Pre-degenerated peripheral nerves co-cultured with bone marrow-derived cells: a new technique for harvesting high-purity Schwann cells
Xiao-pan Wang, Min Wu, Jian-zhong Guan, Zhao-dong Wang, Xu-bin Gao, Yang-yang LiuDepartment of Orthopedics, Bengbu Medical University Affiliated to First Hospital, Bengbu, Anhui Province, China
Pre-degenerated peripheral nerves co-cultured with bone marrow-derived cells: a new technique for harvesting high-purity Schwann cells
Xiao-pan Wang, Min Wu*, Jian-zhong Guan, Zhao-dong Wang, Xu-bin Gao, Yang-yang Liu
Department of Orthopedics, Bengbu Medical University Affiliated to First Hospital, Bengbu, Anhui Province, China
How to cite this article: Wang XP, Wu M, Guan JZ, Wang ZD, Gao XB, Liu YY (2016) Pre-degenerated peripheral nerves co-cultured with bone marrow-derived cells: a new technique for harvesting high-purity Schwann cells. Neural Regen Res 11(10):1653-1659.
Open access statement: This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding: This research was supported by the Key University Natural Science Research Project of Anhui Province of China, No. KJ2016A870.
Min Wu, M.D., Ph.D.,
wmin_2014@126.com.
orcid:
0000-0001-5749-3078
(Min Wu)
Accepted: 2016-08-23
Graphical Abstract
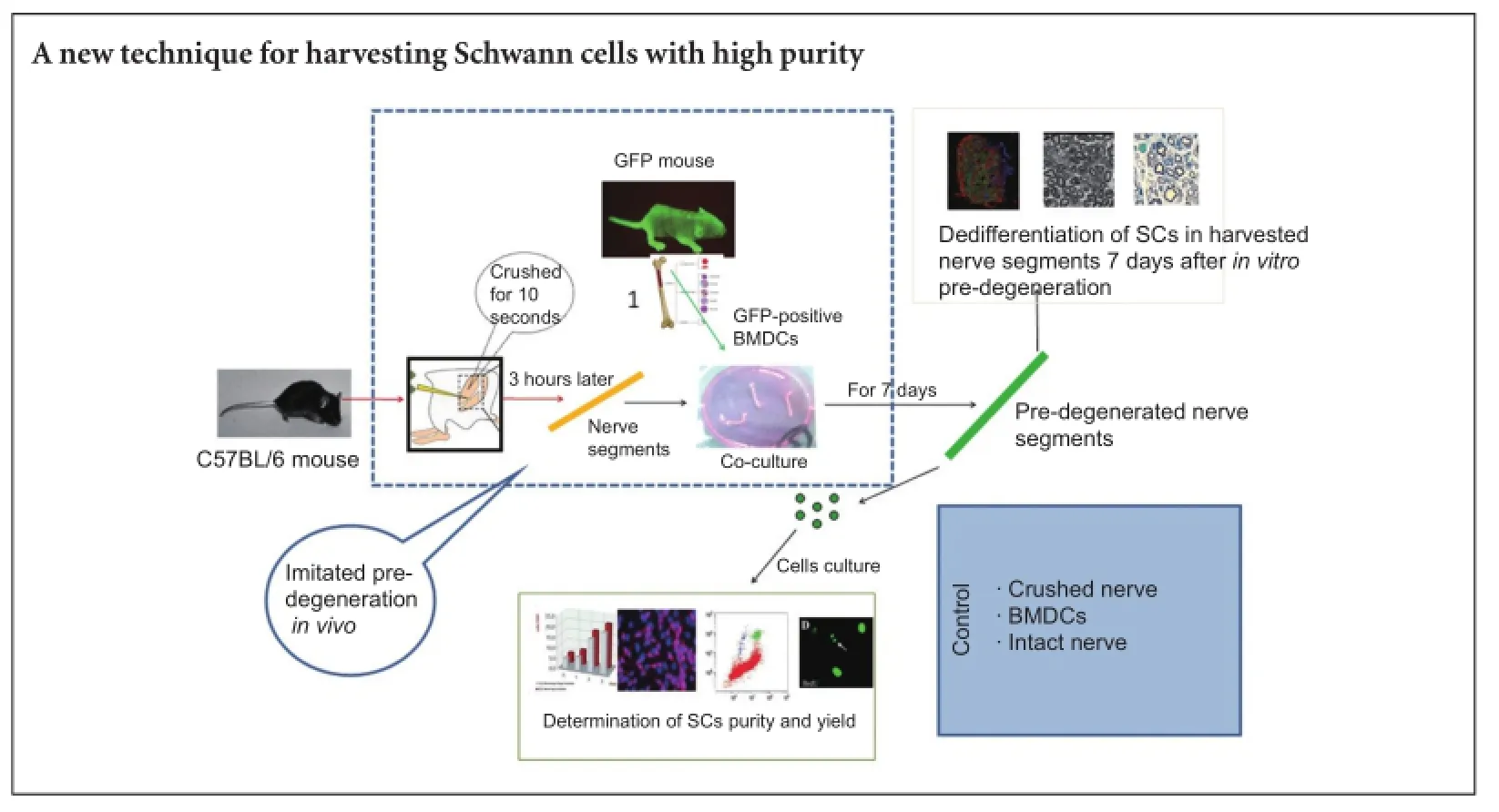
Schwann cells play an important role in the peripheral nervous system, especially in nerve repair following injury, so artificial nerve regeneration requires an effective technique for obtaining purified Schwann cells. In vivo and in vitro pre-degeneration of peripheral nerves have been shown to obtain high-purity Schwann cells. We believed that in vitro pre-degeneration was simple and controllable, and available for the clinic. Thus, we co-cultured the crushed sciatic nerves with bone marrow-derived cells in vitro. Results demonstrated that, 3 hours after injury, a large number of mononuclear cells moved to the crushed nerves and a large number of bone marrow-derived cells infiltrated the nerve segments. These changes promoted the degradation of the nerve segments, and the dedifferentiation and proliferation of Schwann cells. Neural cell adhesion molecule and glial fibrillary acidic protein expression were detected in the crushed nerves. Schwann cell yield was 9.08 ± 2.01 × 104/mg. The purity of primary cultured Schwann cells was 88.4 ± 5.79%. These indicate a successful new method for obtaining Schwann cells of high purity and yield from adult crushed sciatic nerve using bone marrow-derived cells.
nerve regeneration; bone marrow-derived cells; Schwann cells; co-culture; in vitro pre-degeneration; dedifferentiation; glial fibrillary acidic protein; neural cell adhesion molecule; mononuclear cells; neural regeneration
Introduction
The gold standard for peripheral nerve repair is autologous nerve grafting. However, the disadvantages of this method are that it can result in nerve defects at the donor sites and result in only a limited number of successful nerve grafts. Alternative strategies for peripheral nerve repair include creating tubular nerve guidance channels from natural or synthetic materials (Mosahebi et al., 2002; Chalfoun et al., 2006; Li et al., 2006; Cho, 2009). However, a key factor for the successful functioning of artificial nerves is the availability of Schwann cells (SCs). To this end, a reliable method for obtaining SCs in culture is needed.
