAs in pattern 9 were predicted by integrating putative miRNA targets from the miRBase database (http://www.mirbase.org/) with differentially expressed mRNAs identified from the mRNA microarray analysis. These mRNA targets were further analyzed using Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatic resources (https://david.ncifcrf.gov/) to enrich for significant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
Quantitative real-time PCR
RNA isolated from proximal nerve stumps was reverse transcribed using a TaqMan? MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and stem-loop RT primers (Ribobio) to detect the expression levels of miR-30c. For quantification of gene expression, real-time PCR was conducted using SYBR Premix Ex Taq (Tli RNaseH Plus) (TaKaRa) and a StepOnePlus real-time PCR system (Applied Biosystems).e relative expression levels were quantified by the 2-ΔΔCtmethod.
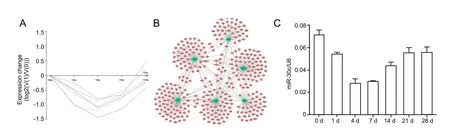
Figure 1 Changes in miRNA expression in proximal nerve stumps following sciatic nerve transaction

Figure 2 Immunostaining of miR-30cmediated myelination of Schwann cells
In vitro experiment
Sciatic nerve sections were collected from 30 neonatal 1-dayold Sprague-Dawley rats after anaesthetized by injection of a mixture of 85 mg/kg trichloroacetaldehyde monohydrate(RichJoint), 42 mg/kg magnesium sulfate (Xilong Scientific),and 17 mg/kg sodium pentobarbital (Sigma-Aldrich). The skin was incised on the lateral side of the hind limb, the sciatic nerve exposed, and an 8 mm-long section of the nerve collected. Primary Schwann cells were isolated from the nerve sections by using trypsin and then treated with anti-Thy1.1 antibody (Sigma) and rabbit complement (Invitrogen, Carlsbad, CA, USA) to remove fibroblasts (Mantuano et al., 2008).is procedure has previously been shown to produce a cell population of > 98% Schwann cells, as indicated by staining with the Schwann cell marker, anti-S100 (DAKO, Carpinte-ria, CA, USA) (Yi et al., 2016a). Primary Schwann cells were passaged no more than three times prior to use and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Invitrogen)in a humidified 5% CO2incubator at 37°C.
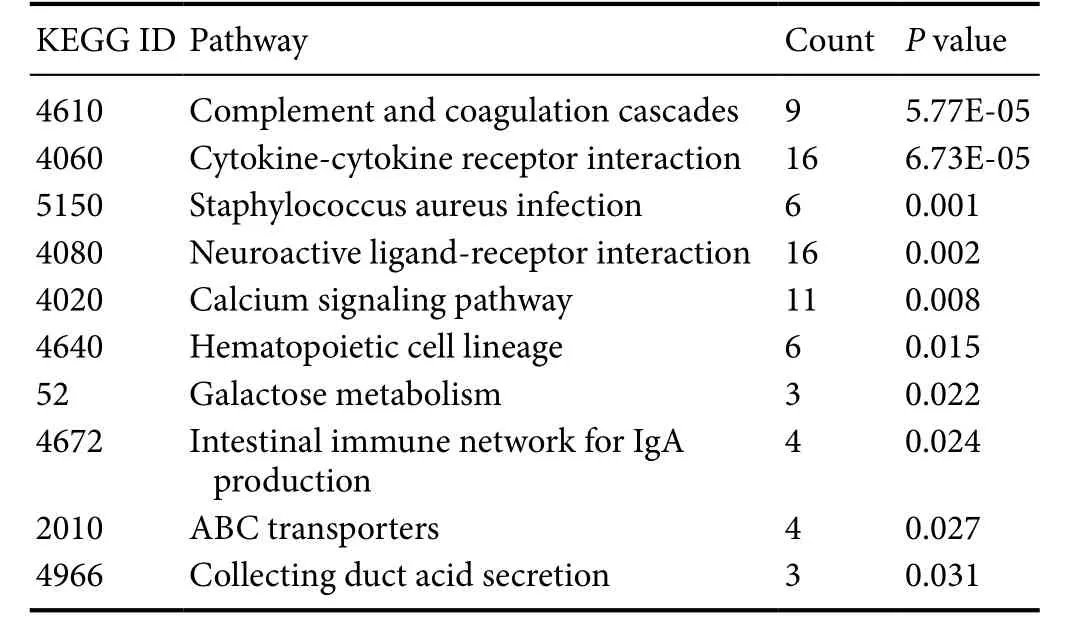
Table 1 Enriched KEGG pathways of intersected genes
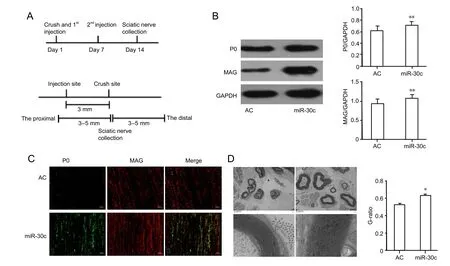
Figure 3 miR-30c promoted myelination of injured sciatic nerve in vivo.
