The Future of Transcatheter Therapy for Mitral Valve Disease
Ted Feldman, MD, FESC, FACC, MSCAI and Mayra Guerrero, MD, FACC, FSCAI
Introduction
The development of transcatheter therapy for mitral valve disease has unfolded over the last decade and a half [1]. All the efforts to develop percutaneous approaches for mitral regurgitation (MR)have been based in some way upon existing surgical procedures. The earliest concepts were based on mitral annuloplasty. Indirect angioplasty utilizing coronary sinus was one of the first entrants into the field. Leaflet repair with the MitraClip device(Abbott Vascular, Santa Clara, CA, USA) has had the greatest development and clinical application.More recently, after the success of transcatheter aortic valve replacement, transcatheter mitral valve replacement has emerged. In looking forward as this field develops, one of the largest questions is what the relative roles of valve repair and valve replacement might be.
In practice today, percutaneous mitral valve therapy is virtually synonymous with MitraClip leaflet repair (Figure 1). Over 25,000 patients have been treated world-wide. The evidence base for the development of this novel therapy includes the initial feasibility study, a randomized trial in surgical candidates, a large prospective United States based registry, and multiple international registry studies.
Percutaneous Leaflet Repair with MitraClip
The EVEREST I (Endovascular Valve Edge-to-Edge REpair STudy) feasibility trial represented the first substantial and successful effort to accomplish percutaneous mitral repair [2]. The initial report included 55 patients. The major finding of the investigation was that this procedure could be performed safely, and resulted in some meaningful reduction in the severity of MR. An important observation that is apparent retrospectively is that the procedural success rate was relatively modest.This is not surprising since this was the first effort with a novel, first in class device. All of the cases in this registry represented the earliest learning curve experience with this device and for these operators. Despite this critical limitation it was clear that the procedure could be performed successfully and safely, that MR was reduced, and most importantly from the clinician viewpoint, patients who had even modest reductions in MR severity had excellent and often dramatic clinical response to this therapy.
The demonstration of feasibility in the EVEREST I experience led to the EVEREST II trial. This was a randomized comparison of MitraClip and conventional surgery including both mitral repair and replacement [3]. Patients included in this trial were all candidates for mitral repair surgery. They were selected to have leaflet morphology acceptable for both percutaneous and surgical repair. The randomization was 2:1 and included 279 patients. The primary composite endpoint for efficacy was freedom from death, surgery for mitral valve dysfunction, and from grade 3+ or 4+ MR at 12 months.The primary safety end point was a composite of major adverse events within 30 days. At 12 months,the rates of the primary end point for efficacy were 55% in the percutaneous repair group and 73% in the surgery group. The respective rates of the components of the primary end point were death, 6% in each group, surgery for mitral valve dysfunction,20% versus 2%, and grade 3+ or 4+ MR, 21% versus 20%. Major adverse events occurred in 15%of patients in the percutaneous repair group and 48% of patients in the surgery group at 30 days.At 12 months, both groups had improved left ventricular (LV) size, New York Heart Association functional class, and quality of life measures, as compared with baseline. The conclusion from this randomized trial was that although percutaneous repair was less effective at reducing MR than conventional surgery, the procedure was associated with superior safety and similar improvements in clinical outcomes. The results have been stable out to 5 years of follow-up [4].