SCs can be obtained from the nerves of neonates as opposed to adult animals, since the former have non-myelinating SCs that can proliferate (Dong et al., 1997; Haastert et al., 2007). Injury to the peripheral nervous system results in Wallerian degeneration of axons, followed by regeneration aTher macrophages penetrate and remove the damaged myelin sheath (Chen et al., 2015). Subsequently, SCs dedifferentiate and re-enter the cell proliferation cycle, forming processes known as Bungner’s bands (Stoll and Muller, 1999) that act as guides for the regenerating axons (Son andThompson, 1995). Mitosis begins 3—5 days aTher injury and is accompanied by changes in SCs such as increases in the expression of the neural cell adhesion molecule (NCAM) and the p75 neurotrophin receptor (p75NTR). Cytoskeletal constituents such as glial fibrillary acidic protein (GFAP) and vimentin also increase (Neuberger and Cornbrooks, 1989; Jessen et al., 1990; Martini, 1994). All are essential for nerve regeneration. Wallerian degeneration is a systematic process that involves a variety of cell types. Signals from degenerating axons and SC chemokines and cytokines recruit and activate macrophages from bone marrow (Lee et al., 2006). These produce factors that promote myelin phagocytosis (Boivin et al., 2007) and SC dedifferentiation and proliferation (Muller, 1996; Stoll et al., 2002).
A method for obtaining SC-enriched cultures, based on SC dedifferentiation and proliferation caused by Wallerian degeneration, has been described (Stoll and Muller, 1999).The distal stump of a nerve was leTh in the animal for several days before being harvested for implantation or culture, by a process known as in vivo pre-degeneration. ATher dissociation and culture, SCs of highly purity and proliferative capability were obtained (Keilhoff et al., 1999; Komiyama et al., 2003; Mauritz et al., 2004; Pannunzio et al., 2005). However, in vitro pre-degeneration involves the dissection, dissociation, and culture of nerve segments for 7 or more days (Kraus et al., 2010). Although the efficiency is low for in vitro degeneration compared with in vivo, it is simple and controllable, therefore more suitable for clinical application. To increase the efficiency of in vitro methods, the present study investigated whether bone marrow-derived cells (BMDCs) can promote in vitro pre-degeneration of peripheral nerves and subsequent activation of SCs. We established a new model in which the sciatic nerve of mice was crushed for 3 hours, leading to the recruitment of mononuclear cells. Crushed nerve segments were then co-cultured with BMDCs to induce in vitro pre-degeneration. We then evaluated the degree of degeneration, dedifferentiation and proliferation of SCs as well as their purity and yield.