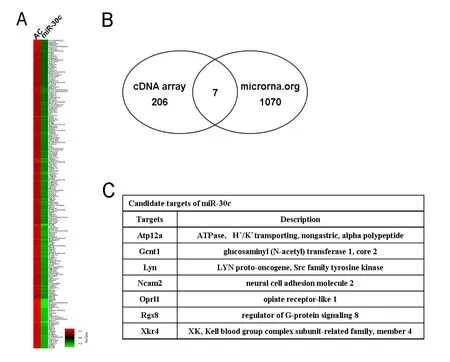
Figure 4 Identification of candidate miR-30c targets.
Purified Schwann cells were transfected with miR-30c agomir (20 nmol/L; Ribobio, Guangzhou, Guangdong Province, China), negative control (20 nmol/L; Ribobio),miR-30c inhibitor (100 nmol/L; Ribobio), or inhibitor control (100 nmol/L; Ribobio) over3 days using Lipofectamine RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s instructions (Zhou, et al., 2014). Agomir is very stable and is suitable for long-term miRNA gain of function studies both in vitro and in vitro (Li et al.,2015).
The co-culture of rat DRGs and Schwann cells was performed as previously described (Saitoh and Araki, 2010).Briefly, DRGs were isolated from embryonic day 15 Sprague-Dawley rats, digested in 1% collagenase, and plated on 24-well plates coated with extracellular matrix laminin(Invitrogen) and poly-L-lysine (Sigma) at 2 × 105cells per well in neurobasal medium supplemented with 2% B27 and 100 ng/mL nerve growth factor. Transfected Schwann cells (3× 105cells per well) were then plated onto established neuronal cultures at a DRG: Schwann cell ratio of 1:5 in DMEM supplemented with 10% FBS and 100 ng/mL nerve growth factor. Seven days later, the mixed cultures of Schwann cells and neurons were treated with 50 μg/mL ascorbic acid (Sigma) to induce myelination. Myelination was allowed to proceed for up to 28 days, and the culture medium was renewed every 2–3 days.
In addition, Schwann cells transfected with miR-30c agomir or non-targeting negative control were isolated for cDNA array analysis. mRNAs that were down-regulated more than 2-fold were selected for miR-30c candidate target gene prediction.
In vivo experiment
Twelve adult male Sprague-Dawley rats were anaesthetized and their sciatic nerves were crushed with forceps as previously described (Yi et al., 2015). Rats were randomly divided into two groups (n = 6 per group) to receive injection of miR-30c agomir or agomir control. Five nmol of miR-30c agomir or negative control were dissolved in 16 μL saline and injected perineurally at the injury site on the same day and 7 days aer nerve crush. Fourteen days aer nerve injury, the rats were sacrificed by decapitation and their sciatic nerve stumps were harvested and subjected to immunohistochemistry, western blot analysis, and transmission electron microscopy observation.e miR-30c agomir was directed at the mature miRNA sequence,synthesized by chemical methods, and treated with specific chemical modification methods by Ribobio. It can be applied by systemic or local injection, inhalation delivery,or oral administration and is able to cross cell membranes(Su et al., 2015).
