Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: a safety analysis of 14 patients
,
1 Regenerative Medicine Center, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Neurological Department, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
3 Electromyography Department, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: a safety analysis of 14 patients
Xiao-yan Li1, Zhan-hua Liang2, Chao Han1, Wen-juan Wei1, Chun-li Song3, Li-na Zhou3, Yang Liu1, Ying Li1, Xiao-fei Ji2, Jing Liu1,*
1 Regenerative Medicine Center, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Neurological Department, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
3 Electromyography Department, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
How to cite this article:Li XY, Liang ZH, Han C, Wei WJ, Song CL, Zhou LN, Liu Y, Li Y, Ji XF, Liu J (2017) Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: a safety analysis of 14 patients. Neural Regen Res 12(3):493-498.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This study was supported by the National Natural Science Foundation of China, No. 81471308; a grant from the Science and Technology Plan Project of Dalian City in China, No. 2015F11GH094.
Graphical Abstract
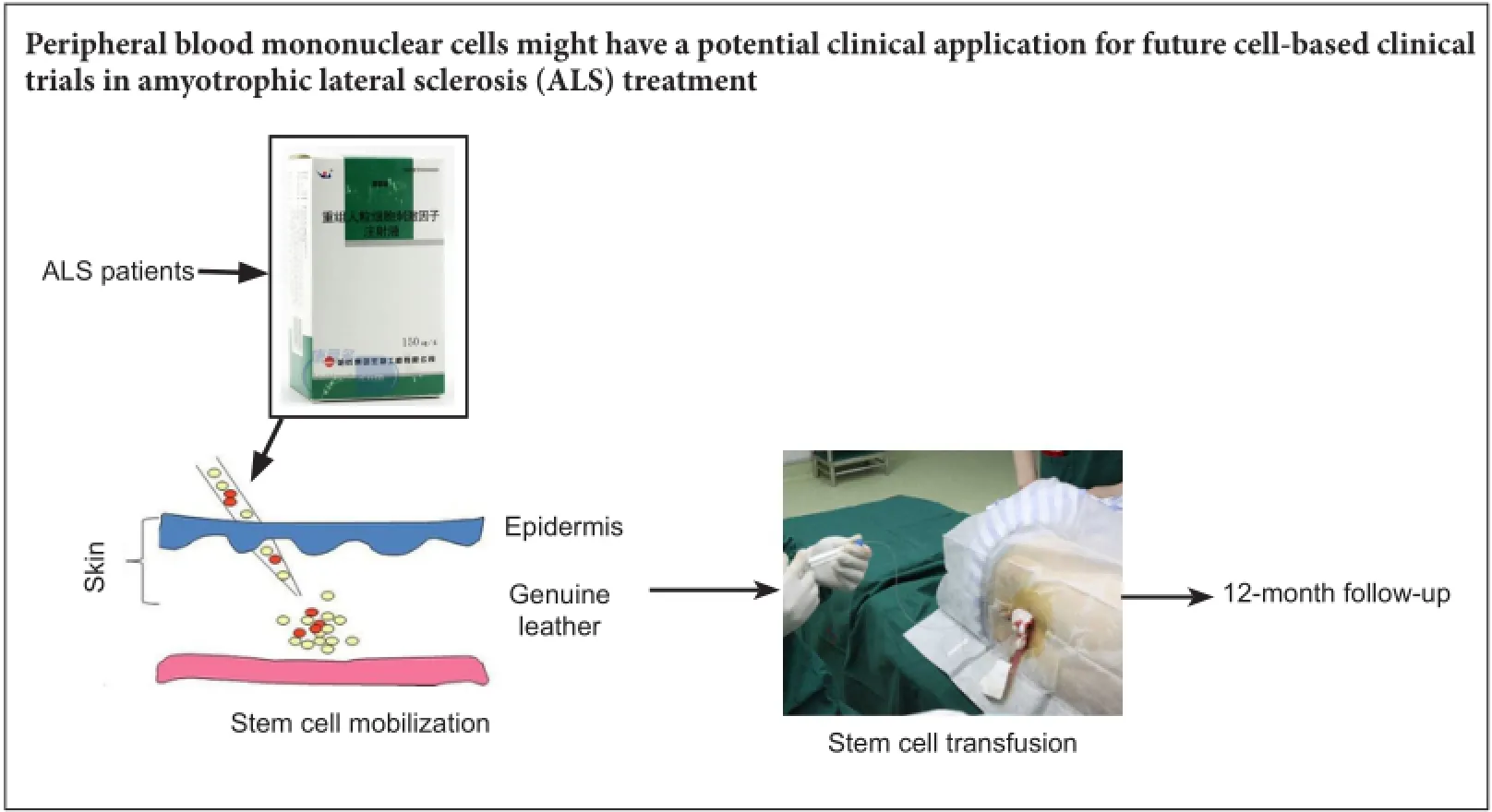
There is a small amount of clinical data regarding the safety and feasibility of autologous peripheral blood mononuclear cell transplantation into the subarachnoid space for the treatment of amyotrophic lateral sclerosis. The objectives of this retrospective study were to assess the safety and efficacy of peripheral blood mononuclear cell transplantation in 14 amyotrophic lateral sclerosis patients to provide more ob9jective data for future clinical trials. After stem cell mobilization and collection, autologous peripheral blood mononuclear cells (1 × 10) were isolated and directly transplanted into the subarachnoid space of amyotrophic lateral sclerosis patients. The primary outcome measure was incidence of adverse events. Secondary outcome measures were electromyography 1 week before operation and 4 weeks after operation, Functional Independence Measurement, Berg Balance Scale, and Dysarthria Assessment Scale 1 week preoperatively and 1, 2, 4 and 12 weeks postoperatively. There was no immediate or delayed transplant-related cytotoxicity. The number of leukocytes, serum alanine aminotransferase and creatinine levels, and body temperature were within the normal ranges. Radiographic evaluation showed no serious transplant-related adverse events. Muscle strength grade, results of Functional Independence Measurement, Berg Balance Scale, and Dysarthria Assessment Scale were not significantly different before and after treatment. These findings suggest that peripheral blood mononuclear cell transplantation into the subarachnoid space for the treatment of amyotrophic lateral sclerosis is safe, but its therapeutic effect is not remarkable. Tus, a large-sample investigation is needed to assess its efficacy further.
nerve regeneration; amyotrophic lateral sclerosis; peripheral blood mononuclear cells; subarachnoid space transplantation; autologous; clinical research; safety; adverse events; neural regeneration