Materials and Methods
Animals
Sixty-four healthy male and female, adult (7—8 weeks) C57Bl/6 mice, weighing 23—27 g, and six male and female adult (7—8 weeks) C57BL/6-Tg (CAG-EGFP) C14-Y01-FM131Osb mice expressing green fluorescent protein (GFP), weighing 22—25 g, were provided by Shanghai SLAC Laboratory Animal Co., Ltd., China (license No. SYXK (Hu) 2012-0001). All mice were housed in the Central Laboratory of Bengbu Medical University of China in a 12-hour light/ dark cycle at 22°C, with a humidity of 40—67%, under specific-pathogen-free conditions. All procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China (2006). Animal experiments were approved by the Institutional Review Committee of Bengbu Medical College of China.
Isolation of mouse BMDCs
BMDCs were obtained from femur and tibia bone marrow of the six transgenic GFP mice. The bone marrow was lysed by adding erythrocyte lysis buffer and extracted in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin. Homogeneous cell suspensions were then reserved for co-culture with sciatic nerve segments.
Sciatic nerve segments
Sciatic nerve segments were harvested from 64 7—8-week-old C57BL/6 mice that were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). The sciatic nerves were exposed bilaterally and one side crushed from the distal to the proximal end across the sciatic notch for 10 seconds with fine forceps. Three hours later, a nerve segment was grasped with micro tweezers and proximally dissected up to its point of transition into the lumbar plexus where it was cut. Distally directed dissection of the nerve was carried out beyond the level of the knee to the end of the skin incision, where a distal cut was made. The contralateral uncrushed sciatic nerve was harvested as a control.
In vitro pre-degeneration
ATher removal of connective tissue, the nerve segments were placed in a 6-well plate with pre-degeneration medium consisting of DMEM, 10% FBS, and 1% penicillin/streptomycin.The medium was changed every 2 days throughout the 7-day pre-degeneration process. Samples were assigned to four groups (n = 8 nerve segments each). In the crushed nerve + BMDCs group, the BMDCs suspensions were first seeded in a 6-well plate, and then crushed nerves were placed directly for co-culturing with BMDCs. In the crushed group, crushed nerves were cultured in medium only. In the BMDCs group, intact nerves were co-cultured with BMDCs. In the intact nerve group, intact nerves were cultured in medium only.
Immunohistochemistry
Following pre-degeneration, part of each sciatic nerve was rinsed with 0.1 M phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. The tissue was cryoprotected by immersion in increasing concentrations of sucrose (10—30%) over several days, then blocked, embedded in optimal cutting temperature compound (SAKURA, Oakland, CA, USA), and frozen on dry ice. Frozen specimens were sectioned on a cryostat at a thickness of 12 μm in the longitudinal plane,and serial sections were collected on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA, USA). The sections were washed in PBS, permeabilized with 0.3% Triton X-100, blocked with 10% goat serum in PBS, and incubated overnight with the following primary antibodies against dedifferentiated SCs: p75NTR, NCAM, and GFAP (all rabbits, 1:1,000; Dako, Glostrup, Denmark), as well as antibodies against macrophage/mononuclear cells, i.e., F4/80 and SMI-94 (both 1:200; Sternberger Monoclonals Inc., Baltimore, MD, USA). Alexa Fluor 546 secondary antibodies (goat and rabbit 1:1,000; Invitrogen, Carlsbad, CA, USA) were used to visualize immunoreactivity, and incubated for 30 minutes at 37°C. Samples were observed and photographically imaged with a confocal laser scanning microscope (TCS SP5, Leica, Germany). The fluorescence intensities of p75NTR, NCAM, and GFAP were analyzed with Image-Pro Plus soThware (Media Cybernetics, Rockville, MD, USA) in each group from at least three random photo-areas at 200× magnification for each experiment. GFP-positive cells were also counted.
Transmission electron microscopy
Two nerve segments for electron microscopy were obtained at the end of pre-degeneration in each group. Tissue specimens were fixed in 1% buffered glutaraldehyde/1% paraformaldehyde and processed as previously described. Ultra-thin sections were cut and analyzed with a transmission electron microscope (JEOL, Tokyo, Japan). The intact myelin was counted in each group from at least three random photo-areas at 1,000× magnification for each experiment.