Immunohistochemistry and immunofluorescence
Co-cultured DRGs and Schwann cells and crushed proximal nerve stumps were collected and processed as previously described (Zanazzi et al., 2001). For immunostaining, cells and tissue slices were fixed with 4% paraformaldehyde/PBS for 5 minutes at room temperature, blocked with 5% goat serum for 30 minutes at room temperature and incubated with primary antibodies: anti-myelin basic protein (MBP)(1:500; AB62631, Abcam St Louis, MO, USA), anti-peripheral myelin protein zero (P0) (1:125; AB31851, Abcam),or anti-myelin-associated glycoprotein (MAG) (1:200;AB89780, Abcam) overnight at 4°C. Specimens were then incubated with Cy3-conjugated goat anti-rabbit secondary antibody (1:1,000; Sigma) for 2 hours at room temperature,and were then mounted on microscope slides. Samples were visualized and images captured using an optical and epifluorescence microscope (Axio Imager M2, Carl Zeiss Microscopy GmbH, Jena, Germany). All assays were performed in triplicate.
western blot analysis
Protein samples were extracted from cultured cells or crushed proximal sciatic nerve stumps, and lysed in Laemmli sample buffer containing 2% SDS, Tris-HCl (52.5 mmol), and protein inhibitors. Protein concentration was determined using a micro BCA protein assay kit (Pierce, Rockford, IL,USA). Extracted protein lysates were mixed with β-mercaptoethanol, glycerin, and bromophenol-blue and then incubated at 95°C for 5 minutes. Equal amounts of protein were then separated by 10% SDS-PAGE and electro-transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA,USA).e membranes were blocked in 5% nonfat dried milk for 2 hours at room temperature, probed with the following primary antibodies: anti-P0 (1:400), anti-MAG (1:600), or anti-GAPDH (1:4,000; AB8245, Abcam) overnight at 4°C,incubated with goat anti-rabbit HRP-conjugated secondary antibodies (1:5,000; Pierce) for 1 hour at room temperature,and then developed with enhanced chemiluminescence reagent (Pierce). Quantification was determined using Quality One soware (Bio-Rad, Hercules, CA, USA). All assays were performed in triplicate
Transmission electron microscopy
The crushed sciatic nerve stumps were harvested, fixed in 4% glutaraldehyde, post-fixed in 1% osmium tetroxide,dehydrated stepwise in increasing concentrations of ethanol, and then embedded in Epon 812 epoxy resin (Sigma).Aer staining with lead citrate and uranyl acetate, ultrathin sections were observed under a transmission electron microscope (JEOL Ltd., Tokyo, Japan). Images were taken at a magnification of × 1.2 k and × 20.0 k. The diameters of myelinated nerve fibers and corresponding axons were measured using Leica QWin V3 (Leica, Wetzlar, Germany) and the G ratio (axonal diameter/outer diameter of the whole nerve fiber) was calculated.