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly evolving, fatal neurodegenerative disease resulting from the degeneration of cortical, bulbar and spinal motor neurons (Poppe et al., 2014; Carreras, 2016; Giacoppo and Mazzon, 2016). The disease progresses inexorably to death, usually because of failure of respiratory function, with a median duration of 3 years.
Recent clinical trials using various types of stem cells, including mesenchymal stromal cells (Mazzini et al., 2012), neural stem cells (Glass et al., 2012), and peripheral blood mononuclear cells (PBMCs) (Janson et al., 2001; Martínez et al., 2012), represent promising strategies for stem cell-based treatment in ALS. It has been demonstrated that the inflammation and neuronal death were reduced in ALS patients after bone marrow transplantation (Mesentier-Louro et al., 2016). In addition, the incidence of immune response was decreased by autologous transplantation of bone marrow cells in ALS patients (Gubert and Satiago, 2016). PBMCs are multi-potent stem cells that are very attractive for a cell therapy approach in ALS because of their plasticity and ability to provide the host tissue with growth factors or modulate the host immune system (Cashman et al., 2008). PBMCs were used clinically and few adverse effects were attributed to their administration (Janson et al., 2001). Early clinical investigations indicated that the transplantation of autologous PBMCs into the dura is feasible in ALS patients; however, one study was limited to three patients (Janson et al., 2001) and the other recruited eight patients (Cashman et al., 2008). There are still many questions regarding the intrathecal transplantation of PBMCs for ALS. We, therefore, performed a retrospective study to assess further the safety and efficacy of the procedure and to test the impact of a cell therapy approach in ALS patients.
Subjects and Methods
Design
A retrospective, self-control study was conducted at the First Affiliated Hospital of Dalian Medical University, China. Fourteen ALS patients who received PBMC autotransplantation were enrolled. The primary outcome measure was the incidence of adverse events. Secondary outcome measures were electromyography (EMG) 1 week before operation and 4 weeks after operation, Functional Independence Measurement (FIM), Berg Balance Scale, and Dysarthria Assessment Scale 1 week preoperatively and 1, 2, 4 and 12 weeks postoperatively. The research procedure is shown inFigure 1.
Subjects
This study was registered with ClinicalTrials.gov (NCT 03085706). Overall, 14 patients aged 31 to 75 years old were eligible if they had definite or probable sporadic ALS and had been treated over 3 months.
Inclusion criteria included: (1) all subjects had a verifiable diagnosis of ALS for 0.5 to 2 years based on a diagnosis using the Revised Criteria of the World Federation of Neurology (Brooks et al., 2000). The grades of diagnosis were clinically definite ALS or clinically probable ALS; (2) ALS was mild-to-moderate based on the ALS Functional Rating Scale-Revised (Kollewe et al., 2008). Electrophysiological features showed compound muscle action potential (CMAP) amplitude of motor nerve normal or mild declining; (3) serum creatine kinase was normal or mild upper, less than 500 U/L.
