Food restriction affects maternal investment but not neonate phenotypes in a viviparous lizard
Yang Wang, Zhi-Gao Zeng, Liang Ma, Shu-Ran Li, Wei-Guo Du,*
?
Food restriction affects maternal investment but not neonate phenotypes in a viviparous lizard
Yang Wang1,2, Zhi-Gao Zeng1, Liang Ma1,2, Shu-Ran Li1,2, Wei-Guo Du1,*
1Key Laboratory of Animal Ecology and Conservational Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China2University of Chinese Academy of Sciences, Beijing 100049, China
Food availability significantly affects an animal’s energy metabolism, and thus its phenotype, survival, and reproduction. Maternal and offspring responses to food conditions are critical for understanding population dynamics and life-history evolution of a species. In this study, we conducted food manipulation experiments in field enclosures to identify the effect of food restriction on female reproductive traits and postpartum body condition, as well as on hatchling phenotypes, in a lacertid viviparous lizard from the Inner Mongolian desert steppe of China. Females under low-food availability treatment (LFT) had poorer immune function and body condition compared with those under high-food availability treatment (HFT). The food availability treatments significantly affected the litter size and litter mass of the females, but not their gestation period in captivity or brood success, or the body size, sprint speed, and sex ratio of the neonates. Females from the LFT group had smaller litter sizes and, therefore, lower litter mass than those from the HFT group. These results suggest that female racerunners facing food restriction lay fewer offspring with unchanged body size and locomotor performance, and incur a cost in the form of poor postpartum body condition and immune function. The flexibility of maternal responses to variable food availability represents an important life strategy that could enhance the resistance of lizards to unpredictable environmental change.
; Food availability; Hatchling; Lizard; Reproductive output
INTRODUCTION
In nature, the food available to an animal exhibits spatio-temporal variation, significantly affecting its energy metabolism and consequently its phenotype, survival, and reproduction (Acheamponget al, 2011; Douhardet al, 2014; Du, 2006; Hoyet al, 2016). Food availability not only affects maternal fitness-related traits (e.g. body size, growth, and reproduction), but might also induce phenotypic variations in offspring (Ballinger & Congdon, 1980; Hillesheim & Stearns, 1991; Jones et al., 2015; Vaissi & Sharifi, 2016). For example, food availability can lead to variations in maternal reproduction (Ballinger, 1977) and offspring growth and survival in lizards(Dunham, 1978; Warneret al, 2015). Therefore, maternal and offspring responses to food conditions are critical for understanding ecological and evolutionary processes, such as population dynamics and life-history evolution.
Food availability can significantly affect female reproductive strategies, such as reproductive timing, investment, and output (offspring number and size) (Ballinger, 1977; Du, 2006; Ramírez-Pinilla, 2006). Two kinds of trade-offs regarding energy allocation are faced by a mother when the energy available to her is limited. First, a mother has to decide on energy allocation for multiple tasks, such as maintenance, growth, and reproduction, leading to important life-history trade-offs (e.g., maintenancereproduction) (Hegemannet al, 2013; Rollinson & Rowe, 2016); for instance, the tropical house wren () decreases parental reproductive investment (i.e. nestling feeding frequency), but does not alter self-maintenance (metabolic rate and body condition) when the cost of activity increases during reproduction (Tielemanet al, 2008). Second, a mother needs to decide on the distribution of energy among offspring within a clutch, thus leading to a trade-off between clutch size and offspring size (Bleuet al, 2013; Duet al, 2005; Olssonet al, 2002). The “optimal egg size theory” assumes that one optimal egg size is appropriate under certain maternal and environmental conditions to maximize the fitness of offspring (Einum & Fleming, 1999; Smith & Fretwell, 1974; Williams, 1966). Accordingly, a mother might give priority of energy allocation to offspring size rather than to clutch size when faced with food restriction, especially in those species whose fitness is determined primarily by egg size (Styrskyet al, 2000). However, other studies have shown that offspring size is not always optimized, and can be highly condition-dependent in some species (Krist & Munclinger, 2015; Rollinson & Hutchings, 2013), with the shift in size being a function of maternal reproductive investment (Caley et al. 2001). In such cases, a mother might change both offspring and clutch sizes in response to food restriction. Despite extensive studies regarding the effect of food availability on maternal reproductive traits (e.g. Bonnetet al, 2001; Du, 2006; Hogstedt, 1981; Kitayskyet al, 1999; Warner & Lovern, 2014; Warneret al, 2015), how female reproduction responds to food availability might differ among species and deserves further investigation.