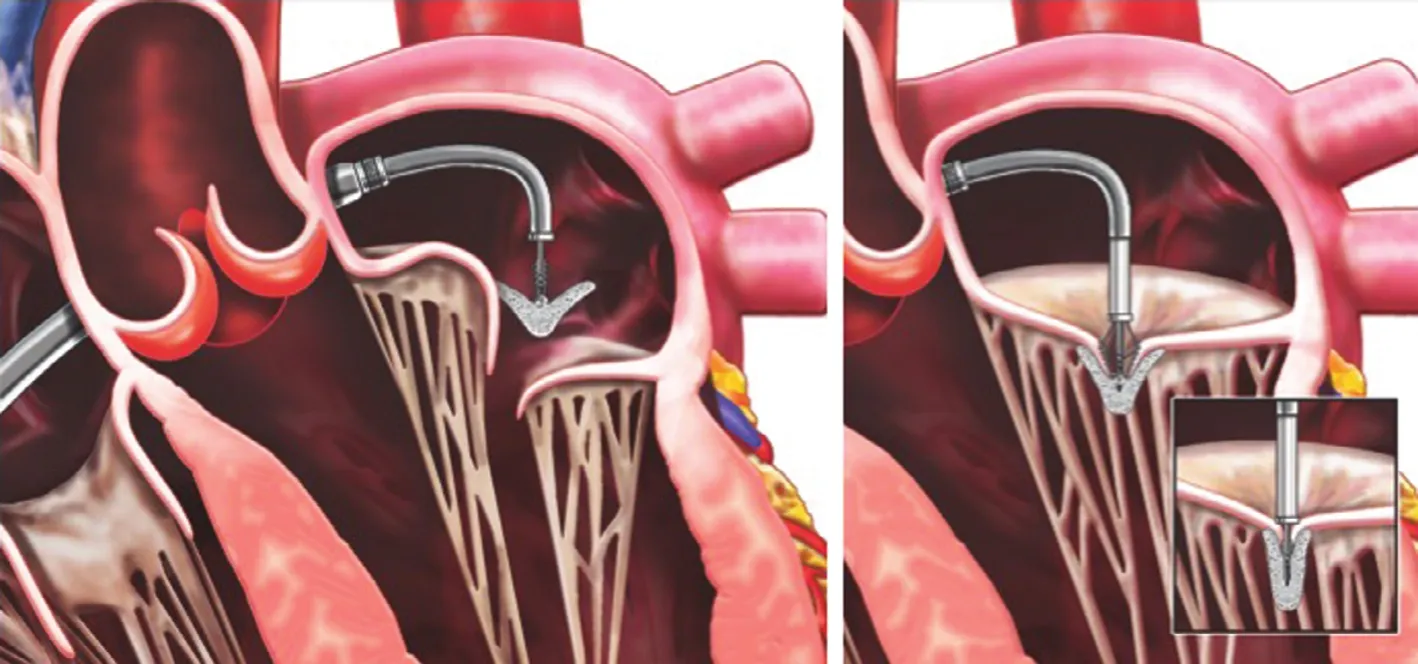
Figure 1 The MitraClip Device.To introduce the Clip, the clip delivery system (CDS) is advanced through the Guide into the left atrium (Left). Under echocardiographic and fluoroscopic guidance, the Clip is aligned perpendicular to the valve plane, with the Clip Arms perpendicular to the line of coaptation. It is then advanced into the left and then slowly retracted to grasp the leaflets (Right). The Clip is closed(Right, inset), and if reduction of mitral regurgitation is satisfactory, it is released. From Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol 2014;63:2057–68. Artwork by Craig Skaggs.
The EVEREST II trial was conducted in the population of patients who were suitable for surgical mitral repair. Surgical repair therapy has developed over more than 50 years and is clearly associated with excellent immediate outcomes and long-term durability, despite the lack of prospective or randomized trials and the absence of core lab echocardiographic follow-up. Thus MitraClip is a completely new therapeutic approach that was compared with an established surgical procedure. The majority of patients treated in EVEREST II had degenerative MR and a smaller proportion had functional MR. It was clear during the trial that these functional MR patients had both safety and efficacy outcomes that were similar to the degenerative MR patients. It was also apparent during the screening process that there were patients with mitral morphology suitable for repair but high risk for surgery characteristics that precluded standard surgery. This observation identified an important group of patients with severe symptomatic MR and no option for surgical therapy.
The REALISM registry was created to evaluate the outcome MitraClip in this high-risk patients.The initial report in this group included 78 patients.Patients with severe symptomatic MR and an estimated surgical mortality rate of 12% were enrolled[5]. A comparator group of patients screened concurrently but not enrolled were identified retrospectively to compare survival in patients treated by standard care. The mean age was 77 years, half had previous cardiac surgery, 59% had functional and 41% degenerative MR. MitraClip devices were successfully placed in 96% of patients. Protocolpredicted surgical mortality rate in the high risk and concurrent comparator group was 18.2% and 17.4%,respectively, and Society of Thoracic Surgeons calculator estimated mortality rate was 14.2% and 14.9%, respectively. The 30-day procedure-related mortality rate was 7.7% in the MitraClip and 8.3%in the comparator group. The 12-month survival rate was 76% in the HRS and 55% in the concurrent comparator group. In surviving patients with matched baseline and 12-month data, 78% had an MR grade of 2+ or less. Left ventricular enddiastolic and end-systolic volumes improved. New York Heart Association functional class improved from III/IV at baseline in 89% to class I/II in 74%.Quality of life evaluated with Short Form-36 physical and mental component scores were improved at 12 months. Most importantly the annual rate of hospitalization for congestive heart failure in surviving patients with matched data decreased from 0.59 to 0.32 hospitalizations per year. The MitraClip procedure was performed without any impact on early mortality and the device reduced MR in a majority of patients deemed at high risk of surgery, resulting in improvement in clinical symptoms and signifi-cant LV reverse remodeling over 12 months.