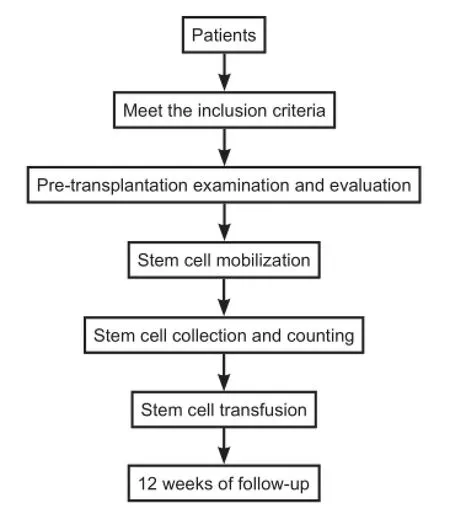
Figure 1 Flow chart of transplantation with autologous peripheral blood mononuclear cells for the treatment of amyotrophic lateral sclerosis patients.
Exclusion criteria included: (1) use of any other investigational agent within 30 days before our treatment; (2) severe cardiac, pulmonary, hepatic or/and hematic disease; (3) human immunodeficiency virus positivity or signs and symptoms consistent with human immunodeficiency virus infection; (4) pregnant or nursing women; (5) history of cancer with less than 5 years documentation of a disease-free state; (6) history of anaphylactic reaction or hypersensitivity to granulocyte colony-stimulating factor (G-CSF); (7) alcohol or drug abuse in recent 1 year; (8) cannot understand or obey the rules of treatment; and (9) blood donor in recent 30 days.
All patients had consecutively visited our treatment center. The mean age of the patients and duration of the disease from diagnosis were 51.7 ± 12.99 years (range: 31-75 years), and 2.33 ± 1.76 years (range: 0.75-8 years), respectively. The mean FIM score at entry was 79.91 ± 18.46 (range: 40-99). All patients received ordinary medical treatment. There were no local or general complications during PBSC separation.
Informed consent was structured as an interview that clearly stated the experimental and preliminary nature of the clinical study and the risks associated with the procedure. Each question was discussed by the neurologist with the patients and their relatives. Subjects were made aware that their participation was entirely voluntary and that participation or non-participation would not interfere with their ongoing clinical care. Before signing, patients and close relatives were offered the possibility of meeting separately with their family physician, the neurosurgeon, and a consultant neurologist, who was not the neurologist in charge, to discuss all pending issues. The study was approved and monitored by the Ethics Committees of the Dalian Medical University (approval number: LCKY-2011-11-01). All patients provided written informed consent.
Isolation of PBMCs
Recombinant human granulocyte colony-stimulating factor injection (rhG-CSF; Harbin Pharmaceutical Group Biological Engineering Co., Harbin, Heilongjiang Province, China) 10 μg/kg was used daily for 4 days to mobilize peripheral blood CD34+mononuclear cells before transplantation. The number of leukocytes met the collection requirements (≥25 × 109), with a mean number of 32.53 ± 8.79 × 109on the morning of the surgery before cell collection. COBE SpectraTMApheresis System (COBE Spectra 6.1, Gambro BCT, Inc., Lakewood, CO, USA) was used to collect the mononuclear cell suspension from the peripheral blood by centrifugation at 2,400 r/min, in a volume of about 50 mL. The cell collection circuit was established by harvesting from the median elbow vein with an 18-gauge needle (Figure 2). During collection, intravenous administration, such as the supplementation of calcium, could be performed (Figure 2A). The mononuclear cell suspension was centrifuged at 2,500 r/min and 4°C, for 10 minutes, and purified. The number of mononuclear cells was counted by Automated cell counter (Z1, Beckman Coulter Inc., Brea, CA, USA). CD34+cells were analyzed by flow cytometry (FACS AriaII, BD Inc., Franklin Lakes, NJ, USA), then resuspended in 10 mL saline. Before transplantation, a fixed volume at a concentration of 1 × 109in 5 mL was delivered to the surgery site.
PBMC transplantation
Lumbar puncture was performed after measuring the opening pressure with a measuring tube. The patient was allowed to relax, and was checked for good respiration to ensure that the needle was properly positioned.
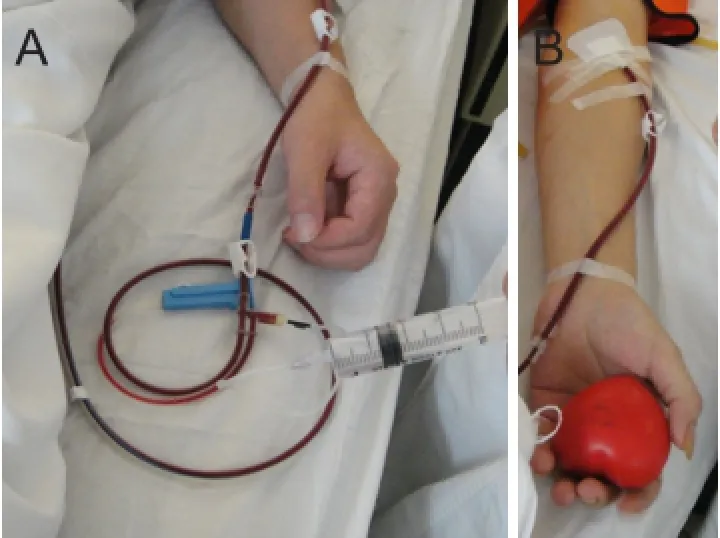
Figure 2 Isolation of peripheral blood mononuclear cells.