In addition to maternal reproductive traits, food availability can also profoundly affect offspring phenotypes and fitness. Previous studies have shown that a variety of phenotypic traits of offspring (e.g., body size, locomotor performance, and growth rate) can be affected by maternal food availability (Du, 2006; Haferet al, 2011; Ravehet al, 2016; Warneret al, 2015), likely through a maternal effect—a phenomenon in which environmental information passes through generations by means of plasticity rather than direct genetic transmission (Fox & Mousseau, 1998; Hsuet al, 2016); for example, low food availability compromises the snout-vent length (SVL) of offspring, as well as the mass, performance ability, and fat reserves of the viviparous lizard(Itonagaet al, 2012b). Offspring sex is one of the most interesting phenotypes affected by maternal food availability. The “sex allocation hypothesis” suggests that females can manipulate the sex ratio of their offspring, contingent upon local conditions, to maximize offspring fitness (Rosenfeld & Roberts, 2004; Trivers & Willard, 1973). Food availability can affect the offspring sex ratio in several avian species; for example, when provided with supplementary food, the kakapo () female produces an excess of males (Cloutet al, 2002). However, such studies are scarce for reptilian species (Wapstra & Warner, 2010; Warneret al, 2007).
Viviparous lizards make an excellent model for studying maternal and offspring responses to food availability because many lizards experience fluctuating food resources (Meserveet al, 2016; Zhuet al, 2014), and viviparous species retain their eggs for a longer period than oviparous species do, with higher locomotor and thermoregulatory costs (Le Galliardet al, 2003; Shine, 2003). Moreover, most ectothermic vertebrates are considered capital breeders, in which reproduction is financed from stored energetic capital (Bonnet et al1998), which makes the direct causal relationship between food availability and reproduction harder to detect. Conversely, many viviparous reptiles are considered income breeders because they can use maternal nutrients (matrotrophy) to supplement or replace yolk nutrients (lecithotrophy) for embryonic development (Bonnet et al2001; Ramírez-Pinilla, 2006; Winneet al, 2006). As a result, viviparous lizards may confront more severe trade-offs between reproduction and self-maintenance when experiencing limited resources during gestation, and thus provide a unique system to increase our understanding of the effects of food on life history strategies in animals. In this study, we conducted food manipulation experiments in field enclosures to identify the effect of food restriction on female reproductive traits (such as gestation period in captivity and litter size) and postpartum body condition, as well as hatchling phenotypes (body size and locomotor performance and immune response) in a lacertid viviparous lizard from the Inner Mongolian desert steppe of China. We aimed to address the following questions: (1) How does female reproductive investment respond to food restriction? (2) Does food restriction affect postpartum body condition of females? (3) How does maternal food restriction influence neonate phenotypes?
MATERIALS AND METHODS
Study species
The multi-ocellated racerunner () is a small viviparous lizard that inhabits desert or semiarid areas, with the SVL of adults ranging from 58 to 73 mm. This species is the main lizard fauna in our field study site at the Shierliancheng Field Station, Institute of Grassland Research of the Chinese Academy of Agricultural Sciences (Zenget al, 2016), located in Ordos, Inner Mongolia, China (N40°12¢17¢¢, E111°07¢43¢¢; elevation 1036 m). Thermal and hydric environments significantly affect the reproductive traits and offspring phenotypes in different populations of this species, with the offspring sex ratio biased towards males when gravid females are kept at high temperatures (Tanget al, 2012; Wanget al, 2016).