Cell culture
An enzyme solution for tissue digestion was prepared by dissolving collagenase NB4 (Serva, Heidelberg, Germany) in DMEM at a concentration of 0.2% (0.27 U/mL). ATher 7 days of pre-degeneration, sciatic nerve samples were rinsed with PBS and cut into 2-mm pieces. After adding enzyme solution, samples were incubated at 37°C for 60 minutes. An equal volume of 0.05% trypsin—EDTA (Gibco, Grand Island, NY, USA) was added to the reaction. The nerve segments were mechanically dissociated by pipetting for 3—5 minutes. A 10-mL volume of FBS was added to stop the digestion and the mixture was centrifuged at 600 × g for 10 minutes. ATher removing the supernatant, the cell pellet was resuspended in SC culture medium consisting of DMEM supplemented with 10% FBS, 2 μM forskolin (Sigma, St. Louis, MO, USA), 10 ng/mL heregulin-β-1 (PeproTech, Rocky Hill, NJ, USA), and 50 ng/mL basic fibroblast growth factor (PeproTech). The cell suspension was seeded in a laminin-coated 25-cm2flask at a density of 2.0 × 104cells/cm2and incubated at 37°C in a humidified atmosphere of 5% CO2.
Morphological observation of SCs
SC morphology was assessed by phase contrast microscopy (Olympus, Tokyo, Japan) at 100× magnification. Cells obtained by enzymatic digestion were resuspended in SC culture medium. Most cells adhered to the laminin-coated flasks within 48 hours and had one of two distinct shapes corresponding to two cell types: SCs were small, bipolar or tripolar, and refractile, while fibroblasts had a flat, polygonal shape with an oval nucleus and blunt cytoplasmic processes.
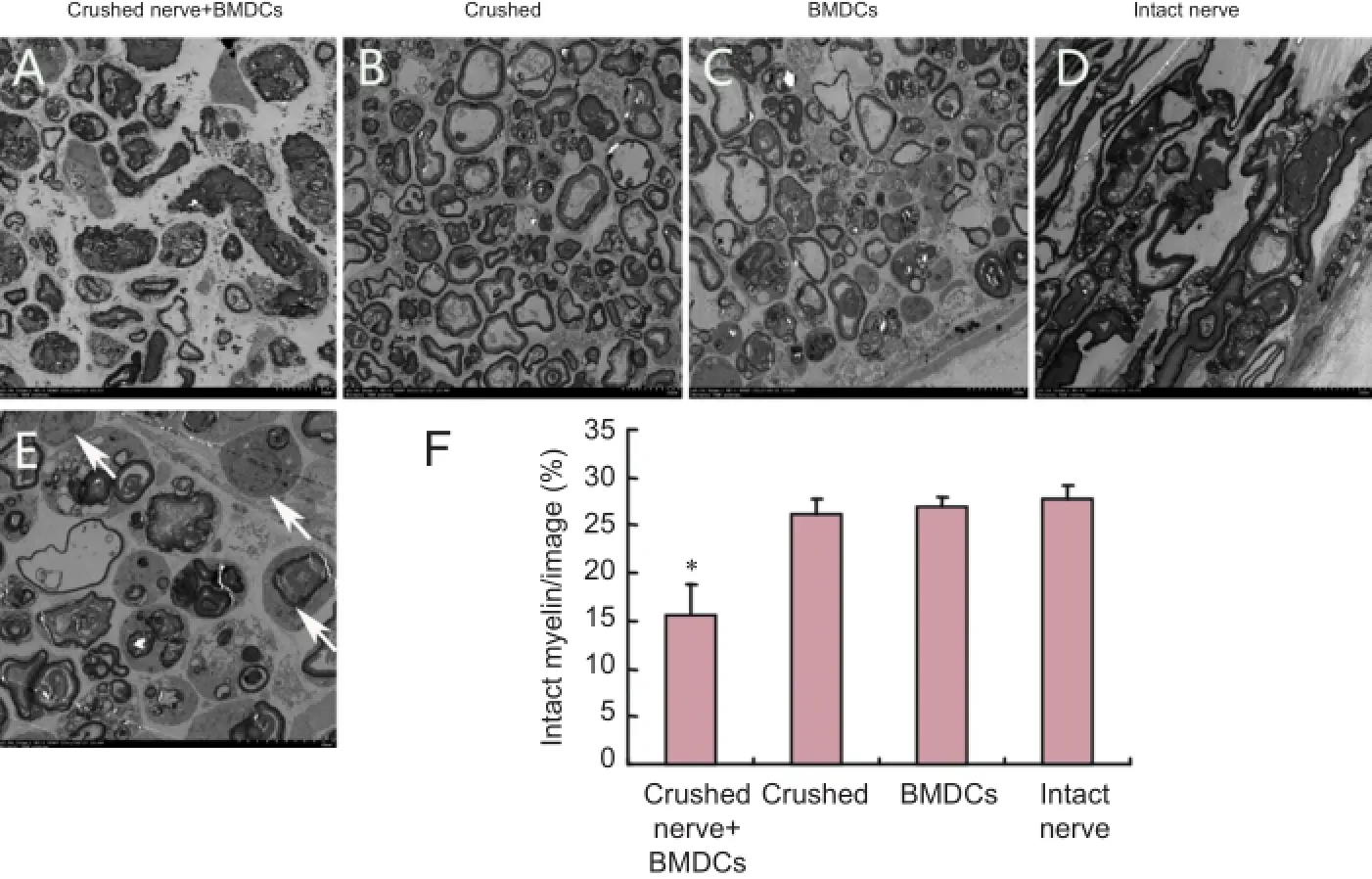
Figure 3 Ultrastructural changes in nerves 7 days aTher in vitro pre-degeneration (transmission electron microscopy).