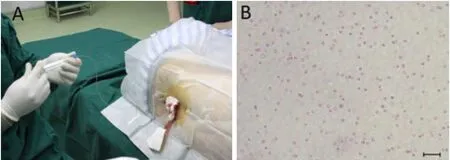
Figure 3 Peripheral blood mononuclear cell transplantation.
Autologous cerebrospinal fluid and PBMC suspension were mixed at a ratio of 1:1. A total mixture of 10 mL with 1 × 109cells was infused slowly into the subarachnoid space through the lumbar (L4-5) spine for approximately 20 minutes (Figure 3). The needle was withdrawn without replacing the stylet, and the puncture site was dressed with a bandage. The patient lay in bed for a few hours.
Follow up
To estimate the disease progression rate, patients were assessed every week during the first month, and then every 3 months until death.
Assessment
The primary outcome measure was adverse events of autologous PBMC mobilization, mononuclear cell collection, and post transplantation. The secondary outcome measures were as follows: FIM was used to assess the self-care ability of daily living. The Berg Balance Scale was utilized to assess the trunk balance capability and limb movement function. The Dysarthria Assessment Scale was employed to assess the progression of bulbar paralysis (Tomik and Guiloff, 2010). The follow-up evaluation was mainly completed while the patients visited the hospital in accordance with the schedule time. We also used home visits to complete the follow-up evaluation if the patient’s activity was poor.
To assess changes in neurological function, needle EMG was performed by a trained electromyographer, with more than 10 years experience, at 1 week pre-operation and 4 weeks post-operation. EMG was performed with an electromyograph and Evoked Potential Equipment Synergy Electrical trigger locator (M153635-Medelec Synergy, Oxford, UK), and the CMAPs of the bilateral median nerve, ulnar nerve, tibial nerve and common peroneal nerve were measured. The EMG was independently performed by a neurologist and a physiotherapist and the mean value of the scores obtained by two assessors was considered in the analysis. The patients were evaluated by EMG once preoperatively and then regularly every month following transplantation, and CMAP amplitudes were useful parameters for evaluating motor neuron loss.
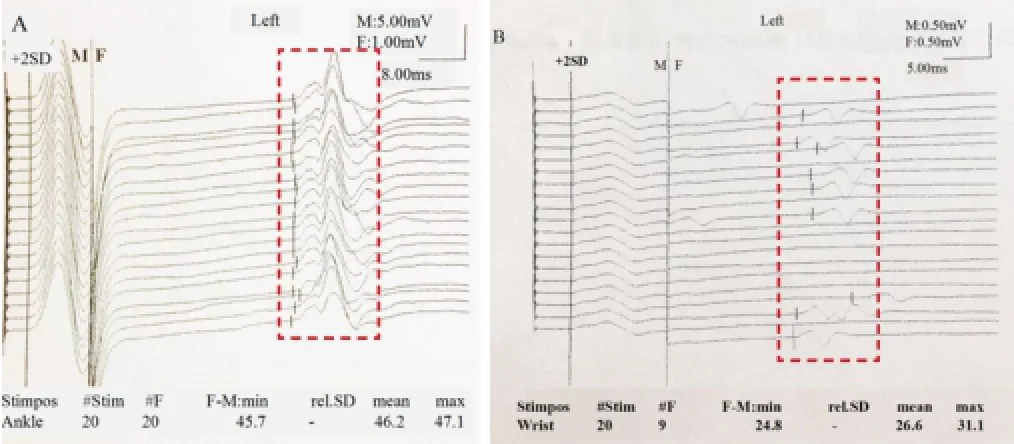
Figure 4 Different EMG results between ALS patients and normal subjects.