Maternal food treatment in field enclosures
Adult(16 males and 48 females) were collected from our field study site and were housed in round enclosures (high×diameter=40 cm×180 cm) built at the field site (one male and three females in each enclosure) from May 20 to May 30, 2014, which is the beginning of the reproductive season for this species. All females had bitemarks on their belly, suggesting that they had mated in the field. The enclosures were covered with plastic nets to avoid predation by birds.
On June 1, 2014, the SVL and body mass of all females were measured, after which each female was randomly assigned to a treatment group with either high (=24) or low (=24) prey availability. Lizards in the high-food availability treatment (HFT) group were fed every other day with 0.05 g of mealworms per gram mass of female per day (amounting to 120% of the average consumption of a gravid female, as measured prior to the experiment), whereas those in the low-food availability treatment (LFT) group were fed at the same intervals with 0.025 g of mealworms per gram mass of female per day (amounting to 60% of the average consumption of a gravid female). Each treatment was replicated eight times (for a total of 16 enclosures). Given that all viviparous amniotes have a placenta that transfers maternal nutrients to the embryo (Flemming et al., 2003), viviparousshould be matrotrophic. The females of this species start to copulate in May, and give birth in July and August, with a gestation period of about 54 days (Tang et al., 2012). Our food treatments lasted 30 days (from June 1 to June 30), covering the middle half of the gestation period.
Female reproductive traits and phenotypes of neonates
On July 1, 2014, females (16 females from HFT and 15 females from LFT) in the field enclosures were retrieved and transferred to the laboratory for measurement of reproductive traits. Females were maintained in small cages (long×wide×high= 310×210×180 mm) with a substrate of sand and two small pieces of brick as shelter. The cages were exposed to the natural light regime of the field station, and a 60W incandescent light bulb was suspended 5 cm above each cage for thermoregulation from 0800 to 1200h. Food (mealworms and crickets dusted with additional vitamins and minerals) was provided daily. Each cage contained two females and was checked once per day for neonates, and four times per day following first parturition.
Immediately after a female produced a litter of neonates, the mother was measured and weighed. The SVL and body mass of neonates were then measured to 0.01 mm and 0.001 g accuracy after the absorption of the yolks one day later. Litter size was determined as the number of neonates and litter mass was calculated as the total mass of neonates produced by a female (Li et al, 2006; Ramírez-Bautistaet al, 2000). The gestation period in captivity was calculated as the days between the initiation of treatment and female parturition, which did not include the gestation period in the field before the females were collected. To measure the locomotor performance, each neonate was made to run on a racetrack (80 cm long, marked at 20-cm intervals), within 2–3 days of birth, by stimulation with a soft paintbrush (Irschick & Losos, 1998). This procedure was repeated twice at 30±1 °C allowing a break of 1 h between each test. For quantitating locomotor performance, the sprint speed was determined by averaging the fastest speeds for covering a distance of 20 cm in each race. The sex of neonates was identified by observing the preanal scales—males have large, square, regularly-distributed preanal scales, whereas females have small, round, and scattered preanal scales (Wanget al, 2016).
A total of 12 and 14 females produced offspring from the LFT and HFT groups, respectively. Offspring from four clutches in the LFT group and one clutch in the HFT group were stillborn and excluded from further analysis of locomotor performance and offspring sex. The brood success of females was calculated as the number of females producing live neonates / total number of females.
