FGF-2 is required to prevent astrogliosis in the facial nucleus after facial nerve injury and mechanical stimulation of denervated vibrissal muscles
Arzu Hizay, Mark Seitz, Maria Grosheva, Nektarios Sinis, Yasemin Kaya, Habib Bendella, Levent Sarikcioglu, Sarah A. Dunlop, Doychin N. Angelov
1Department of Anatomy, Akdeniz University, Faculty of Medicine, Dumlupinar Bulvari 07058 Kampus, Antalya, Turkey;
2Department of Anatomy I, University of Cologne, Joseph-Stelzmann-Strasse 9, D-50924 Cologne, FR Germany;
3 Department of Oto-Rhino-Laryngology, University Hospital Cologne, Joseph-Stelzmann-Strasse 9, D-50924 Cologne, FR Germany;
4Depeartment of Plastic-, Hand- and Reconstructive Microsurgery, Berlin, FR 12249, Germany;
5 Department of Neurosurgery, Hospital Merheim, University of Witten-Herdecke, Ostmerheimer Straβe 200, 51109 Cologne, Germany;
6 Experimental and Regenerative Neurosciences, School of Animal Biology, University of Western Australia (M092) 35 Stirling Highway, CRAWLEY WA 6009, Australia.
FGF-2 is required to prevent astrogliosis in the facial nucleus after facial nerve injury and mechanical stimulation of denervated vibrissal muscles
Arzu Hizay1,Δ, Mark Seitz2,Δ, Maria Grosheva3, Nektarios Sinis4, Yasemin Kaya1, Habib Bendella5, Levent Sarikcioglu1, Sarah A. Dunlop6, Doychin N. Angelov2,?
1Department of Anatomy, Akdeniz University, Faculty of Medicine, Dumlupinar Bulvari 07058 Kampus, Antalya, Turkey;
2Department of Anatomy I, University of Cologne, Joseph-Stelzmann-Strasse 9, D-50924 Cologne, FR Germany;
3 Department of Oto-Rhino-Laryngology, University Hospital Cologne, Joseph-Stelzmann-Strasse 9, D-50924 Cologne, FR Germany;
4Depeartment of Plastic-, Hand- and Reconstructive Microsurgery, Berlin, FR 12249, Germany;
5 Department of Neurosurgery, Hospital Merheim, University of Witten-Herdecke, Ostmerheimer Straβe 200, 51109 Cologne, Germany;
6 Experimental and Regenerative Neurosciences, School of Animal Biology, University of Western Australia (M092) 35 Stirling Highway, CRAWLEY WA 6009, Australia.
Recently, we have shown that manual stimulation of paralyzed vibrissal muscles after facial-facial anastomosis reduced the poly-innervation of neuromuscular junctions and restored vibrissal whisking. Using gene knock outs, we found a differential dependence of manual stimulation effects on growth factors. Thus, insulin-like growth factor-1 and brain-derived neurotrophic factor are required to underpin manual stimulation-mediated improvements, whereas FGF-2 is not. The lack of dependence on FGF-2 in mediating these peripheral effects prompted us to look centrally, i.e. within the facial nucleus where increased astrogliosis after facial-facial anastomosis follows "synaptic stripping". We measured the intensity of Cy3-fluorescence after immunostaining for glial fibrillary acidic protein (GFAP) as an indirect indicator of synaptic coverage of axotomized neurons in the facial nucleus of mice lacking FGF-2 (FGF-2-/-mice). There was no difference in GFAP-Cy3-fluorescence (pixel number, gray value range 17–103) between intact wildtype mice (2.12± 0.37×107) and their intact FGF-2-/-counterparts (2.12±0.27×107) nor after facial-facial anastomosis +handling (wildtype: 4.06±0.32×107; FGF-2-/-: 4.39±0.17×107). However, after facial-facial anastomosis, GFAP-Cy3-fluorescence remained elevated in FGF-2-/--animals (4.54±0.12×107), whereas manual stimulation reduced the intensity of GFAP-immunofluorescence in wild type mice to values that were not significantly different from intact mice (2.63±0.39×10 ). We conclude that FGF-2 is not required to underpin the beneficial effects of manual stimulation at the neuro-muscular junction, but it is required to minimize astrogliosis in the brainstem and, by implication, restore synaptic coverage of recovering facial motoneurons.