Similar results were noted after more experience was obtained with this high-risk group, with over 350 patients reported in 2014 [6]. The EVEREST II High-Risk registry and REALISM Continued Access Study High-Risk Arm were prospective registries of patients who received the MitraClip device for MR in the United States. One year outcomes were reported for patients with 3–4+ MR and an overall surgical mortality risk of >12%, based on the society of thoracic surgeons risk calculator.Patients were elderly, mean age 76 years, with 70%having functional MR and 60% having prior cardiac surgery. The mitral valve device reduced MR to≤2+ in 86% of patients at discharge. Major adverse events at 30 days included death in 4.8%, myocardial infarction in 1.1%, and stroke in 2.6%. At 12 months, MR was ≤2+ in 84% and LV end-diastolic volume improved from mean 161 mL to 143 mL.The annual hospitalization rate for heart failure fell from 0.79% pre-procedure to 0.41% per year postprocedure. The Kaplan-Meier survival estimate at 12 months was over 77%. These findings of safety,favorable LV remodeling, improved symptoms, and reduced heart failure hospitalizations in a high-risk group of patients with no other options for therapy are the basis for most of the use of MitraClip and global practice.
Numerous prospective registries have validated these findings [7–9]. In addition to the safety of the procedure in a high-risk population, the length of hospital stay is remarkably short, averaging<3 days. The rate of discharge to home exceeds 80% in this elderly patient group with multiple comorbidities.
In the United States, FDA approval was given for high-risk patients with DMR. Surgical mitral valve repair remains the gold standard for severe DMR.A prohibitive risk DMR cohort was identified by a multidisciplinary heart team selected from high-risk DMR patients, enrolled in the EVEREST II studies[10]. A total of 127 patients were retrospectively identified as having prohibitive risk. Patients were elderly with a mean age of 82.4 years, severely symptomatic, and at prohibitive surgical risk with STS score mean 13.2%. MitraClip was successfully performed in 95.3%. The hospital stay was only 2.9±3.1 days. At 30 days the mortality was 6.3%. At 1 year the mortality was 23.6%. At 1 year, the severity of MR in 82.9% of patients remained ≤2+ and hospitalizations for heart failure were reduced in patients whose MR was reduced. It was concluded that after 1 year in prohibitive surgical risk patients MitraClip is associated with safety and good clinical outcomes, including decreases in rehospitalization,functional improvements, and favorable ventricular remodeling. This study defined the approved indication for MitraClip in the United Sates.
The MitraClip procedure is performed using general anesthesia and transesophageal echocardiographic (TEE) guidance (Figures 2–4). The use of general anesthesia is driven by the need for TEE. In current practice, typical procedure times range from 1 to 2 h. Occasional procedures are protracted. Left atrial access is achieved using transseptal puncture.It is clear that patient selection is critical for achieving good outcomes, and that operator experience is also an important variable determining procedure success.
Despite the highly successful registry experience using this device for FMR, randomized trial data is being accumulated to clearly define the role of MitraClip in high-risk FMR. The COAPT trial is ongoing in the United States. This randomized comparison of MitraClip and guideline directed medical therapy will compare the two treatments in 430 patients. Rigorous patient selection criteria including history of heart failure with either elevated BNP or a recent heart failure hospitalization, LV ejection fraction <50%, and the use of cardiac resynchronization pacing therapy when indicated, in conjunction with best medical therapy have resulted in slow enrollment. As a result, final results will be not available for some time. Several other randomized trials are ongoing internationally to address and better define the role of MitraClip for FMR.