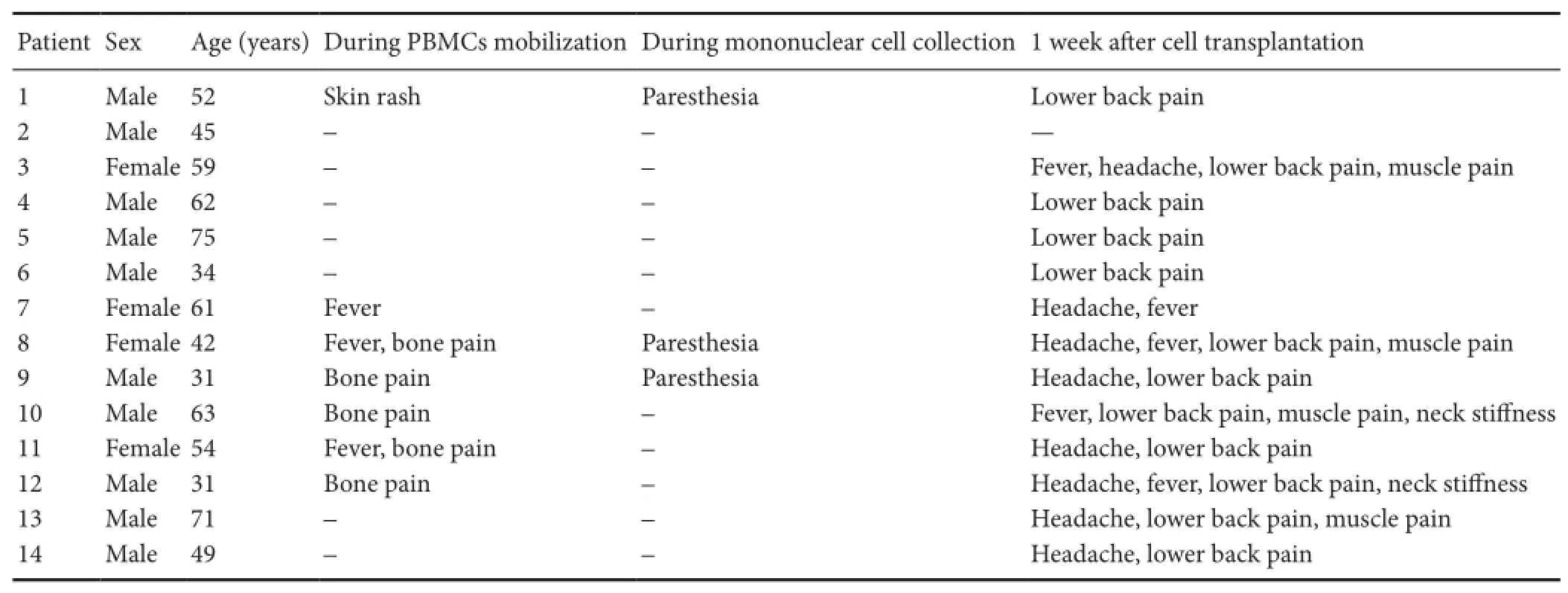
Table 1 Adverse effects after the intrathecal transplantation of autologous PBMCs
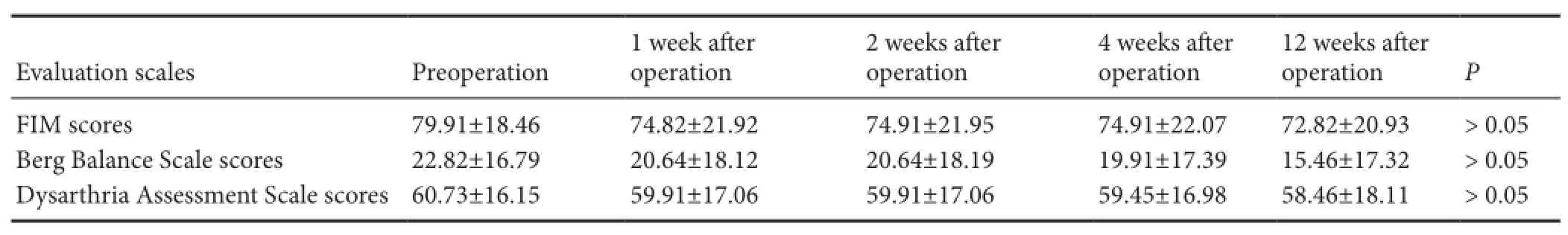
Table 2 Clinical characteristics of patients before and after transplantation
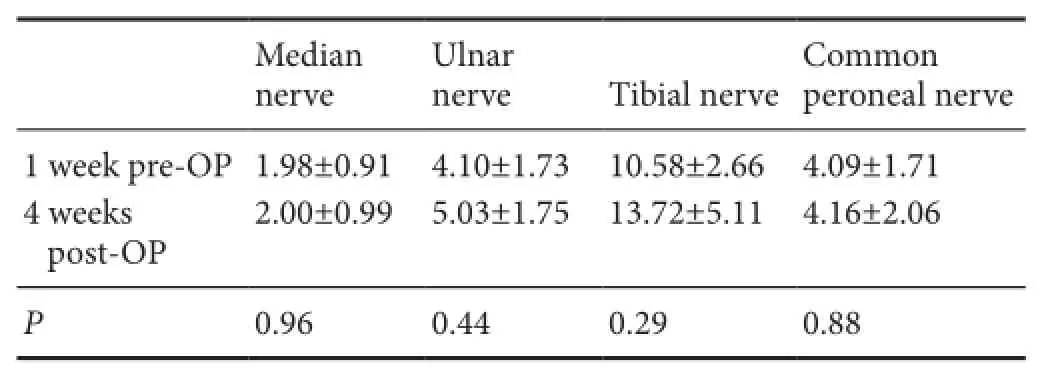
Table 3 Comparison of compound muscle action potential (ms) between pre-operation and post-operation
Statistical analysis
Data, expressed as the mean ± SD, were analyzed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed by paired samplet-test. A value ofP< 0.05 was considered statistically significant.