FGF-2, facial nerve, axotomy, astrogliosis, whisking function, polyinnervation
Introduction
Restoration of function after transection of peripheral nerves is poor. Occurrence of "post-paralytic syndromes" such as paresis, synkinesis and dysreflexia are inevitable. Although axonal regrowth is robust, a large body of evidence points to poor recovery being attributable, at least in part, to extensive sprouting and therefore inaccurate reinnervation of target muscles . Indeed, axonal sprouting occurs at a number of locations en route along the axis of the facial nucleus - facial-nerve trunk - facial nerve fascicles - facial, [2] muscles .
The quality of peripheral nerve regeneration, both within the nerve and at the motor end-plate/terminal Schwann cell complex, can be improved by various non-invasive therapies. Muscles with flaccid paralysis can be stimulated electrically or by exercise, procedures which inhibit intramuscular axonal sprouting and diminish motor-end-plate polyinnervation, thereby improving reinnervation quality[3].
With respect to functional improvements, we have recently shown that after facial nerve injury, manual stimulation (MS) of denervated whisker pads reduces the proportion of polyinnervated neuro-muscular junctions (NMJ). Furthermore, the shift towards the normal monoinnervated state is associated with improved whisking function and blink reflexes . Factors contributing to this beneficial effect could involve the denervated muscles themselves, which produce numerous short-range diffusible sprouting with various neurotrophic factors being identified as possible candidates stimuli[6–7].
We have also recently showed that after facial nerve injury and MS [(facial-facial anastomosis (FFA)+MS] in mice deficient in insulin-like growth factor-1 (IGF-1+/-) or brain-derived neurotrophic factor (BDNF+/-) and its Trk-B receptor, reinnervation of NMJ was highly inaccurate and vibrissal whisking was poor . We thus concluded that the deficiency of both growth factors was involved in lack of functional recovery.
By contrast, mice lacking fibroblast growth factor-2 (FGF-2-/-) recovered well after FFA+MS (but not after FFA only), indicating that FGF-2 may not be required to underpin the beneficial "peripheral" effects of MS[10]. We therefore decided to explore whether there were any central effects of MS by examining the facial motoneurons in the brainstem. After facial nerve injury, activated (GFAP+) astrogliocytes reversibly displace perisomatic synapses from the neuronal surface and induce "synaptic stripping"[11]. Hence, the amount of GFAP-expressing astroglia provides indirect information about the status of synaptic coverage of facial neurons.
In the present study, we therefore quantified the intensity of Cy3 fluorescence after immunostaining for GFAP as a measure of the total amount of activated astrocytes in the facial nucleus. We examined FGF-2-/-mice subjected to facial nerve injury and subsequent MS of the vibrissal muscles or handling alone (i.e. no treatment). Wildtype (WT) littermates were used as controls.
Material and methods
Experimental procedures
All experiments were conducted in accordance with the German Law on the Protection of Animals and procedures were approved by the local Animal Care Committee, University of Cologne. Guidelines were identical to those of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996, the UK Animals (Scientific Procedures) Act 1986 and associated guidelines and the European Communities Council Directive of 24 November 1986 (86/609/ EEC). Before and after surgery, animals were fed standard laboratory food (Ssniff, Soest, Germany), provided tap water ad libitum and kept in an artificial light-dark cycle of 12 hours light on and 12 hours off.
Animals and groups
Homozygous mice constitutively lacking all iso- forms of FGF-2, strain Fgf2tm1Zllr C57/Bl6[12]were used as well as WT animals (C57/Bl6). Genotyping was performed by PCR (Cycling conditions: 30 minutes 95°C, 30 cycles: 30 seconds 95°C, 30 seconds 61°C, 90 seconds 72°C, final elongation for 10 minutes 72°C) using the HotStarTaq Master Mix Kit (Qiagen) from mouse tail DNA and subsequent agarosegel-electrophoresis. To differentiate between WT and transgenes, we used the following primers: - neo6: 5′-GAT CTG GAC GAA GAG CAT CAG GGG-3′, - wt6: 5′-CAA GTT TCT AAC TTT CTC CGC TCC TGC-3′, and - wt5: 5′-CAA TCT ATT GGG GTC AAG CCT ATT GGG-3′.
The transgene allele yielded an amplicon of 750 bp and the wildtype 344 bp.Thirty-six mice were divided into six groups with each group consisting of six animals (Table 1). Groups 1 and 2 were intact WT or homozygous knockouts (FGF-2'). Groups 3–6 comprised WT (Groups 3 and 4) or FGF-2-/-(Groups 5 and 6) mice which underwent FFA. Following FFA, animals either received MS (see below, Groups 4 and 6) or served as "handling" controls (see below; Groups 3 and 5).