Immunocytochemistry
Cells cultured on cover slips were fixed with 4% paraformaldehyde for 20 minutes, washed three times with PBS, and then blocked with 10% goat serum (Sigma) in PBS for 30 minutes at 37°C. Samples were treated with rabbit polyclonal anti-p75NTRantibody (Abcam, Cambridge, UK) diluted 1:500 in PBS at 37°C for 1 hour. As a control, samples were incubated without primary antibody. After three washes with PBS, samples were treated with Alexa Fluor 546 goat anti-rabbit IgG (Invitrogen) diluted 1:1,000 in PBS for 30 minutes at 37°C. Cell nuclei were counterstained with 1 μM Hoechst 33343 (Sigma) for 10 seconds. ATher a final wash inPBS, samples were mounted with mounting medium (Dako) and visualized under a fluorescence microscope (Olympus). Images were captured and processed with Image-Pro Plus software (Media Cybernetics). The cell morphologies were observed and classified, and the SC purity was calculated based on p75NTRimmunostaining.
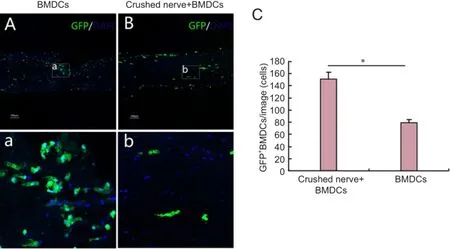
Figure 2 BMDCs infiltration into harvested nerve segments aTher 7 days of in vitro pre-degeneration (confocal laser scanning microscope).

Figure 4 Dedifferentiation of SCs in harvested nerve segments 7 days aTher in vitro pre-degeneration (immunofluorescence staining, confocal laser scanning microscopy).
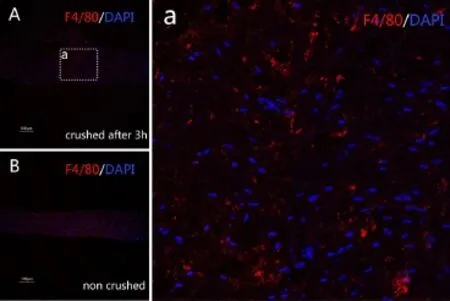
Figure 1 Mononuclear cells in harvested nerve segments 3 hours aTher injury (immunofluorescence staining, confocal laser scanning microscope).
Determination of SC purity and yield
ATher immunostaining with antibodies, SCs and fibroblasts were identified based on their morphologies. Cells with a bipolar or tripolar shape were recognized as SCs, whereas flat or polygonal cells were recognized as fibroblasts. The purity of SCs was derived from the calculation of the percentage of p75NTR-positive cells with respect to the total number of counted Hoechst-positive cells:
(1) SCs purity (%) = p75NTR-positive cells/Hoechst-positive cells × 100%.
(2) Cell yield was determined by cell counts using a hemocytometer following trypsinization and then the final cell yield was calculated as follow: Cell yield (104/mg) = number of cells/weight of nerve segments in each group.