Table 2 Dysregulated miRNAs in pattern 9 aer sciatic nerve transection
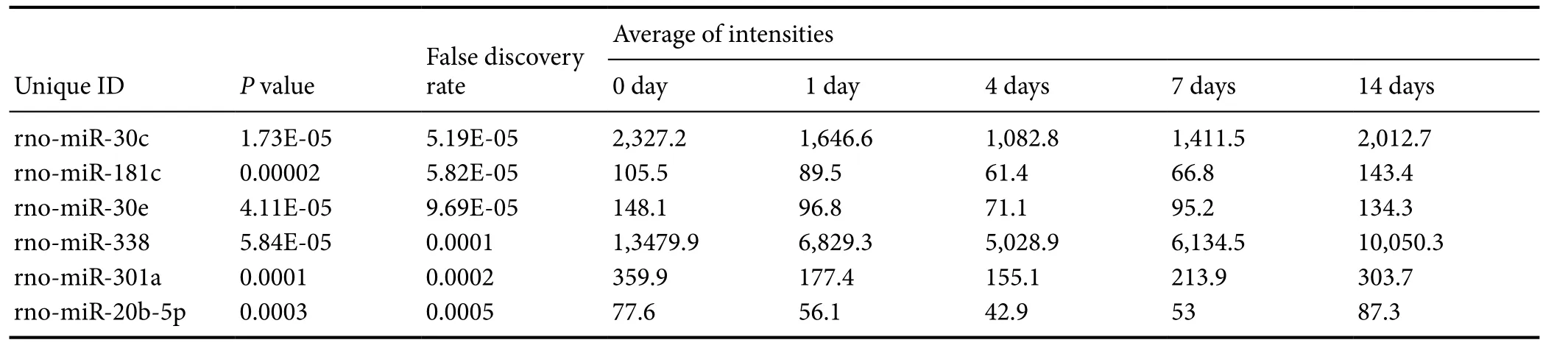
Table 2 Dysregulated miRNAs in pattern 9 aer sciatic nerve transection
False discovery rate Unique IDP value Average of intensities 0 day 1 day4 days7 days14 days rno-miR-30c1.73E-055.19E-052,327.21,646.61,082.81,411.52,012.7 rno-miR-181c0.000025.82E-05105.589.561.466.8143.4 rno-miR-30e4.11E-059.69E-05148.196.871.195.2134.3 rno-miR-3385.84E-050.00011,3479.96,829.35,028.96,134.510,050.3 rno-miR-301a0.00010.0002359.9177.4155.1213.9303.7 rno-miR-20b-5p0.00030.000577.656.142.95387.3
Statistical analysis
Statistical analyses were performed using SPSS 21.0 (SPSS,IBM, NY, USA). Student’s t-test was used for comparison between two groups. A P value < 0.05 was considered statistically significant. All data are expressed as the mean ± SEM.
Results
Numerous miRNAs were down-regulated and then up-regulated in nerve stumps aer sciatic nerve transection
Pattern 9 contained six dysregulated miRNAs: miR-30c,miR-181c, miR-30e, miR-338, miR-301a, and miR-20b-5p. The expression levels of miRNAs in this pattern were decreased from day 1 post-injury and reached a minimum on day 4. Levels then increased and reached normal levels on day 14 (Figure 1A). We compared miRs in the miRBase database with differentially expressed mRNAs (identified by the adjusted F-test with the Random Variation Model) and identified 347 putative target genes. A regulatory network among miRNAs in pattern 9 and their target genes were constructed, which showed that several mRNA targets were regulated by multiple miRNAs (Figure 1B). KEGG pathway analysis identified critical signaling pathways and biological functions involved in these target genes (Table 1). Among enriched KEGG pathways, cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, and calcium signaling pathways include numerous target genes, suggesting that these miRNAs and target mRNAs may regulate neuroprotection and regeneration.
miR-30c was differentially expressed in sciatic nerve stumps at different time points following nerve transection
Firstly, the expression profile of miR-30c was determined by quantitative real-time PCR (Figure 1C). Consistent with the microarray results, the expression level of miR-30c was immediately downregulated post nerve transection, reaching a minimum on day 4, and expression then gradually increased up to 28 days post nerve transection, indicating that miR-30c was downregulated in the acute phase post injury and then slowly returned to normal levels during the regeneration phase.
miR-30c regulated Schwann cell myelination in vitro
miR-30c agomir was transfected into cultured Schwann cells to examine its effect on Schwann cell myelination. Aer the co-culture of Schwann cells with DRGs, myelin sheath began to form.e miR-30c agomir-transfected Schwann cells,showed a higher expression level of MBP (a myelin-associated protein) compared with cells transfected with negative control (Figure 2A). In contrast, cells transfected with miR-30c inhibitor had a reduced expression level of MBP (Figure 2B).ese findings indicated that miR-30c exerted a positive effect on Schwann cell myelination.
miR-30c accelerated sciatic nerve myelinationin vivo
Identification of candidate miR-30c targets
miRNAs function by binding to and regulating their target genes. We attempted to identify candidate miR-30c target genes by performing cDNA microarray analysis together with MiRanda prediction. Schwann cells transfected with miR-30c agomir yielded 206 genes that were down-regulated more than 2-fold (Figure 4A).e miRNA target prediction program, MiRanda, identified 1,070 putative target genes.e joint use of cDNA microarray and MiRanda-based analysis identified seven candidate target genes, Atp12a, Gcnt1,Lyn, Ncam2, Oprl1, Rgs8, and Xkr4 (Figure 4B, C).