Figure 2 Fluoroscopic Images From a MitraClip Procedure in a COAPT Patient.The patient is a 72-year-old man with severe MR, elevated BNP, multiple heart failure hospitalizations, and additional risks of prior coronary bypass surgery, carotid endarterectomy, and chronic kidney disease. The left ventricular ejection fraction is 45%. The STS risk score for mitral valve replacement is 10.9%. The upper left panel shows the placement of a MitraClip with transesophageal echo guidance (TEE). The white arrow shows the MitraClip device still attached to the delivery system. The upper panel shows placement of the second clip, noted by the arrow. This second clip is being passed lateral to the first, in a closed configuration. Lower left shows the second clip opened in the left ventricle prior to being pulled back to grasp the mitral leaflets. The lower right panel shows both clips after final release. The distal marker at the tip of the guide catheter is seen at the left side of the picture (arrow). From Feldman T, Mehta A, Guerrero M, Levisay JP, Salinger MH. MitraClip therapy for mitral regurgitation: secondary MR. In Interventional Cardiology Clinics; Matthew Price, Editor-in-Chief, and Jason Rogers,Editor-special issue on Transcatheter Mitral Valve Intervention, Interv Cardiol Clin 2015;5:83–92.
For the present, MitraClip remains the best validated catheter-based MR therapy. Several tens of thousands of patients have been treated with this technology, compared to only several hundred with all of the other percutaneous mitral repair technologies combined.
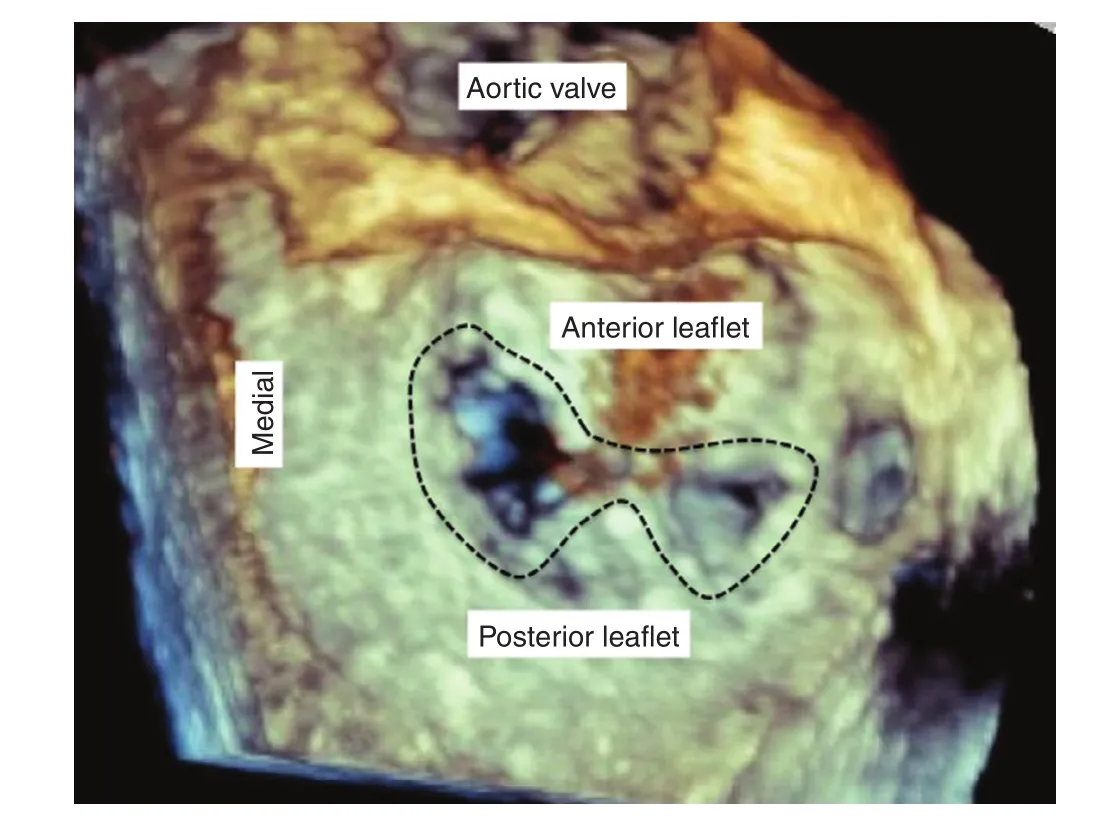
Figure 3 3-Dimensional Transesophageal Echocardiographic Image Obtained From the Left Atrial Side, the So-Called “Surgeons Review”.The aortic valve is at the top of the picture. The dotted black line encircles the double orifice created by placement of the MitraClips. From Feldman T, Mehta A, Guerrero M,Levisay JP, Salinger MH. MitraClip therapy for mitral regurgitation: secondary MR. In Interventional Cardiology Clinics; Matthew Price, Editor-in-Chief, and Jason Rogers, Editor-special issue on Transcatheter Mitral Valve Intervention, Interv Cardiol Clin 2015;5:83–92.