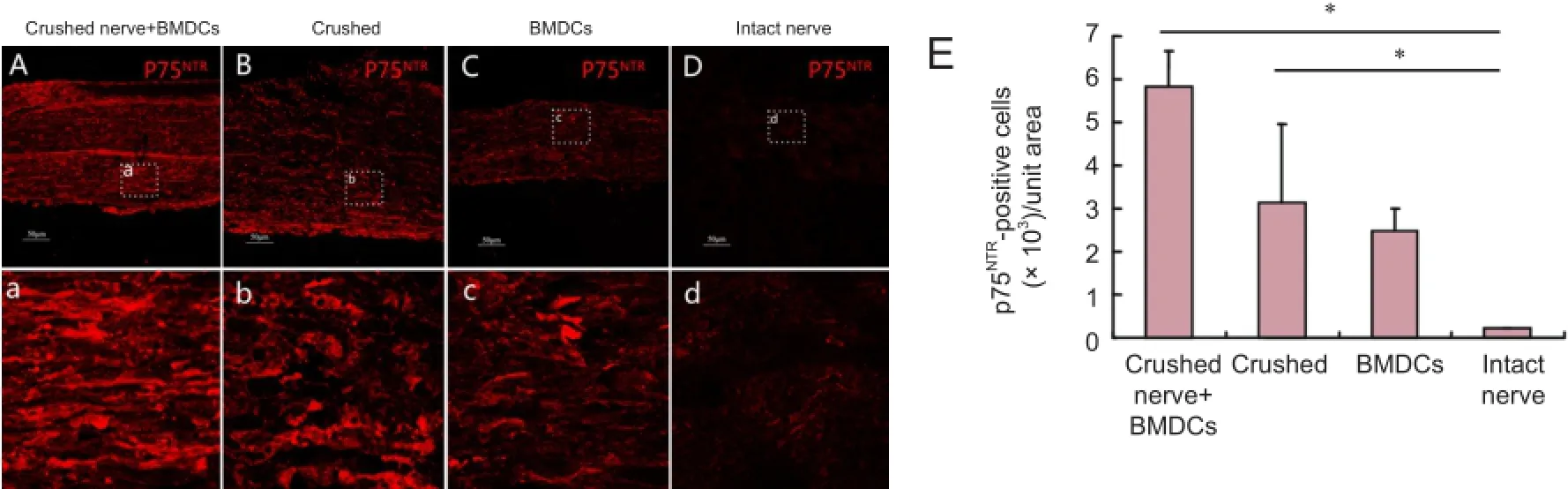
Figure 5 Dedifferentiation of SCs in harvested nerve segments 7 days aTher in vitro pre-degeneration (immunofluorescence staining, confocal laser scanning microscopy).
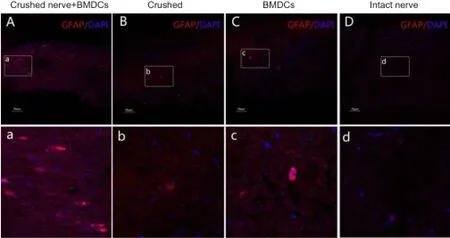
Figure 6 Dedifferentiation of SCs in harvested nerve segments 7 days aTher in vitro pre-degeneration (immunofluorescence staining, confocal laser scanning microscopy).

Figure 7 SCs aTher primary culture for 48 hours.
Statistical analysis
All data are expressed as the mean ± SD. For quantitative comparison and analysis, the values were subjected to two-sample t-test, one-way analysis of variance and the least significant difference test using SPSS 18.0 software (SPSS, Chicago, IL, USA). Statistical significance was set at P < 0.05.
Results
Mononuclear cells were recruited to the site of nerve injury
Following injury to the peripheral nerve, SC- and macrophage-derived cytokines and chemokines recruit mononuclear cells from the blood that phagocytose debris while activating SCs during Wallerian degeneration. Indeed, we observed many F4/80-positive cells in crushed nerves, but not in intact nerves (Figure 1).
BMDCs infiltrated into nerve segments in co-cultures with injured nerve segments
BMDCs were obtained from transgenic GFP mice so that they could be visualized in co-cultures with harvested nerve segments. We observed many infiltrated GFP-positive cells in nerve segments of crushed nerve + BMDCs and BMDCs groups, particularly in the crushed nerve + BMDCs group (P< 0.05; Figure 2).
BMDCs promoted the degeneration of co-cultured injured nerve segments
Nerve segments in all groups showed degeneration 7 daysaTher in vitro pre-degeneration, including of axons and myelin sheaths. The extent of degeneration and the number of phagocytic cells were greater in the crushed nerve + BMDCs group than in the crushed nerve only, BMDCs and intact nerve groups (P < 0.05; Figure 3). There were no differences in the degree of degeneration or the number of phagocytic cells among the crushed, BMDCs and intact nerve groups.

Table 1 Cell yield in the four experimental groups 7 days following in vitro pre-degeneration and purity of SCs aTher primary culture for 48 hours
BMDCs promoted the dedifferentiation of SCs from injured nerve segments
During Wallerian degeneration, SCs re-expressed embryonic cell surface molecules, such as NCAM and p75NTR, and upregulated cytoskeletal components, such as GFAP as a part of the dedifferentiation process. We detected NCAM, p75NTR, and GFAP expression in SCs in all groups 7 days after pre-degeneration, with the highest levels observed in crushed nerve + BMDCs group (Figures 4—6). The levels of NCAM, p75NTR, and GFAP proteins were similar among crushed, BMDCs and intact nerve groups.
Characterization of dedifferentiated SCs
Nerve segments were dissociated 7 days aTher in vitro pre-degeneration and the number of cells was counted before seeding in 6-well plates. The cumulative cell counts were significantly higher in the crushed nerve +BMDCs group than in the crushed, BMDCs and intact nerve groups (P < 0.05; Table 1).