Surgical procedures
FFA involved transection and end-to-end suture of the right facial nerve under surgical anesthesia (Ketamin/ Xylazin; 100 mg Ketanest?, Parke-Davis/ Pfizer, Karlsruhe, Germany, and 5 mg Rompun?, Bayer, Leverkusen, Germany, per kg body weight; i.p.) and was undertaken by a trained surgeon (M. Grosheva). The trunk of the facial nerve was exposed and transected close to its emergence from the foramen stylomastoideum. The proximal and distal stumps were immediately reconnected using two 11-0 atraumatic sutures (Ethicon, Norderstedt, Germany).
Manual stimulation of vibrissal muscles and handling controls
At one day after surgery and continuing for two months, mice receiving MS (groups 4 and 6) were subjected to gentle rhythmical stroking of the right whisker pad for 5 minutes per day. Handling controls (group 3 and 5) were carefully removed from the cage by an investigator and held for 5 minutes as if they were to receive MS[14].
Analysis of vibrissal motor performance
We used our established technique of video-based motion analysis of explorative vibrissal motor performance?. At the conclusion of the experiment (2 months after surgery), animals were videotaped for 35 minutes during active exploration (Digital Camcorder: Panasonic NV DX-110 EG; 50 Hz; 50 fields per second; shutter open for 4 ms per cycle). Selected sequences (1.5 sec) containing the most pronounced whisking (vibrissal bouts) were captured by a 2D/ Manual Advanced Video System (PEAK Motus 2000, PEAK Performance Technologies, Inc., Englewood, CO, USA). Bouts on both the operated (ipsilateral to surgery) and intact (contralateral to surgery) sides were subjected to motion analysis using specific reference points to evaluate: (i) whisking frequency: cycles of protraction (forward movement) and retraction (backward movement) per second, (ii) angle at maximal protraction, (iii) amplitude: the difference between maximal retraction and maximal protraction, (iv) angular velocity during protraction, and (v) angular acceleration during protraction. Measurements were performed by two observers (Mark Seitz and Srebrina Angelova) blinded to the treatment.
Evaluation of the intensity of fluorescence
Fixation and tissue preparation
Two months after surgery, all animals were anaesthetized and transcardially perfused (4% paraformaldehyde in phosphate buffered saline pH 7.4). Brainstems were sectioned coronally at 50 μm using a vibratome. Immunohistochemical staining for GFAP was performed on every second section through the facial nucleus (according to the fractionator sampling strategy, the facial nucleus consisted of 23–25 sections) in one incubation batch.
Immunohistochemistry
Sections were washed in Tris buffered saline (TBS) solution (0.1 mol/L, pH 7.4) for 10 minutes × 2 times and blocked with 5% bovine serum albumin (BSA, Sigma) in TBS (for 30 minutes at room temperature). Incubation with the primary antibody against GFAP (guinea-pig; Serum GF52, Progen; 1:2,000 diluted in TBS in 0.8% BSA) was for 2 hours. After a thorough rinse (4 × 10 minutes in TBS), the second block with 5% normal goat serum (NGS in 0.1 mol/L TBS for 15 minutes) was followed by incubation with Alexa-Fluor 568-conjugated anti-guinea pig IgG from goat (1:400, Invitrogen, Carlsbad, CA, USA) for 1 hour. Sections were rinsed, washed in distilled water and coverslipped with flouromount.
Fluorescence microscopy
A Zeiss microscope equipped with a CCD Video Camera System combined with the analyzing software Image-Pro Plus 6.2 (Media Cybernetics, Silver Spring, MD, USA) was used to quantify fluorescence intensity. Fluorescent images captured via the rhodamine filter were compared using the 8 BPP gray scale format whereby each pixel contains 8 bits of information encoding brightness, with a range of 0 to 255. The scale for pixel brightness, or pixel gray value, is constructed so that the higher numbers indicate greater pixel brightness. Digital images were captured with a slow scan CCD camera (Spot RT, Diagnostic Instruments, Scientific Instrument Company, Inc., Campbell, CA, USA).
For quantification of pixel brightness, images were captured using a × 25 objective and Image-Pro Plus Software Version 6.2 (Media Cybernetics Rockville, MD, USA). Exposure time was optimized to ensure that only few pixels were saturated at 255 gray value. However, all images representing the same labeling were taken under the same conditions of exposure (duration).
The lateral facial subnucleus was selected as an area of interest that could be unambiguously and consistently identified between animals (AOI; white ellipse in Fig. 1). Using a magnification of × 25, we positioned the long diameter of the ellipse parallel to the readily identifiable ventral margin of the brainstem and thenplaced the ellipse immediately adjacent to the lateral edge (transition zone ventral to lateral, indicated in Fig. 1 by an asterisk).