Coronary Sinus Indirect Annuloplasty with Carillon
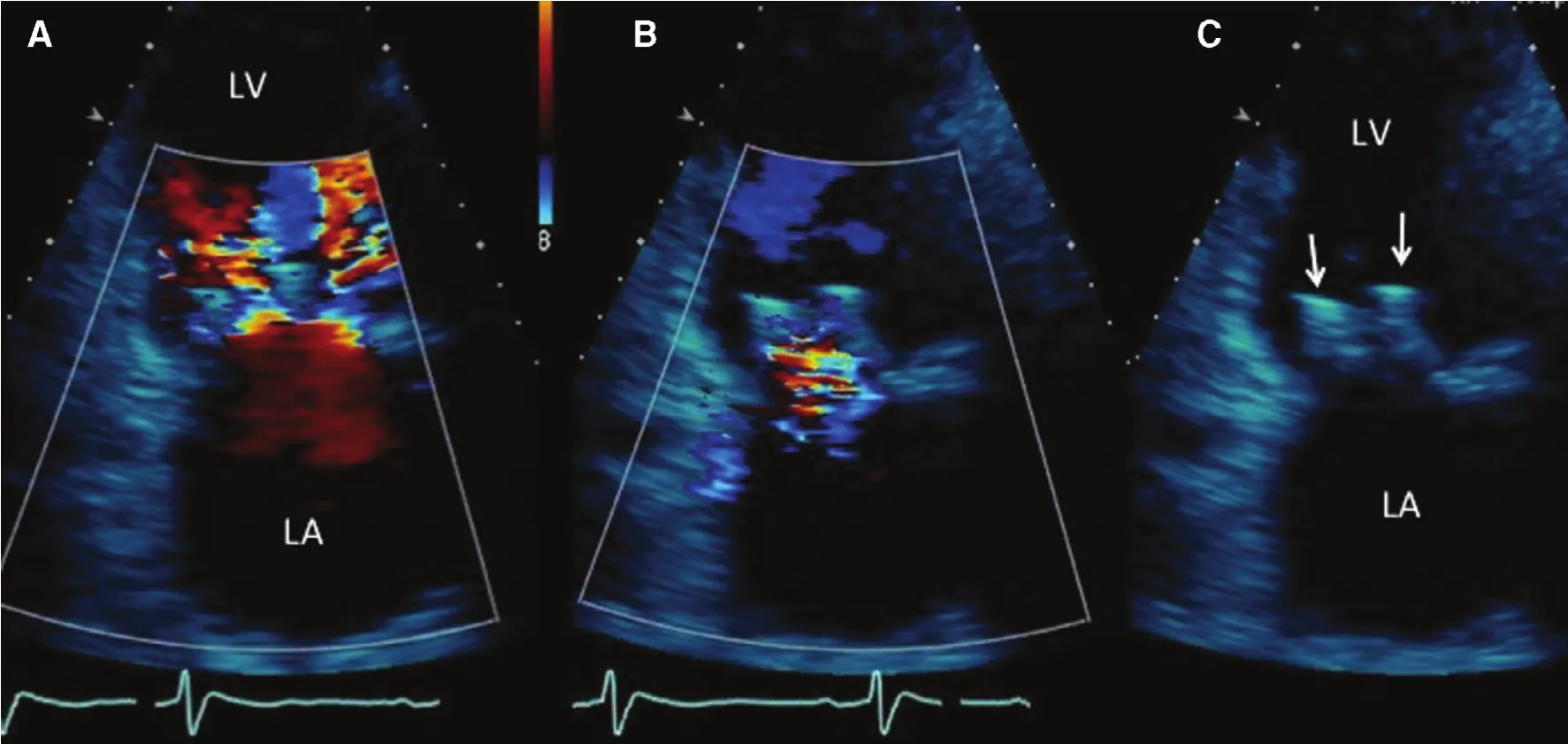
Figure 4 Transthoracic Echocardiogram Obtained Before Discharge.This four-chamber view shows the left ventricle (LV) at the top of the picture, and the left atrium (LA) at the bottom of the picture. On the left panel (A) shows the double jets of diastolic inflow into the left ventricle created by the double orifice. Panel(B) shows mild MR into the left atrium. On the right side, panel (C) shows the echo appearance of the two MitraClips, shown by the arrows. From Feldman T, Mehta A, Guerrero M, Levisay JP, Salinger MH. MitraClip therapy for mitral regurgitation:secondary MR. In Interventional Cardiology Clinics; Matthew Price, Editor-in-Chief, and Jason Rogers, Editor-special issue on Transcatheter Mitral Valve Intervention, Interv Cardiol Clin 2015;5:83–92.