ATher enzymatic dissociation, cells were resuspended in SC culture medium; most adhered to flasks within 48 hours and had one of two shapes. Fibroblasts were flat and polygonal with an oval nucleus and blunt cytoplasmic processes, while SCs were small, elongated, fusiform, and bipolar or tripolar. Based on these characteristics, most cells in the crushed nerve + BMDCs group were SCs and few were fibroblasts. The percentage of fibroblasts was higher in the crushed, BMDCs and intact nerve groups than in the crushed nerve + BMSCs group. The identity of SCs was confirmed by epifluorescence microscopy using antibodies against p75NTR(Figure 7). Primary cultures of SCs purity was significantly higher in the crushed nerve + BMDCs group (88.4% ± 5.79% based on P75NTRexpression) than that in the crushed, BMDCs and intact nerve groups (P < 0.05; Table 1).
Discussion
In the present study, we designed a new model for in vitro pre-degeneration. in vivo pre-degeneration is an efficient method for culturing SCs (Keilhoff et al., 1999; Tomita et al., 2009). However, in vivo pre-degeneration requires two separate surgical procedures and is unacceptable in humans for ethical reasons. Thus, the in vitro approach may be widely applicable, because it is simple and controllable, although the efficiency is low. Therefore, we designed our new model of crushed nerve segments co-cultured with BMDCs so as to imitate the in vivo pre-degeneration.
In this study, the new model imitated the partial process of in vivo pre-degeneration successfully, in which mononuclear cells are recruited to the crushed nerve. Moreover, BMDCs infiltrated nerve segments 7 days after co-culture, which was consistent with the process of in vivo pre-degeneration. It was previously shown that peripheral nerves crushed for 3 hours and co-cultured with BMDCs induced the expression of leukemia inhibitory factor and interleukin-6 in SCs. This was followed by activation and recruitment of blood mononuclear cells, which may in turn recruit BMDCs and promote SC dedifferentiation and proliferation (Tofaris et al., 2002). Matrix metalloproteinases have been shown to play a critical role in this process (La Fleur et al., 1996). In particular, matrix metalloproteinase-9 expression aTher a nerve is crushed is associated with the breakdown of the blood-nerve barrier and mononuclear cell invasion (Shubayev and Myers, 2000; Siebert et al., 2001). Taking these points into consideration was crucial for our study to succeed.
During nerve degeneration, cytokines released by mononuclear phagocytes are critical for SC dedifferentiation and re-entry to the cell cycle for their proliferation; all necessary for the culture of SCs. For example, interleukin-1 stimulates nerve growth factor production (Lindholm et al., 1987, 1988), and induces the expression of integrin-β1 on fibroblasts-like cells (Santala and Heino, 1991). Integrin-β1 is temporally correlated with macrophage recruitment to the endoneurium (Taskinen et al., 1995). Transforming growth factor-β, another macrophage-derived cytokine, is a mitogen for SCs (Ridley et al., 1989). Moreover, transforming growth factor-β released by SCs acts a macrophage chemoattractant (Stoll et al., 1989). Thus, mutually regulatory interactions exist between macrophages and SCs.
In our model, mononuclear cells were recruited and BMDCs were infiltrated in the crushed nerve segments and clearly promoted the degeneration of the nerve segments.The axons and the myelin sheets were disrupted and then removed. SCs dedifferentiated and proliferated, regaining the expression of NCAM and p75NTRand upregulating GFAP.This cell culture has proved an effective technique to obtain Schwann cells. In our study, the cumulative cell count in the culture was 9.08 ± 2.01 × 104/mg and the purity was 88.4 ± 5.79 % in primary culture.
In summary, our study demonstrates a method for obtain-ing a highly pure SC culture from crushed sciatic nerve by co-culturing with BMDCs that can potentially be used for artificial nerve regeneration following nerve injury.
Acknowledgments: We thank Dr. Zun-li Shen and Yu-qing Jin from Department of Plastic and Reconstructive Surgery, Shanghai First Hospital, Shanghai Jiao Tong University School of Medicine, China, for their kind supply of GFP-positive mice.
Author contributions: XPW designed the study, conducted the experiments, analyzed the data, and wrote the paper. MW obtained the funding and provided the critical revision of the paper. JZG designed the study. ZDW and XBG participated in nerve pre-degeneration and co-culture with BMDCs. YYL participated in cell culture and animal administration. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S (2007) Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery aTher peripheral nerve injury. J Neurosci 27:12565-12576.
Chalfoun CT, Wirth GA, Evans GR (2006) Tissue engineered nerve constructs: where do we stand? J Cell Mol Med 10:309-317.
Chen S, Chen ZG, Dai H, Ding JX, Guo JS, Han N, Jiang BG, Jiang HJ, Li J, Li SP, Li WJ, Liu J, Liu Y, Ma JX, Peng J, Shen YD, Sun GW, Tang PF, Wang GH, Wang XH, et al. (2015) Repair, protection and regeneration of peripheral nerve injury. Neural Regen Res 10:1777-1798.