Cellular immune response of postpartum females
Cellular immune response was assessed by administering an injection (20 μL) of 50 mg of phytohemagglutinin (PHA) in the right foot of postpartumfemales. The thickness of the right and left feet were measured every 12 h from 0 to 36 h following the PHA injection. The difference in foot thickness was considered as an index of immune response (Brownet al, 2011; Vinkleret al, 2010). The largest difference in thickness (i.e., peak immune response) was found at 12 h. Thus, PHA-induced skin swelling at 12 h was used to identify the effect of food availability on the immune response of females.
Statistical analysis
All analyses were performed with SPSS Statistics software (ver. 22; IBM Corp. 2014). Data were normalized by log-transformation when necessary. Maternal body condition was calculated as a residual score from the regression of body mass on SVL (Jakobet al, 1996). Treatment effects on maternal body condition, PHA response, reproductive output, and neonate traits were evaluated by one-way analysis of variance (ANOVA). Clutch means were calculated for neonate traits to avoid pseudo-replication. Pearson-Square test was used for comparing between-treatment differences in brood success.
RESULTS
At the beginning of the experiment, both male and female body sizes did not differ between food treatments (Table 1). However, the postpartum body conditions were worse for females in the LFT group than for those in the HFT group (1,24=7.928,=0.010) (Figure 1A), although initial body condition did not differ between the two food treatment groups (1,24=1.162,=0.292). In addition, females from the LFT group had poorer immune function than those from the HFT group, as indicated by the lower PHA response of LFT group females (1,23=7.214,=0.014, Figure 1B).
Food availability treatment did not affect brood success or gestation period in captivity of females, but did significantly affect both litter size and litter mass (Table 1). The females from the LFT group had smaller litter sizes and, therefore, lower litter mass compared with those for females from the HFT group (Table 1). Moreover, maternal food availability did not affect the body size (SVL and body mass), sprint speed, or sex ratio of the neonates (Table 1).
DISCUSSION
When facing food restriction, female racerunners lay fewer offspring with unchanged body size and locomotor performance, but at a cost in terms of poor postpartum body condition and immune function. These maternal and offspring responses to food restriction have interesting implications for our understanding of the reproductive strategies of lizards under temporally fluctuating food abundance in nature.
A female must decide how to allocate limited resources to the processes of self-maintenance and reproduction (Du, 2006; Hegemannet al, 2013; Itonagaet al, 2012a). Obviously, female racerunners from the LFT group produced neonates with a similar body size to those produced by females from the HFT group, but at the cost of poor body condition and immune function. Understandably, lizards adopt a strategy of keeping reproductive investment relatively constant and sacrificing otherrequirements like those of immune function and energy storage under constrained food conditions, because maintaining acompetent immune system is a nutritionally demanding process that necessitates trade-off decisions with competing nutrient demands, such as those of growth, reproduction, and thermal regulation (Sheldon & Verhulst, 1996; Ulleret al, 2006; Zamora-Camachoet al, 2016). This strategy of females giving priority to reproduction rather than to self-maintenance has also been reported in other reptiles and vertebrate species; for example, the body condition of female jacky dragons () fed a low-quality diet declined dramatically throughout the reproductive season, whereas their hatchlings were larger and in better body condition than those produced from females fed a high-quality diet (Warneret al, 2007). Such a life strategy helps the reproduction and recruitment of populations, but may significantly weaken the immune response and, therefore, the survival of females via elevated risks of predation or starvation in their natural habitat (Iglesias-Carrascoet al, 2016; Neuman-Leeet al, 2015).
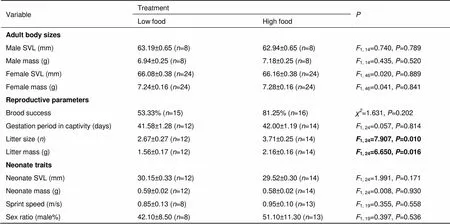
Table 1 Reproductive traits of Eremias multiocellata under high- and low-food availability treatment
Values are expressed as means±. One-way ANOVA and Fisher’s Exact Test were used to compare the between-treatment differences in reproductive traits. Brood success=number of females producing live neonates/total number of females. Neonate traits (except for sex ratio) were calculated as clutch means to avoid pseudo-replication. SVL, snout-vent length.