Results
Safety of autologous PBMC transplantation for ALS patients
Table 1shows all reported adverse events. All symptoms were noted immediately after surgery. No correlation was found between the severity and duration of side effects and cell doses.
Adverse events occurred during the G-CSF-based mobilization of autologous PBMC transplantation. The G-CSF infusion was well tolerated by ALS patients without any significant adverse effects (Table 1). Of 14 patients, five had mild bone pain that was most frequently recorded during G-CSF administration. No patients received any analgesic therapy and the pain disappeared shortly after the cessation of G-CSF administration. Tree patients had a mild self-limited febrile reaction (temperature ≤ 38°C) which lasted for 8 to 24 hours after G-CSF injection. Skin rash was observed in one patient at the injection site (recovered after 30 minutes). The patients tolerated all the procedures outlined in the therapeutic schedule without difficulty.
Adverse events during cell collection based on the use of acid-citrate-dextrose A (ACD-A) are shown inTable 1. Among the 14 patients, three had mild perioral paresthesia followed by digital numbness caused by hypocalcemia, which recovered after 1-2 g calcium gluconate was administered by intravenous infusion (Figure 1A).
During transplantation, the PBMC suspension was injected into the lumbar (L4-5) spine. No adverse events occurred. All patients were followed postoperatively every week during the first month, and then every 3 months until death. Participants were evaluated for adverse events, and serial measure scales.Table 1summarizes the data of adverse events of all patients up to 1 week after the surgery. Of the 14 patients, 12 (86%) had lower back pain, eight (57%) had headache, five (36%) had mild fever, four (29%) had muscle pain in both lower limbs, and two (14%) had neck stiffness. Their body temperature was below 38°C, and the mean was 37.03 ± 0.42°C of the 14 patients at 6 hours after surgery. However, none of these events was considered definitely related to the cells. Adverse events were usually temporary and disappeared within 1 week after surgery. The main indicators by laboratory testing were normal. Alanine aminotransferase was 18.80 ± 4.96 IU/L, and the mean value of serum creat-inine was 63.00 ± 20.94 μM before PBMC transplantation. On day 1 after PBMC transplantation, the mean number of leukocytes was 8.07 ± 2.09 × 109; alanine aminotransferase was 18.00 ± 5.89 IU/L, and the mean value of serum creatinine was 62.75 ± 20.60 μM. There were no severe complications after discharge from the hospital.
Efficacy of autologous PBMC transplantation in ALS patients
Two patients were lost during follow up, and one patient died from cerebral hemorrhage within 1 month after the first transplantation. Therefore, 11 patients were included, and followed at regular intervals of every week during the first month, and then every 3 months until death after transplantation.Table 2shows the clinical characteristics of patients at the time of admission to hospital and follow ups. The mean scores of evaluation scales decreased with time after PBMC transplantation. However, there was no significant difference in preoperative and postoperative mean scores at 1, 2, 4 and 12 weeks (P> 0.05;Table 2).
Of 14 patients, only nine accepted EMG examination (Figure 4) at 1 week preoperatively and 4 weeks postoperatively.Table 3summarizes the CMAPs from different areas of the nine patients. There was no significant difference for the median nerve, ulnar nerve, tibial nerve, and common peroneal nerve between pre-operation and post-operation timepoints (P> 0.05).
In addition, the clinical syndromes of seven patients decreased, including increased muscle strength and decreased muscle fasciculation 1 to 3 weeks post-operatively. Of the 11 patients evaluated, nine patients died. Eight patients died of respiratory failure associated with disease progression between 6 and 12 months after surgery. Only one patient had a repeated injection three times a year, and they had a longer survival of 20 months after surgery. Two patients who remained alive had a longer disease course prior to surgery (7 years of known disease), indicating that the clinical syndromes were related to ALS itself, rather than transplantation.
Discussion
Questions related to the optimal number of cells to engraft, the location and the selection of patients are still unanswered. The aim of the present study was to evaluate the safety and tolerability of the intrathecal transplantation of autologous PBMCs in 14 ALS patients. Participants were evaluated for adverse events, physical examination and serial measure scales during the different periods of the therapy.