An interactive threshold was used to detect the pixel brightness of the minimum fluorescence. Threshold values ensured the inclusion of the entire signal range in the sample. This value was further used to extract and compare the pixel number between animals of the same group and between experimental groups. A scale with a minimum of 17 and maximum of 103 was used for graphical representation of the results. Using this scale, sections without fluorescence received 17 points and those with very strong fluorescence received 103 points.
Statistical analysis
Data were analyzed using one-way ANOVA with post-hoc Tukey test and a significance level of 0.05 (Statistica 6.0 software; StatSoft, Tulsa, OK, USA).
Results
Vibrissal whisking
Intact mice
In intact WT and FGF-2-/-animals, whisking involves active exploration whereby mystacial vibrissae sweep back and forth with a frequency of about 5–6 Hz and a maximal protraction (a rostrally open angle between the vibrissal shaft and the sagittal plane) of about 55°.Protraction is mediated by striated muscle fibers that form a sling around the rostral aspect of each hair follicle; contraction pulls the base of the follicle caudally and moves the distal whisker tips forward. The mean amplitude was approximately 55° (WT: 52.2 ± 7.4°; FGF-2-/-: 56.3 ± 6.7°), the angular velocity about 800° and the angular acceleration about 60,000°/sec2.
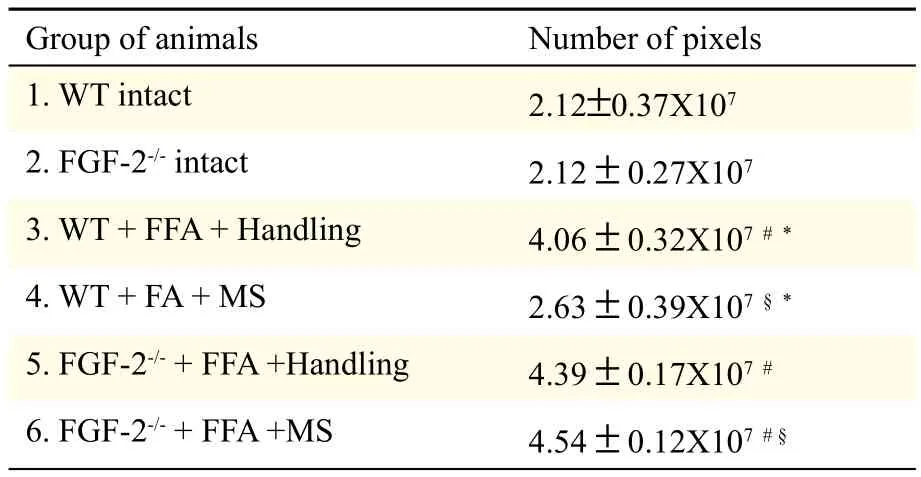
Table 1 Intensity of Cy3-fluorescence according to a gray scale (range 17-103) in aged-matched WT (FGF-2+/+) and FGF-2-/-female mice after facial-facial anastomosis (FFA) with and without manual stimulation (MS) of the vibrissal muscles.
Mice subjected to FFA and subsequent handling
Following FFA, both WT and FGF-2-/-mice receiving handling only had large functional deficits compared to intact mice. The main functional deficiency was seen in the significantly smaller amplitudes of vibrissal movements (WT: 23.4 ± 4.1°; FGF-2-/-12.8 ± 3.2°; P<0.05 compared to intact counterparts). Moreover, the mean amplitude measured in the FGF-2-/-animals was significantly smaller compared to WT- mice (FGF-2-/-: 12.8 ± 3.2° compared to WT: 23.4 ± 4.1°; P<0.05). The reduced whisking amplitude in FGF-2-/-animals suggests that this growth factor is required to promote functional recovery, at least in the absence of any other intervention such as MS.
Mice subjected to FFA and manual stimulation
Compared to their respective counterparts subjected only to handling, MS appeared to be effective in both WT and FGF-2-/-mice. Thus, whisking amplitude was significantly increased in WT mice (MS: 36.3 ± 6.4° compared to handling only: 23.4 ± 4.1°; P<0.05). Similarly, despite the deficiency of FGF-2, whisking amplitude was significantly increased by MS in the FGF-2-/-mice (MS: 35.3 ± 5.1° compared to handling only: 12.8 ± 3.2°: P<0.05).