Figure 1 Effects of food availability treatment on female postpartum body condition (A) and immune function (B)
Data are summarized as means±. Immune function represents cellular immune response assessed by phytohemagglutinin (PHA)-induced skin swelling within 12 h of PHA application. LFT, low-food availability treatment; HFT, high-food availability treatment.
The pre-breeding nutritional condition of females is closely related to the production of neonates in oviparous vertebrates, with reduced fecundity under conditions of food limitation (Du, 2006; Donelsonet al, 2008; Johnsonet al, 2014; Lehman & Smith, 1988; Warner & Lovern, 2014). In response to decreased resources, the females of multiple-clutch species can reduce their reproductive output by decreasing litter size (Sunet al, 2002), offspring size (Abell, 1999), or reproductive frequency (Du, 2006). The viviparous racerunners in our study produced one litter per reproductive season, and decreased their litter size rather than offspring size in response to food restriction. This reproductive strategy reflects the highly critical reproductive investment for each hatchling, suggesting that an optimal neonate body size could exist independent of nutritional conditions. This is consistent with the optimal offspring size theory in which females reproducing in a given environment divide available resources into optimally-sized offspring (Sinervo & Licht, 1991; Smith & Fretwell, 1974). Nonetheless, further studies on the relationship between initial body size and fitness of neonates are needed to verify this hypothesis. At the same time, the reduction in clutch size under conditions of low food availability could be an effective strategy to improve offspring fitness. Fewer offspring means more abundant food per neonate in an environment with finite resources, which will reduce competition and maximize the survival and reproduction of descendants (Bartlett, 1988).
The traditional sex allocation theory generates two conflicting predictions on how maternal nutrition affects the offspring sex ratio (Hamilton, 1967; Trivers & Willard, 1973). Females may produce higher numbers of male offspring under low-food conditions because males might have higher fitness or are more likely to disperse under stressful food conditions (Komdeuret al, 1997; Warneret al, 2007). Alternatively, the opposite pattern may occur, because producing more daughters under low-food conditions will gain greater fitness return given that offspring fitness is less dependent on body size (hence, reproductive energy input) in daughters than in sons (Krist & Munclinger, 2015; Trivers & Willard, 1973). In the current case, however, females did not adjust the sex ratio of their offspring, giving no support to either prediction above.
Overall, female viviparous lizards may sacrifice their own health to produce high-quality offspring and maintain the sex ratio, which could maximize their reproductive success andindividual fitness when experiencing low food availability. In addition to maternal and offspring responses to environmental factors like temperature and precipitation (Maet al, 2014; Wanget al, 2016), the flexibility of maternal responses to variable food availability represents an important life strategy that might enhance the resistance of lizards to unpredictable environmental change. Understanding maternal and offspring responses to the combined impact of these biotic and abiotic factors is a considerable challenge and should be of great interest for future investigations.
Acknowledgements
We thank Shao-Yong Chen and Zhi-Liang Jie for their assistance in the field and laboratory.
Abell AJ. 1999. Variation in clutch size and offspring size relative to environmental conditions in the lizard Sceloporus virgatus.33(2): 173-180.
Acheampong E, Campbell RW, Diekmann ABS, St John MA. 2011. Food availability effects on reproductive strategy: The case of(Copepoda: Calanoida).428: 151-159.
Ballinger RE. 1977. Reproductive strategies: food availability as a source of proximal variation in a lizard.58(3): 628-635.
Ballinger RE, Congdon JD. 1980. Food resource limitation of body growth rates in(Sauria, Iguanidae).1980(4): 921-923.
Bartlett J. 1988. Male Mating success and paternal care in(Coleoptera, Silphidae).23(5): 297-303.