Another early concept for mitral repair is based on the close anatomic proximity of the coronary sinus to the mitral annulus. The coronary sinus encircles a substantial part of the circumference of the posterior mitral leaflet. Thus a device that tensions the coronary sinus might be effective at reducing the mitral circumference and facilitating leaflet approximation. The coronary sinus approach is attractive because of its centricity. Vascular access requires only a nine French guide catheter placed via internal jugular venous access. The distal anchor of the Carillon device is implanted in the distal coronary sinus (Cardiac Dimension Inc., Kirkland, WA, USA)(Figure 5). Before the proximal anchor is released,traction is placed on the anchor by simply pulling on the guide catheter. Reductions in MR severity can be watched in real time using transesophageal echo. One potential limitation is that the coronary sinus crosses over the circumflex coronary artery or an obtuse marginal branch in a significant number of patients. Thus, if coronary compromise is noted during device implantation, repositioning can be used to minimize or eliminate this problem. In the most problematic situations the Carillon can be removed since the implant process is completely reversible until the time of final device release [11].
The Carillon has been evaluated in the AMADEUS,TITAN and TITAN-II registry trials including 113 patients. After 1 year following implantation in all three reports, there were improvements in indices of MR severity, favorable LV remodeling, and improvements in quality of life and exercise capacity. One interesting finding that is consistent in all three registries has been the continued improvement and these measures of effectiveness over the course of the year after implantation. In the TITAN trial, the Carillon Mitral Contour System was evaluated in 53 heart failure patients with at least moderate FMR [12]. Patients in whom the device was placed then acutely recaptured for clinical reasons served as a control group for comparison. Thirtysix patients received a permanent implant while 17 had the device recaptured. The 30-day major adverse event rate was 1.9%. In contrast to the comparison group, the implanted cohort demonstrated significant reductions in regurgitant volume (baseline 34.5±11.5 mL to 17.4±12.4 mL at 12 months).There was also a reduction in LV diastolic volume[baseline 208.5±62.0 mL to 178.9±48.0 mL at 12 months (P=0.015)] and systolic volume [baseline 151.8±57.1 mL to 120.7±43.2 mL at 12 months(P=0.015)], compared with progressive LV dilation in the comparator patients. The 6-min walk distance improved for the implanted patients by 102.5±164 m at 12 months (P=0.014) and 131.9±80 m at 24 months (P<0.001). Significant clinical improvements persisted up to 24 months. TITAN II studied 50 patients and similar results have been presented,but not yet published. These studies establish that the Carillon can be used safely in a heart failure population with measurable improvements in MR severity,LV remodeling and quality of life.

Figure 5 Coronary Sinus Annuloplasty: The Cardiac Dimensions Carillon Device: The Guide Catheter is Introduced Through Jugular Venous Access.The device is delivered in the distal coronary sinus andf the distal anchor is released (left panel), and then the guide catheter is pulled back to release the proximal anchor in the coronary sinus ostium. The right panel shows the wireform, made of nitinol wire, after release in the coronary sinus. Cinching pf the mitral annulus results in compression of the septal-lateral dimension and thus the regurgitant orifice. From Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation.J Am Coll Cardiol 2014;63:2057–68. Artwork by Craig Skaggs.