Measurements of GFAP-immunofluorescence
In intact mice, there was no difference in the amount of GFAP-Cy3-fluorescence (pixel number within a gray value range of 17-103) between intact WT animals (2.12 ± 0.37 × 107) and FGF-2-/--mice (2.12 ± 0.27 × 107) (Fig. 1A,1B; Table 1). Furthermore, there were also no differences after FFA + handling between WT animals (4.06 ± 0.32 × 107) and FGF-2-/--mice (4.39 ± 0.17 × 107) (Fig. 1C,1D; Table 1). In addition, in WT-mice, MS resulted in an intensity of fluorescence (2.63 ± 0.39 × 107) which was not significantly different from intact WT mice (P<0.05). However, in the FGF-2-/-homozygotes, the intensity of GFAP-immunofluorescence remained high (4.54 ± 0.12 × 107) (Fig. 1E,1F; Table 1).
Discussion
Our finding that recovery of target muscle reinnervation after facial nerve injury is poor in FGF-2 deficient mice supports other work on the pivotal role ofthis growth factor in peripheral nerve development as well as regeneration. Thus, FGF-2 supports survival of Schwann cell precursors in embryonic mice[13]and in rats, induces proliferation of Schwann cells in vitro[14]. FGF-2 is also expressed in motor and sensory neurons as well as in Schwann cells in both the developing and adult peripheral nervous system. In agreement with these observations, FGF-2 stimulates axon regrowth in vivo and contributes to the enlargement and maintenance of axon caliber[15].
Following injury to peripheral nerves, such as the facial and hypoglossal nerves, reactive gliosis occurs remotely within central nuclei[16]as well as in transynaptically linked regions such as the motor cortex[17]. Within motor nuclei, reactive astrogliosis has been shown to reversibly displace perisomatic synapses from the neuronal surface ("synaptic stripping")[11,18]. Such synaptic stripping presumably also underlies the lack of functional recovery observed after peripheral nerve injury[12].
The discrepancy between persistent astrogliosis in the facial nucleus of the operated FGF-2-/-mice and the good recovery of whisking function cannot be explained easily. There should be no doubt that all facial perikarya underwent ("synaptic stripping") after facial nerve injury. In response to transection of the facial nerve, the resident microglia show a dramatic increase in mitotic activity, rapidly migrate towards the neuronal cell surface and displace the afferent synaptic terminals. The axotomized motoneurons "respond" to their deafferentation with a decrease in the synthesis of transmitter- related compounds, e.g. muscarinicand glycine receptors and a decrease in activity of enzymes involved in the biosynthesis of transmitters, e.g. dopamine-β-hydroxylase, tyrosinehydroxylase, cytochromeoxidase and acetylcholinesterase. These changes correspond to the electrophysiological status of regenerating neurons: increased excitability with preserved integrity of the dendritic input[3].
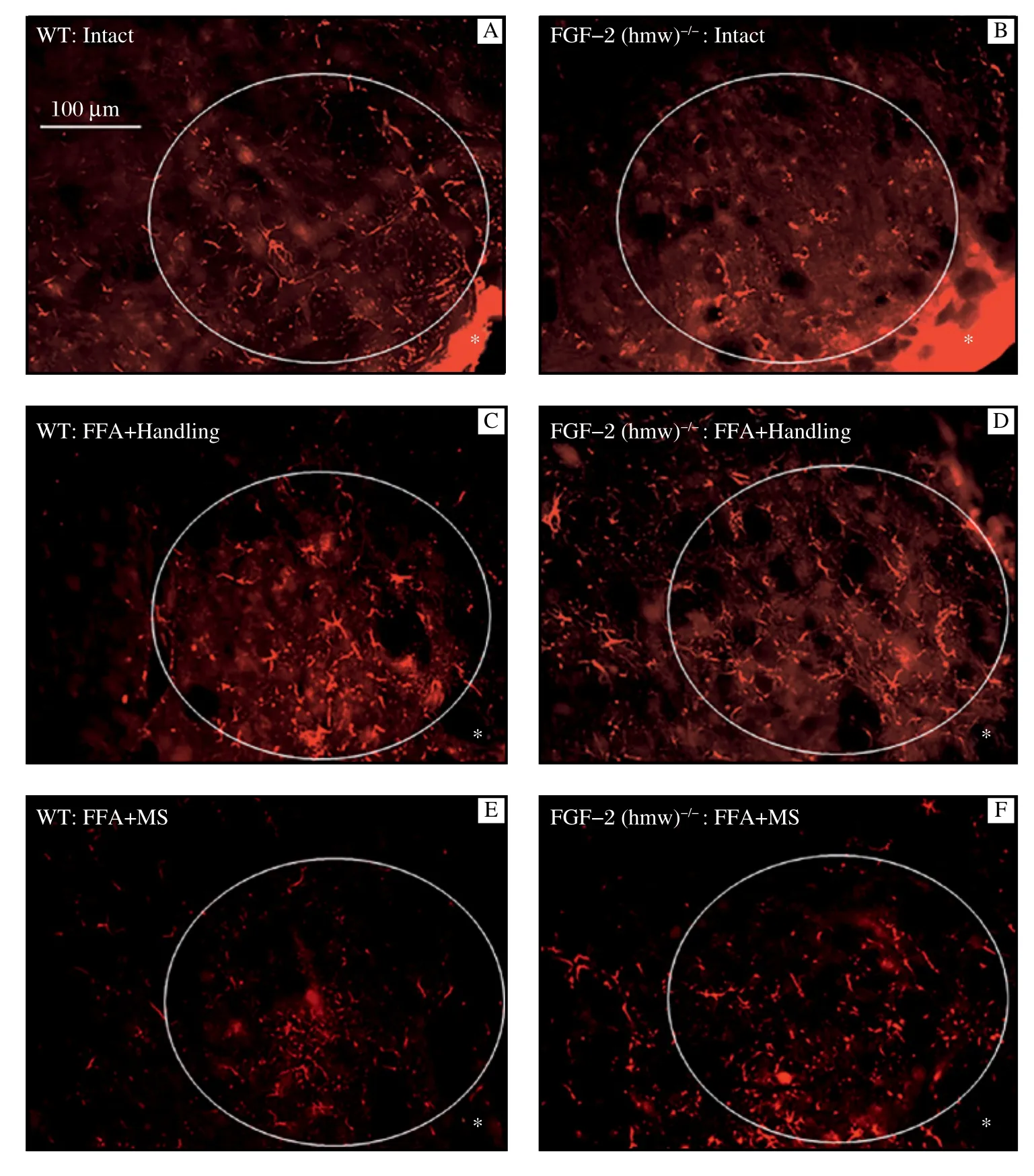
Fig. 1 Representative microphotographs showing GFAP-Cy3-immunofluorescence in the lateral facial subnucleus of WT-(A, C, E) and FGF-2-/-(B, D, F) mice. The asterisk indicates the transition zone between the ventral and lateral edge of the 50 μm thick vibratome sections.