Discussion
Successful repair of peripheral nerve injury relies on a series of complicated cellular events, including timely dedifferentiation and redifferentiation of Schwann cells. We previously identified a group of differentially expressed miRNAs (in patterns 6, 26, 76, and 9) in proximal nerve stumps aer sciatic nerve injury. Two of the upregulated miRs in pattern 76,miR-221/222, advanced Schwann cell proliferation and migration by targeting longevity assurance homologue 2 (Lass2)post injury (Yu et al., 2012b). miR-9, a pattern 6 miRNA that was downregulated post injury, inhibited Schwann cell migration by targeting collagen triple helix repeat containing protein 1 (Cthrc1) (Zhou et al., 2014). Other studies also indicated regulatory roles of miRNAs in Schwann cells, such as the beneficial effect of miR-340 on Schwann cell migration, miR-1 and miR-sc3 effects on Schwann cell proliferation and migration, and the inhibitory effect of miR-sc8 and Let-7 on Schwann cell proliferation and migration (Gu et al.,2015; Li, et al, 2015, 2017; Yi et al., 2016b).
These findings mainly identified effects of miRNAs on Schwann cell dedifferentiation (proliferation and/or migration), which is critical in the acute phase post peripheral nerve injury.e redifferentiation of Schwann cells is equally important for peripheral nerve regeneration as proper and timely myelin formation and axon wrapping is necessary for functional recovery. Notably, several studies indicate that the differentiation and myelination processes of oligodendrocytes (glial cells in the central nervous system) can be regulated by miRNAs (Dugas et al., 2010; Emery 2010; Zhao et al., 2010). However, the regulatory effect of miRNAs on the differentiation of and myelination by glial cells in the peripheral nervous system remains unknown.
Considering the importance of Schwann cell redifferentiation, miRNAs in pattern 9 attracted our attention. miRNAs in this pattern exhibited a U-shaped expression pattern and might be related to the phenotype switch of Schwann cells.Post nerve injury, long-term up-regulated miRNAs in this pattern might contribute to Schwann cell redifferentiation and myelin sheath formation (Xu et al., 2014; Yu et al.,2015). Here, we studied the dynamic changes and cellular functions of miR-30c in detail. An in vitro study showed that miR-30c robustly increased the amount of MBP in co-cultured DRGs and Schwann cells. An in vitro study further indicated that miR-30c promoted Schwann cells myelination and thus contributes to nerve regeneration.
Further studies have identified the importance of miR-30c in the nervous system. Sun et al. (2016 a, b) showed that miR-30c promoted the cell cycle of stem cells and enhanced adult neurogenesis in the brain by targeting semaphorin 3A.Li et al. (2015) demonstrated that miR-30c protected the spinal cord from ischemia/reperfusion injury. Our study indicated that miR-30c might also play significant roles in the peripheral nervous system. In the future, we will perform luciferase assays, candidate gene transfection, and rescue assays, to determine the direct target gene(s) of miR-30c.
In summary, we show that miR-30c is downregulated following sciatic nerve injury, reaching a minimum on day 4. Gradual recovery of miR-30c expression promotes the myelination of Schwann cells.e functional study of miR-30c provides a better understanding of the regulatory role of miRNAs in peripheral nerve regeneration and might provide new avenues for the treatment of peripheral nerve injury.
Author contributions:SY, BY, SLZ conceived and designed the experiments. SY, QHW, LLZ, JQ, YXW, and SLZ performed the experiments.SY, YXW, BY, and SLZ analyzed the data. SY, BY, and SLZ contributed to reagents/materials/analysis tools. SY, BY, and SLZ wrote the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest:None declared.