Bleu J, Le Galliard JF, Fitze PS, Meylan S, Clobert J, Massot M. 2013. Reproductive allocation strategies: A long-term study on proximate factors and temporal adjustments in a viviparous lizard.171(1): 141-151.
Bonnet X, Bradshaw D, Shine R. 1998. Capital versus income breeding: An ectothermic perspective., 83(2): 333-342.
Bonnet X, Naulleau G, Shine R, Lourdais O. 2001. Short-term versus long-term effects of food intake on reproductive output in a viviparous snake,.92(2): 297-308.
Brown GP, Shilton CM, Shine R. 2011. Measuring amphibian immunocompetence: Validation of the phytohemagglutinin skin-swelling assay in the cane toad,.2(4): 341-348.
Caley MJ, Schwarzkopf L, Shine R. 2001. Does total reproductive effort evolve independently of offspring size?55(6): 1245-1248.
Clout MN, Elliott GP, Robertson BC. 2002. Effects of supplementary feeding on the offspring sex ratio of kakapo: A dilemma for the conservation of a polygynous parrot.107(1): 13-18.
Donelson JM, McCormick MI, Munday PL. 2008. Parental condition affects early life-history of a coral reef fish.360(2): 109-116.
Douhard M, Plard F, Gaillard JM, Capron G, Delorme D, Klein F, Duncan P, Loe LE, Bonenfant C. 2014. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate.281(1785): 20140276.
Du WG, Ji X, Shine R. 2005. Does body-volume constrain reproductive output in lizards?1(1): 98-100.
Du WG. 2006. Phenotypic plasticity in reproductive traits induced by food availability in a lacertid lizard,.112(2): 363-369.
Dunham AE. 1978. Food availability as a proximate factor influencing individual growth rates in the iguanid lizard.59(4): 770-778.
Einum S, Fleming IA. 1999. Maternal effects of egg size in brown trout (): norms of reaction to environmental quality.266(1433): 2095-2100.
Flemming A F, Blackburn D G. 2003. Evolution of placental specializations in viviparous African and South American lizards., 299A(1): 33–47.
Fox CW, Mousseau TA. 1998. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects.. New York: Oxford University Press, 159-177.
Hafer N, Ebil S, Uller T, Pike N. 2011. Transgenerational effects of food availability on age at maturity and reproductive output in an asexual collembolan species.7(5): 755-758.
Hamilton WD. 1967. Extraordinary sex ratios.156(3774): 477-488.
Hegemann A, Matson KD, Flinks H, Tieleman BI. 2013. Offspring pay sooner, parents pay later: Experimental manipulation of body mass reveals trade-offs between immune function, reproduction and survival.10: 77.
Hillesheim E, Stearns SC. 1991. The responses ofto artificial selection on body weight and its phenotypic plasticity in two larval food environments.45(8): 1909-1923.
Hogstedt G. 1981. Effect of additional food on reproductive success in the Magpie ().50(1): 219-229.
Hoy SR, Millon A, Petty SJ, Whitfield DP, Lambin X. 2016. Food availability and predation risk, rather than intrinsic attributes, are the main factors shaping the reproductive decisions of a long-lived predator.85(4): 892-902.
Hsu BY, Dijkstra C, Darras VM, de Vries B, Groothuis TGG. 2016. Maternal adjustment or constraint: Differential effects of food availability on maternal deposition of macro-nutrients, steroids and thyroid hormones in rock pigeon eggs.6(2): 397-411.
Iglesias-Carrasco M, Head ML, Cabido C. 2016. Habitat dependent effects of experimental immune challenge on lizard anti-predator responses.70(11): 1931-1939.
Irschick DJ, Losos JB. 1998. A comparative analysis of the ecological significance of maximal locomotor performance in Caribbean anolis lizards.52(1): 219-226.
Itonaga K, Jones SM, Wapstra E. 2012a. Do gravid females become selfish? Female allocation of energy during gestation.85(3): 231-242.