This post-traumatic deafferentation is reversible if target reinnervation occurs. Quantitative electron microscopic analysis of regenerated cat gastrocnemius motoneurons has, however, revealed that restoration of synaptic inputs is incomplete in several respects. Thus, for example, total synaptic frequency (number of synapses per unit membrane length) and total synaptic coverage (percent of membrane length covered by synapses) estimated for motoneuron cell somata and proximal, intermediate and distal dendritic segments recover to 60% - 81% and 28% - 48% of normal, respectively.
Finally, reinnervation of motor targets (with accompanying recovery of the perisomatic synaptic density) does not automatically mean recovery of function. Voluminous work (also from our laboratory) has shown that polyinnervation of the NMJ is a major critical factor for recovery of coordinated motor performance[4–5-,8–9].
FGF-2 is a member of a large family of small peptide growth factors found in neurons and glia and has multiple roles. For neurons, FGF-2 is involved in maintaining neurogenic niches in vivo[19]and in neuroprotection following nervous system injury[20]. Similarly, FGF-2 appears to be important for glia[21]. Several studies have highlighted a role for FGFs in region-specific regulation of glial differentiation. Thus, using the FGF-2 null mouse, Irmady et al.[22]demonstrated that FGF-2 is critical for cortical astrocyte differentiation. Additionally, following nervous system trauma, reactive astrocytes show increased FGF-2 immunoreactivity[23,24].
Following facial nerve transection and repair, increased GFAP immunoreactivity was observed within 2-3 days after axotomy in the facial nucleus on the lesioned side[16]. This reactive change lasted longer (up to 1 year) when axon regeneration was prevented or delayed by placing a metal clip on the proximal nerve stump. Although GFAP immunoreactivity was also studied in shorter times (7-11 days) after nerve lesion, we evaluated GFAP immunoreactivity after two months to observe its distribution in the facial nucleus after injury. Facial nerve injury was evaluated after two months since the same time was used to evaluate facial nerve recovery in our previous studies. Similarly, and not unexpectedly, we observed reactive gliosis (as evidenced by elevated GFAP-Cy3-fluorescence) in WT mice following FFA + Handling. However, somewhat surprisingly, given that FGF-2 appears to be involved in astrogliosis, we also observed elevated GFAP-Cy3-fluorescence (i.e. astrogliosis) in FGF-2-/-mice following FFA + Handling. Our data therefore suggest that factors in addition to FGF-2 must also be involved in astrogliosis. Indeed, ATP activation of P2X receptors appears to be an alternative pathway to FGF-2 in mediating astrocyte proliferation[27]. Another alternative to FGF-2 is the PKCepsilon protein kinase which regulates morphological stellation as well as multiple astrocytic signalling pathways.