Research ethics:The study protocol was approved by Administration Committee of Experimental Animals of Nantong University (20150304-003). All animal care and animal surgeries were performed in accordance with the Guide for Care and Use of Laboratory Animals (Public Health Service, 19965, NIH Publication No. 85-23).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review: Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Shan-hui Hsu, National Taiwan University, China.
Ambros V (2004) The functions of animal microRNAs. Nature 431:350-355.
Barres BA (2010) Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron 65:597-611.
Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G,Julien JP, Lacroix S (2008) Requirement of myeloid cells for axon regeneration. J Neurosci 28:9363-9376.
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297.
Bremer J, O’Connor T, Tiberi C, Rehrauer H, Weis J, Aguzzi A (2010)Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination. PLoS One 5:e12450.
Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW (2005) Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol 64:613-622.
Chen ZL, Yu WM, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209-233.
Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Zhao X, He X, Han X, Yu Y, Ye F,Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR(2010) MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65:612-626.
Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330:779-782.
Freidin M, Asche S, Bargiello TA, Bennett MV, Abrams CK (2009)Connexin 32 increases the proliferative response of Schwann cells to neuregulin-1 (Nrg1). Proc Natl Acad Sci U S A 106 :3567-3572.
Geuna S, Gnavi S, Perroteau I, Tos P, Battiston B (2013) Tissue engineering and peripheral nerve reconstruction: an overview. Int Rev Neurobiol 108:35-57.
Gu XS, Ding F, Yang YM, Liu J (2011) Construction of tissue engineered nerve gras and their application in peripheral nerve regeneration. Prog Neurobiol 93:204-230.
Gu Y, Chen C, Yi S, Wang S, Gong L, Liu J, Gu X, Zhao Q, Li S (2015)miR-sc8 inhibits Schwann cell proliferation and migration by targeting Egfr. PLoS One 10:e0145185.
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, Liao EY, Luo XH (2015) MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest 125:1509-1522.
Li L, Jiang HK, Li YP, Guo YP (2015) Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J Biomed Sci 22:50.
Li S, Wang X, Gu Y, Chen C, Wang Y, Liu J, Hu W, Yu B, Wang Y,Ding F, Liu Y, Gu X (2015) Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Moler 23:423-433.
Li S, Zhang R, Yuan Y, Yi S, Chen Q, Gong L, Liu J, Ding F, Cao Z, Gu X (2017) MiR-340 regulates fibrinolysis and axon regrowth following sciatic nerve injury. Mol Neur 54:4379-4389.
Liu K, Tedeschi A, Park KK, He Z (2011) Neuronal intrinsic mechanisms of axon regeneration. AnnuRev Neurosci 34:131-152.
Mantei N, Meijer D, Suter U (2010) Dicer in Schwann cells is required for myelination and axonal integrity. J Neurosci 30:6763-6775.
Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM (2008)e hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein.J Neurosci 28:11571-11582.
Pereira JA, Baumann R, Norrmen C, Somandin C, Miehe M, Jacob C, Luhmann T, Hall-Bozic H, Yun B, Anderegg A, Menichella D,Wrabetz L, Feltri ML, Awatramani R (2010) MicroRNA-deficient Schwann cells display congenital hypomyelination. J Neurosci 30:7722-7728.
Quintes S, Brinkmann BG, Ebert M, Frob F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D, Meijer D, Yu B, Zhou S, Yi S, Gu X (2015)e regulatory roles of non-coding RNAs in nerve injury and regeneration. Prog Neurobiol 134:122-139.
Saitoh F, Araki T (2010) Proteasomal degradation of glutamine synthetase regulates schwann cell differentiation. J Neurosci 30:1204-1212.
Su X, Wang H, Ge W, Yang M, Hou J, Chen T, Li N, Cao X (2015) An in vivo method to identify microRNA targets not predicted by computation algorithms: p21 targeting by miR-92a in cancer. Cancer Res 75:2875-2885.
Sun T, Li T, Davies H, Li W, Yang J, Li S, Ling S (2016a) Altered morphologies and functions of the olfactory bulb and hippocampus induced by miR-30c. Front Neurosci 10:207.