Cho M (2009) Reconnecting injured nerves. Nat Neurosci 12:1085.
Dong Z, Dean C, Walters JE, Mirsky R, Jessen KR (1997) Response of Schwann cells to mitogens in vitro is determined by pre-exposure to serum, time in vitro, and developmental age. Glia 20:219-230.
Haastert K, Mauritz C, Chaturvedi S, Grothe C (2007) Human and rat adult Schwann cell cultures: fast and efficient enrichment and highly effective non-viral transfection protocol. Nat Protoc 2:99-104.
Jessen KR, Morgan L, Stewart HJ, Mirsky R (1990) Three markers of adult non-myelin-forming Schwann cells, 217c (Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development 109:91-103.
Keilhoff G, Fansa H, Schneider W, Wolf G (1999) In vivo predegeneration of peripheral nerves: an effective technique to obtain activated Schwann cells for nerve conduits. J Neurosci Methods 89:17-24.
Komiyama T, Nakao Y, Toyama Y, Asou H, Vacanti CA, Vacanti MP (2003) A novel technique to isolate adult Schwann cells for an artificial nerve conduit. J Neurosci Methods 122:195-200.
Kraus A, Tager J, Kohler K, Manoli T, Haerle M, Werdin F, Hoffmann J, Schaller HE, Sinis N (2010) Efficacy of various durations of in vitro predegeneration on the cell count and purity of rat Schwann-cell cultures. J Neurotrauma 27:197-203.
La Fleur M, Underwood JL, Rappolee DA, Werb Z (1996) Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med 184:2311-2326.
Lee H, Jo EK, Choi SY, Oh SB, Park K, Kim JS, Lee SJ (2006) Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem Biophys Res Commun 350:742-747.
Li Q, Ping P, Jiang H, Liu K (2006) Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery 26:116-121.
Lindholm D, Heumann R, Hengerer B, Thoenen H (1988) Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem 263:16348-16351.
Lindholm D, Heumann R, Meyer M, Thoenen H (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330:658-659.
Martini R (1994) Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol 23:1-28.
Mauritz C, Grothe C, Haastert K (2004) Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J Neurosci Res 77:453-461.
Mosahebi A, Fuller P, Wiberg M, Terenghi G (2002) Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol 173:213-223.
Muller HW (1996) Gene expression in nerve regeneration. Diabet Med 13:682.
Neuberger TJ, Cornbrooks CJ (1989) Transient modulation of Schwann cell antigens aTher peripheral nerve transection and subsequent regeneration. J Neurocytol 18:695-710.
Pannunzio ME, Jou IM, Long A, Wind TC, Beck G, Balian G (2005) A new method of selecting Schwann cells from adult mouse sciatic nerve. J Neurosci Methods 149:74-81.
Ridley AJ, Davis JB, Stroobant P, Land H (1989) Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol 109:3419-3424.
Santala P, Heino J (1991) Regulation of integrin-type cell adhesion receptors by cytokines. J Biol Chem 266:23505-23509.
Shubayev VI, Myers RR (2000) Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res 855:83-89.
Siebert H, Dippel N, Mader M, Weber F, Bruck W (2001) Matrix metalloproteinase expression and inhibition aTher sciatic nerve axotomy. J Neuropathol Exp Neurol 60:85-93.
Son YJ, Thompson WJ (1995) Schwann cell processes guide regeneration of peripheral axons. Neuron 14:125-132.
Stoll G, Muller HW (1999) Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9:313-325.
Stoll G, Jander S, Myers RR (2002) Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst 7:13-27.
Stoll G, Griffin JW, Li CY, Trapp BD (1989) Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol 18:671-683.
Taskinen HS, Heino J, Roytta M (1995) The dynamics of beta 1 integrin expression during peripheral nerve regeneration. Acta Neuropathol 89:144-151.
The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
Tofaris GK, Patterson PH, Jessen KR, Mirsky R (2002) Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 22:6696-6703.
Tomita K, Hata Y, Kubo T, Fujiwara T, Yano K, Hosokawa K (2009) Effects of the in vivo predegenerated nerve graTh on early Schwann cell migration: quantitative analysis using S100-GFP mice. Neurosci Lett 461:36-40.
Copyedited by Ann Dawes E, Haase R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.193246
*Correspondence to:
- 中國神經(jīng)再生研究(英文版)的其它文章
- Recovery of an injured anterior cingulum to the basal forebrain in a patient with brain injury: a 4-year follow-up study of cognitive function
- Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber's hereditary optic neuropathy
- Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function aTher spinal cord injury
- Human amniotic epithelial cells combined with silk fibroin scaffold in the repair of spinal cord injury
- Electrical stimulation promotes regeneration of injured oculomotor nerves in dogs
- Boric acid reduces axonal and myelin damage in experimental sciatic nerve injury