Earlier studies have demonstrated FGF-2 immunoreactivity in the rat facial nucleus, which gets upregulated after facial nerve injury[25]. We were unable to find direct information about the different isoforms (18, 20.5, 21, 23kD) that may be present in the murine facial nucleus. Anyway, based on earlier work by Allodi et al. showing that 18-kDa-FGF-2 mediates neuritogenesis though with inhibitory effects on the myelination and that 21-/ 23-kDa-FGF-2 mediates long distance myelination of regenerating axons and early recovery of functions, we may assume that all FGF-2 isoforms are present in the murine facial nucleus, based on the successful recovery of vibrissal whisking. A partial support to this assumption was found in the results of Dono et al.[12]demonstrating the presence of all 3 isoforms in the brain of newborn mice.
In previous studies, we showed that MS provided functional recovery of vibrissal muscles and reduced the degree of polyinnervation following facial nerveinjury[4].
Earlier work has shown that motoneurons are dependent on growth factors for their survival both normally and after axotomy[1-2]. Indeed, MS of denervated vibrissal muscles was ineffective in mice deficient in IGF-1 and BDNF[8-9]. By contrast, and surprisingly, following facial nerve transection and immediate repair, MS in FGF-2-/-mice was effective in improving both whisking function and accuracy of target muscle reinnervation[10]. However, as we show here, MS in FGF-2-/-mice failed to prevent gliosis in the facial nucleus.
At first sight, our results seem to contradict earlier work which indicates that cerebral injections of bFGF activated the astroglial reaction. The absence of bFGF in our experimental animals should have impeded the upregulation of GFAP in FGF-2-/-mice. While appreciating the results in this report, we identified four important differences from our present study. First, they used rats and we used mice. Second, they performed direct injury to the central nervous system with a breakdown of the blood-brain barrier, while in our experiments injury to the peripheral facial nerve was followed by the indirect retrograde axon reaction and "chromatolysis" in facial motoneurons. Third, the areas in which Eclancher et al. injected bFGF included the cortex,striatum, hippocampus and corpus callosum; while we studied the facial nucleus in the brainstem. Fourth, following bFGF injections, Eclancher et al. let the animals survive 3-20 days, while our mice lived two months after facial nerve injury. Finally, we may also suppose that FGF-2 is not the only trophic factor responsible for the upregulation of GFAP in the activated astrocytes.
In conclusion, a lack of FGF-2 results in inaccurate target re-innervation and poor functional recovery. In the periphery, but even in the absence of FGF-2, MS can improve the accuracy of reinnervation and restore function[10]. However, centrally, a lack of FGF-2 leads to astrogliosis that cannot be prevented by MS. Nevertheless, sustained astrogliosis in the facial nucleus resulting from of a lack of FGF-2 does not prevent MS from conferring its functional benefit. We therefore conclude that the benefits of MS are not underpinned by FGF-2 in the periphery and that central gliosis resulting from a lack of FGF-2 does not impact on accuracy of reinnervation or functional recovery.
Acknowledgements
This study was financially supported by the K?ln Fortune Programm, the Jean-Uhrmacher Foundation, and Akdeniz University Research Fund. SAD is a Principal Research Fellow of the National Health & Medical Research Council, Australia.
References
[1] English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets[J]. J Comp Neurol, 2005,490(4):427–441.
[2] Angelov DN, Guntinas-Lichius O, Wewetzer K, et al. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats[J]. Adv Anat Embryol Cell Biol, 2005,180(1):1–130.
[3] Brown MC, Holland RL. A central role for denervated tissues in causing nerve sprouting[J]. Nature, 1979,282(5740):724726.
[4] Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking[J]. Neurobiol Dis, 2007,26(1):229–242.
[5] Bischoff A, Grosheva M, Irintchev A, et al. Manual stimulation of the orbicularis oculi muscle improves eyelid closure after facial nerve injury in adult rats[J]. Muscle Nerve, 2009,39(2):197–205.
[6] Slack JR, Pockett S. Terminal sprouting of motoneurones is a local response to a local stimulus[J]. Brain Res, 1981,217(2):368–374.