Sun T, Li W, Ling S (2016b) miR-30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif 49:270-280.
Sundem L, Chris Tseng KC, Li H, Ketz J, Noble M, Elfar J (2016)Erythropoietin enhanced recovery aer traumatic nerve injury: myelination and localized effects. J Hand Surg Am 41:999-1010.
Suter U, Wegner M, Sereda MW, Nave KA (2016) Zeb2 is essential for Schwann cell differentiation, myelinationandnerve repair. Nat Neurosci 19:1050-1059.
Tian F, Yang W, Mordes DA, Wang JY, Salameh JS, Mok J, Chew J,Sharma A, Leno-Duran E, Suzuki-Uematsu S, Suzuki N, Han SS,Lu FK, Ji M, Zhang R, Liu Y, Strominger J, Shneider NA, Petrucelli L, Xie XS, Eggan K (2016) Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging. Nat Commun 7:13283.
Verrier JD, Semple-Rowland S, Madorsky I, Papin JE, Notterpek L(2010) Reduction of Dicer impairs Schwann cell differentiation and myelination. J Neurosci Res 88:2558-2568.
Viader A, Chang LW, Fahrner T, Nagarajan R, Milbrandt J (2011)MicroRNAs modulate Schwann cell response to nerve injury by reinforcing transcriptional silencing of dedifferentiation-related genes.J Neurosci 31:17358-17369.
Wang Y, Zheng Z, Hu D (2013) Inhibition of EphA4 expression promotes Schwann cell migration and peripheral nerve regeneration.Neurosci Lett 548:201-205.
Webber C, Zochodne D (2010)e nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells.Exp Neurol 223:51-59.
Xu Y , Zhang X , Pu S , Wu J , Lv Y , Du D (2014 ) Circulating microRNA expression profile: a novel potential predictor for chronic nervous lesions. Acta Biochim Biophys Sin (Shanghai) 46:942-949.
Yi S, Wang S, Zhao Q, Yao C, Gu Y, Liu J, Gu X, Li S (2016a) miR-sc3,a novel MicroRNA, promotes Schwann cell proliferation and migration by targeting Astn1. Cell Transplant 25:973-982.
Yi S, Yuan Y, Chen Q, Wang X, Gong L, Liu J, Gu X, Li S (2016b) Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor aer peripheral nerve injury.Sci Rep 6:29121.
Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou S, Gu X, Yu B (2015) Deep sequencing and bioinformatic analysis of lesioned sciatic nerves aer crush injury. PLoS One 10:e0143491.
Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding F, Gu X (2012a) miR-182 inhibits Schwann cell proliferation and migration by targeting FGF9 and NTM, respectively at an early stage following sciatic nerve injury. Nucleic Acids Res 40:10356-10365.
Yu B, Zhou S, Wang Y, Qian T, Ding G, Ding F, Gu X (2012b) miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J Cell Sci 125:2675-2683.
Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D,Marchionni MA, Salzer JL (2001) Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol 152:1289-1299.
Zhou S, Gao R, Hu W, Qian T, Wang N, Ding G, Ding F, Yu B, Gu X(2014) MiR-9 inhibits Schwann cell migration by targeting Cthrc1 following sciatic nerve injury. J Cell Sci 127:967-976.
Graphical Abstract
miR-30c promotes Schwann cell remyelination

*Correspondence to:
Song-lin Zhou, M.D.,songlin.zhou@ntu.edu.cn
orcid:
0000-0001-8598-0922
(Song-lin Zhou)
10.4103/1673-5374.217351
Accepted: 2017-06-02
Copyedited by Jeremy A, Li JY, Li CH, Song LP, Zhao M
- 中國神經(jīng)再生研究(英文版)的其它文章
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration
- Effect of glial cells on remyelination after spinal cord injury
- In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity
- End-to-side neurorrhaphy repairs peripheral nerve injury: sensory nerve induces motor nerve regeneration
- Central projections and connections of lumbar primary afferent fibers in adult rats: effectively revealed using Texas red-dextran amine tracing