Itonaga K, Jones SM, Wapstra E. 2012b. Effects of maternal basking and food quantity during gestation provide evidence for the selective advantage of matrotrophy in a viviparous lizard.7(7): e41835.
Jakob EM, Marshall SD, Uetz GW. 1996. Estimating fitness: A comparison of body condition indices.77(1): 61-67.
Johnson JC, Miles LS, Trubl PJ, Hagenmaier A. 2014. Maternal effects on egg investment and offspring performance in black widow spiders.91: 67-73.
Jones SKC, Munn AJ, Penman TD, Byrne PG. 2015. Long-term changes in food availability mediate the effects of temperature on growth, development and survival in striped marsh frog larvae: Implications for captive breeding programmes.3(1): cov029.
Kitaysky AS, Wingfield JC, Piatt JF. 1999. Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes.13(5): 577-584.
Komdeur J, Daan S, Tinbergen J, Mateman C. 1997. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs.385(6616): 522-525.
Krist M, Munclinger P. 2015. Context dependence of maternal effects: Testing assumptions of optimal egg size, differential, and sex allocation models.96(10): 2726-2736.
Le Galliard JF, Le Bris M, Clobert J. 2003. Timing of locomotor impairment and shift in thermal preferences during gravidity in a viviparous lizard.17(6): 877-885.
Lehman S, Smith AA. 1988. Regional differentiation in the stomach of the green anole.220(4): 364-368.
Li H, Ji X, Qu YF, Gao JF, Zhang L. 2006. Sexual dimorphism and female reproduction in the multi-ocellated racerunner () (Lacertidae).52(2): 250-255.
Ma L, Sun BJ, Li SR, Sha W, Du WG. 2014. Maternal thermal environment induces plastic responses in the reproductive life history of oviparous lizards.87(5): 677-683.
Meserve PL, Vásquez H, Kelt DA, Gutiérrez JR, Milstead WB. 2016. Patterns in arthropod abundance and biomass in the semiarid thorn scrub of Bosque Fray Jorge national park, north-central Chile: A preliminary assessment.126: 68-75.
Neuman-Lee LA, Fokidis HB, Spence AR, Van der Walt M, Smith GD, Durham S, French SS. 2015. Food restriction and chronic stress alter energy use and affect immunity in an infrequent feeder.29(11): 1453-1462.
Olsson M, Wapstra E, Olofsson C. 2002. Offspring size-number strategies: Experimental manipulation of offspring size in a viviparous lizard ().16(1): 135-140.
Ramírez-Bautista A, Balderas-Valdivia C, Vitt LJ. 2000. Reproductive ecology of the whiptail lizard(Squamata: Teiidae) in a tropical dry forest.2000(3): 712-722.
Ramírez-Pinilla MP. 2006. Placental transfer of nutrients during gestation in an Andean population of the highly matrotrophic lizard genus mabuya (squamata: scincidae).20(1): 194-204.
Raveh S, Vogt D, K?lliker M. 2016. Maternal programming of offspring in relation to food availability in an insect ().283(1828): 20152936.
Rollinson N, Hutchings JA. 2013. Environmental quality predicts optimal egg size in the wild.182(1): 76-90.
Rollinson N, Rowe L. 2016. The positive correlation between maternal size and offspring size: Fitting pieces of a life-history puzzle.91(4): 1134-1148.
Rosenfeld CS, Roberts RM. 2004. Maternal diet and other factors affecting offspring sex ratio: A review.71(4): 1063-1070.
Sheldon BC, Verhulst S. 1996. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology.11(8): 317-321.
Shine R. 2003. Effects of pregnancy on locomotor performance: An experimental study on lizards.136(3): 450-456.
Sinervo B, Licht P. 1991. Proximate constraints on the evolution of egg size, number, and total clutch mass in lizards.252(5010): 1300-1302.
Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring.108(962): 499-506.