[7] Zhao C, Veltri K, Li S, et al. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection[J]. J Neurotrauma, 2004,21(10):1468–1478.
[8] Kiryakova S, Sohnchen J, Grosheva M, et al. Recovery of whisking function promoted by manual stimulation of the vibrissal muscles after facial nerve injury requires insulinlike growth factor 1 (IGF-1)[J]. Exp Neurol, 2010, 222(2):226–234.
[9] Sohnchen J, Grosheva M, Kiryakova S, et al. Recovery of whisking function after manual stimulation of denervated vibrissal muscles requires brain-derived neurotrophic factor and its receptor tyrosine kinase B[J]. Neuroscience, 2010,170(1):372–380.
[10] Seitz M, Grosheva M, Skouras E, et al. Poor functional recovery and muscle polyinnervation after facial nerve injury in fibroblast growth factor-2-/- mice can be improved by manual stimulation of denervated vibrissal muscles[J]. Neuroscience, 2011,182(1):241–247.
[11] Graeber MB, Kreutzberg GW. Astrocytes increase in glial fibrillary acidic protein during retrograde changes of facial motor neurons[J]. J Neurocytol, 1986,15(3):363–373.
[12] Dono R, Texido G, Dussel R, et al. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice[J]. Embo J, 1998,17(15):4213–4225.
[13] Dong Z, Sinanan A, Parkinson D, et al. J Neurosci Res, 1999,56(4):334–348.
[14] Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells[J]. J Cell Biol, 1990,110(4):1353–1360.
[15] Fujimoto E, Mizoguchi A, Hanada K, et al. Basic fibroblast growth factor promotes extension of regenerating axons of peripheral nerve. In vivo experiments using a Schwann cell basal lamina tube model[J]. J Neurocytol, 1997,26(8):511–528.
[16] Laskawi R, Wolff JR. Changes in glial fibrillary acidic protein immunoreactivity in the rat facial nucleus following various types of nerve lesions[J]. Eur Arch Otorhinolaryngol, 1996,253(8):475–480.
[17] Laskawi R, Rohlmann A, Landgrebe M, et al. Rapid astroglial reactions in the motor cortex of adult rats following peripheral facial nerve lesions[J]. Eur Arch Otorhino laryngol, 1997,254(2):81–85.
[18] Graeber MB, Bise K, Mehraein P. Synaptic stripping in the human facial nucleus[J]. Acta Neuropathol, 1993, 86(2):179–181.
[19] Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone[J]. Dev Neurosci, 2004,26(2–4):181–196.
[20] Lee TT, Green BA, Dietrich WD, et al. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat[J]. J Neurotrauma, 1999,16(5):347–356.
[21] Kang K, Lee SW, Han JE, et al. The complex morphology of reactive astrocytes controlled by fibroblast growth factor signaling[J]. Glia, 2014,62(8):1328–1344.
[22] Irmady K, Zechel S, Unsicker K. Fibroblast growth factor 2 regulates astrocyte differentiation in a regionspecific manner in the hindbrain[J]. Glia, 2011,59(5):708–719.
[23] Clarke WE, Berry M, Smith C, et al. Coordination of fibroblast growth factor receptor 1 (FGFR1) and fibroblast growth factor-2 (FGF-2) trafficking to nuclei of reactive astrocytes around cerebral lesions in adult rats[J]. Mol Cell Neurosci, 2001,17(1):17–30.
ΔThese authors contributed equally and share
ship.
? Doychin N. Angelov, M.D., Ph.D, Department of Anatomy I, University of Cologne, Joseph-Stelzmann-Strasse 9,D-50924 Cologne, Germany; Telephone: +49-221-478-5654; Fax: +49-221-478-87893; Email: angelov.anatomie@uni-koeln.de.
04 March 2014, Revised 28 November 2014, Accepted 10 April 2015, Epub 20 March 2016
R616.2, Document code: A
The authors reported no conflict of interests.
 THE JOURNAL OF BIOMEDICAL RESEARCH2016年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2016年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Health care professional training in biomedical waste management at a tertiary care hospital in India
- Stereotactic guidance for navigated percutaneous sacroiliac joint fusion
- Force degradation behavior of glucocorticoid deflazacort by UPLC: isolation, identification and characterization of degradant by FTIR, NMR and mass analysis
- Cellular metabolic energy modulation by tangeretin in 7,12-dimethylbenz(a) anthracene-induced breast cancer
- Sulindac sulfide selectively increases sensitivity of ABCC1 expressing tumor cells to doxorubicin and glutathione depletion
- Effects of closing and reopening live poultry markets on the epidemic of human infection with avian influenza A virus