Two hematopoietic growth factors have been approved by the US Food and Drug Administration for cell mobilization: G-CSF and granulocyte-macrophage colony-stimulating factor (Hsu and Cushing, 2016). G-CSF is the first-line treatment for hematopoietic stem cell mobilization and has been shown to reduce neutropenia-related infection and enhance posttransplant myeloid recovery (Giralt et al., 2014). It is a major mediator of responses requiring increased neutrophils and host defense (Roberts, 2005). G-CSF stimulation followed by leukapheresis was used in this study, as it is currently the most efficient and safe approach for autologous and allogeneic hematopoietic stem cell harvesting, which might be of value in the treatment of ALS (Cashman et al., 2008). G-CSF-mobilized CD34+cells differentiate into neural cells and may be used to treat ALS. Of 14 patients, five had bone pain (36%), which was most frequently recorded during G-CSF administration, consistent with a previous study (45%) (Samaras et al., 2015). The PBMC mobilization protocol for ALS patients was effective and safe, and sufficient numbers of PBMCs were isolated to meet the needs of autologous transplantation.
We used ACD-A as an anticoagulant, and the ratio of ACD-A to whole blood was 1:12. ACD-A might be a risk factor for anesthesia and bleeding complications. In this study, three patients had anesthesia (finger and mouth) caused by citrate-related toxicity, which is one of the most frequent complications of apheresis. It is caused by the infusion of ACD-A, which chelates with calcium ions. The most commonly reported hypocalcemic symptoms are mild perioral paresthesia followed by digital numbness (Hegde et al., 2016). ACD-A is used to prevent the coagulation of blood during collection. The chelation of calcium causes a wide range of symptoms and signs can be graded into three categories. Tree patients in our study showed mild symptoms of tingling around the mouth and nose, and less often a tingling in the fingers (Grade I). More severe toxicity may cause nausea or vomiting (Grade II) or more rarely, tetany, hypotension and/or cardiac dysrhythmia (Grade III) (Howell et al., 2015). In general, cell collection is well tolerated and related adverse events are mostly restricted to symptoms of citrate toxicity. The ratio of citrate-related complications was less than in other studies (48%) (Buchta et al., 2003), because of the reduced blood flow rate allowing less citrate into the system. All patients had a circulating blood volume of 4,000-6,000 mL in our study. In addition to supplying calcium by vein for the treatment of citrate toxicity, oral calcium before stem cell collection can play a crucial role in step prevention.
During transplantation, the PBMC suspension was injected into the lumbar (L4-5) spine. Adverse events were not determined. Each injection contained the collected cell suspension in a volume of 20 mL. Surgery was carried out every 3 months for patients according to the treatment regimens. In this group, one patient had completed the treatment three times without any complications during transplantation. All patients were evaluated for adverse events until 1 week after surgery. The adverse events were probably caused by transplanted cells, but the exact reasons are still debatable. In June 2016, one study also confirmed that the injection procedure, as well as the introduction of high numbers of human neural stem cells into the spinal cord, was safe (Glass et al., 2016). In addition, they succeeded in moving this complex procedure from a single surgical center to three centers. In general, the surgery is well tolerated. The adverse events were usually temporary and disappeared within 1 week after surgery, and patients were then discharged. There were no severe complications related to the treatment after discharge from the hospital.
ALS can produce EMG signs of motor unit loss and pyramidal signs affecting both the upper and lower limbs. Terefore, using needle EMG to demonstrate a neurogenic pattern in the bulbar or trunk muscles may be essential forthe differential diagnosis of ALS (Lefaucheur and de Carvalho, 2016). EMG can identify subclinical lower motor neuron lesions, thereby providing more sensitive information than clinical examination alone (Pan et al., 2016). The results of CMAPs showed no significant difference between pre-operation and post-operation. This might be explained by unknown etiology and pathological mechanisms in ALS either directly or indirectly. Expanding the sample size and strengthening long-term follow up may improve the accuracy of therapeutic efficacy estimation in autologous PBMC transplantation for treating ALS.
Intrathecal transplantation of autologous PBMCs in ALS patients was safe for at least 12 months in 14 patients. The approach was feasible and well tolerated, and no significant adverse events were reported. However, the treatment results of autologous PBMCs in ALS patients are not clear. Terefore, a long-term clinical trial to examine the therapeutic safety, biological effects, and efficacy is required. Replicable data from a large number of patients with different clinical phenotypes and at various stages of the disease are also needed.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for htheir images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Author contributions:XYL and ZHL performed isolation and transplantation of PBMCs. JL and XYL were responsible for study design. CLS and LNZ obtained patient consent and assessed the changes in neurological function. CH and WJW performed all cell functional tests. YL and YL contributed to the writing of the manuscript. XYL and XFJ performed statistical analysis. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293-299.