Styrsky JD, Dobbs RC, Thompson CF. 2000. Food-supplementation does not override the effect of egg mass on fitness-related traits of nestling house wrens.69(4): 690-702.
Sun LX, Shine R, Zhao DB, Tang ZR. 2002. Low costs, high output: Reproduction in an insular pit-viper (, Viperidae) from north-eastern China.256(4): 511-521.
Tang XL, Yue F, Yan XF, Zhang DJ, Xin Y, Wang C, Chen Q. 2012. Effects of gestation temperature on offspring sex and maternal reproduction in a viviparous lizard () living at high altitude.37(6): 438-444.
Tieleman BI, Dijkstra TH, Klasing KC, Visser GH, Williams JB. 2008. Effects of experimentally increased costs of activity during reproduction on parental investment and self-maintenance in tropical house wrens.19(5): 949-959.
Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring.179(4068): 90-92.
Uller T, Isaksson C, Olsson M. 2006. Immune challenge reduces reproductive output and growth in a lizard.20(5): 873-879.
Vaissi S, Sharifi M. 2016. Changes in food availability mediate the effects of temperature on growth, metamorphosis and survival in endangered yellow spotted mountain newt: Implications for captive breeding programs.71(4): 444-451.
Vinkler M, Bainová H, Albrecht T. 2010. Functional analysis of the skin-swelling response to phytohaemagglutinin.24(5): 1081-1086.
Wang Y, Zeng ZG, Li SR, Bi JH, Du WG. 2016. Low precipitation aggravates the impact of extreme high temperatures on lizard reproduction.182(4): 961-971.
Wapstra E, Warner DA. 2010. Sex allocation and sex determination in squamate reptiles.4(1-2): 110-118.
Warner DA, Lovern MB, Shine R. 2007. Maternal nutrition affects reproductive output and sex allocation in a lizard with environmental sex determination.274(1611): 883-890.
Warner DA, Lovern MB. 2014. The maternal environment affects offspring viability via an indirect effect of yolk investment on offspring size.87(2): 276-287.
Warner DA, Buckelew AM, Pearson PR, Dhawan A. 2015. The effect of prey availability on offspring survival depends on maternal food resources.115(2): 437-447.
Williams LH. 1966. Observations on the life history of the poplar hawk moth,L. Part I. Mating, egg laying and larval development and behaviour.41(7-9): 93-102.
Winne CT, Willson JD, Gibbons JW. 2006. Income breeding allows an aquatic snaketo reproduce normally following prolonged drought-induced aestivation.75(6): 1352-1360.
Zamora-Camacho FJ, Reguera S, Moreno-Rueda G. 2016. Elevational variation in body-temperature response to immune challenge in a lizard.4: e1972.
Zeng ZG, Bi JH, Li SR, Wang Y, Robbins TR, Chen SY, Du WG. 2016. Habitat alteration Influences a desert steppe lizard community: Implications of species-specific preferences and performance.30(1): 34-48.
Zhu H, Wang DL, Wang L, Fang J, Sun W, Ren BZ. 2014. Effects of altered precipitation on insect community composition and structure in a meadow steppe.39(4): 453-461.
10.24272/j.issn.2095-8137.2017.011
24 January 2017; Accepted: 03 March 2017
This study was supported by the grant from the National Natural Science Fund for Distinguished Young Scholars (31525006)
E-mail: duweiguo@ioz.ac.cn
- Zoological Research的其它文章
- Comparative study of the transfection efficiency of commonly used viral vectors in rhesus monkey (Macaca mulatta) brains
- FasParser: a package for manipulating sequence data
- Characterization of cyclophilin D in freshwater pearl mussel (Hyriopsis schlegelii)
- Dynamic changes in DNA demethylation in the tree shrew (Tupaia belangeri chinensis) brain during postnatal development and aging
- The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes
- Author Guidelines for Submitting Manuscripts to Zoological Research