Buchta C, Macher M, Bieglmayer C, H?cker P, Dettke M (2003) Reduction of adverse citrate reactions during autologous large-volume PBPC apheresis by continuous infusion of calcium-gluconate. Transfusion 43:1615-1621.
Carreras FJ (2016) Glaucoma and amyotrophic lateral sclerosis, two kindred diseases? Neural Regen Res 11:1415-1417.
Cashman N, Tan LY, Krieger C, M?dler B, Mackay A, Mackenzie I, Benny B, Nantel S, Fabros M, Shinobu L, Yousefi M, Eisen A (2008) Pilot study of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS). Muscle Nerve 37:620-625.
Giacoppo S, Mazzon E (2016) Can cannabinoids be a potential therapeutic tool in amyotrophic lateral sclerosis? Neural Regen Res 11:1896-1899.
Giralt S, Costa L, Schriber J, DiPersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E, Hosing C, Negrin R, Petersen FB, Rondelli D, Soiffer R, Leather H, Pazzalia A, Devine S (2014) Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 20:295-308.
Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL (2012) Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 30:1144-1151.
Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, Bordeau J, Fournier C, Johe K, Hazel T, Cudkowicz M, Atassi N, Borges LF, Rutkove SB, Duell J, Patil PG, Goutman SA, Feldman EL (2016) Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology 87:392-400.
Gubert F, Satiago MF (2016) Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment? Neural Regen Res 11:1216-1219.
Hegde V, Setia R, Soni S, Handoo A, Sharma SK, Chaudhary D, Kapoor M (2016) Prophylactic low dose continuous calcium infusion during peripheral blood stem cell (PBSC) collections to reduce citrate related toxicity. Transfus Apher Sci 54:373-376.
Howell C, Douglas K, Cho G, El-Ghariani K, Taylor P, Potok D, Rintala T, Watkins S (2015) Guideline on the clinical use of apheresis procedures for the treatment of patients and collection of cellular therapy products. Transfus Med 25:57-78.
Hsu YM, Cushing MM (2016) Autologous stem cell mobilization and collection. Hematol Oncol Clin North Am 30:573-589.
Janson CG, Ramesh TM, During MJ, Leone P, Heywood J (2001) Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res 10:913-915.
Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B (2008) ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J Neurol Sci 275:69-73.
Lefaucheur JP, de Carvalho M (2016) New insights into the clinical neurophysiological assessment of ALS. Neurophysiol Clin 46:157-163.
Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, Gutierrez-Jimenez E, Zazueta-Fierro OE, Meza JA, Couret-Alcaraz P, Hernandez-Torre M (2012) Stem cell transplantation in amyotrophic lateral sclerosis patients: methodological approach, safety, and feasibility. Cell Transplant 21:1899-1907.
Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, Carriero A, Monaco F, Fagioli F (2012) Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 14:56-60.
Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, Santiago MF (2016) Bone marrow-derived cells as a therapeutic approach to optic nerve diseases. Stem Cells Int 2016:5078619.
Pan H, Jian F, Lin J, Chen N, Zhang Z, Wang Y, Cui L, Kimura J (2016) Needle electromyography of the frontalis muscle in patients with amyotrophic lateral sclerosis. Muscle Nerve 54:1093-1096.
Poppe L, Rué L, Robberecht W, Van Den Bosch L (2014) Translating biological findings into new treatment strategies for amyotrophic lateral sclerosis (ALS). Exp Neurol 262 Pt B:138-151.
Roberts AW (2005) G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors 23:33-41.
Samaras P, Pfrommer S, Seifert B, Petrausch U, Mischo A, Schmidt A, Schanz U, Nair G, Bargetzi M, Taverna C, Stupp R, Stenner-Liewen F, Renner C (2015) Efficacy of vinorelbine plus granulocyte colony-stimulation factor for CD34+hematopoietic progenitor cell mobilization in patients with multiple myeloma. Biol Blood Marrow Transplant 21:74-80.
Tomik B, Guiloff RJ (2010) Dysarthria in amyotrophic lateral sclerosis: A review. Amyotroph Lateral Scler 11:4-15.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Jing Liu, M.D., liujing.dlrmc@hotmail.com.
orcid: 0000-0001-5687-4004 (Xian-yan Li)
10.4103/1673-5374.202918
Accepted: 2017-02-13
- 中國神經再生研究(英文版)的其它文章
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Anatomical distributional defects in mutant genes associated with dominant intermediate Charcot-Marie-Tooth disease type C in an adenovirusmediated mouse model
- Mechanisms responsible for the inhibitory effects of epothilone B on scar formation after spinal cord injury
- The mechanism of Naringin-enhanced remyelination after spinal cord injury
- Estrogen affects neuropathic pain through upregulating N-methyl-D-aspartate acid receptor 1 expression in the dorsal root ganglion of rats
- Combination therapy using evening primrose oil and electrical stimulation to improve nerve function following a crush injury of sciatic nerve in male rats