The Carillon received CE approval in 2011.In international use, over 400 patients have been treated with the Carillon device. A randomized study of 120 patients (REDUCE FMR) to compare Carillon treatment against medical control is underway and a trial in the United States is in the planning stages.
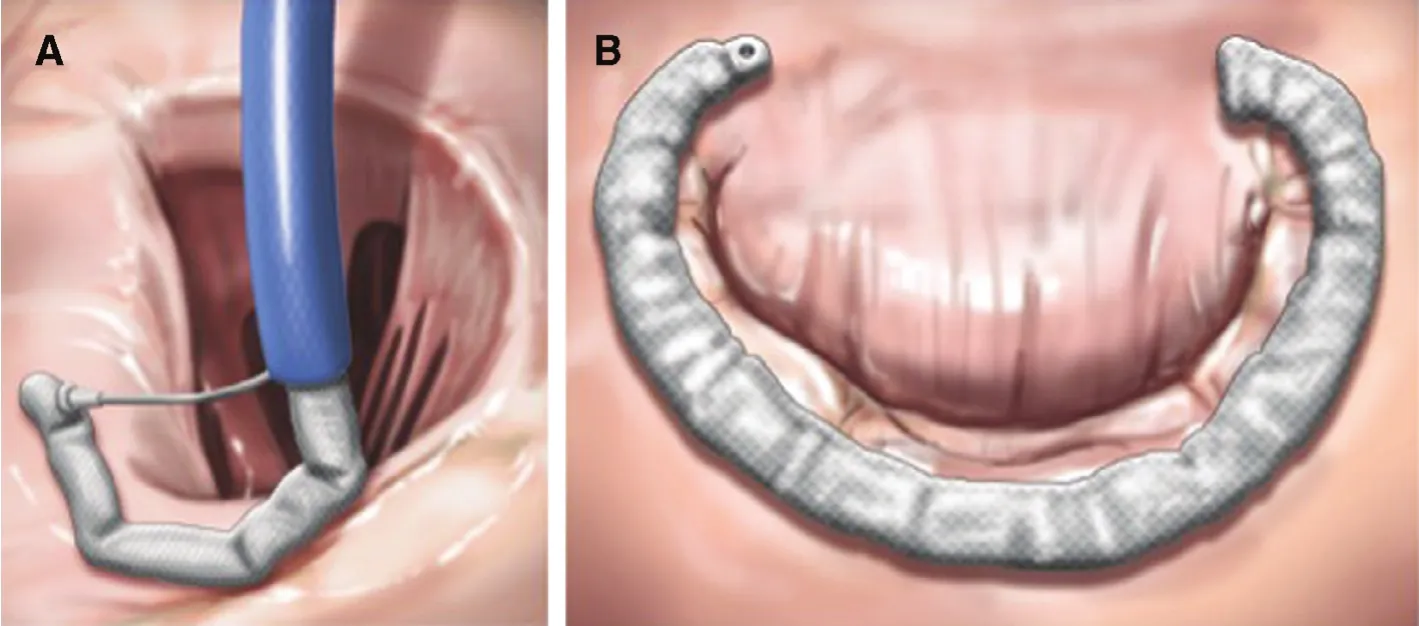
Figure 6 Valtech CardioBand.The left panel (A) shows a transseptal guide catheter delivering the annuloplasty ring in segments. Each segment is sequentially anchored into the annulus. The right panel (B) shows the final annuloplasty ring encircling the posterior leaflet. From Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol 2014;63:2057–68.Artwork by Craig Skaggs.
Direct Annuloplasty with the Valtech Cardioband
The second annuloplasty device to receive CE approval is the Valtech Cardioband (Valtech Cardio,Or Yehuda, Israel) [13]. Among the various percutaneous MR approaches the Cardioband most closely approximates surgical annuloplasty (Figure 6).Using a transseptal approach the band is implanted using screw anchors on the left atrial side of the mitral annulus. The finished implant results in an incomplete annuloplasty ring. The delivery system closely resembles the stabilizer and guide catheter employed in the MitraClip procedure. Results from 45 treated patients have been presented. After 1 year, 94% had MR severity 2+ or less. There was a 20% average decrease in antero-posterior dimension. Six minute walk test distance, Minnesota living with heart failure questionnaire score and New York Heart Association class all improved signifi-cantly [14]. While there have not been comparisons among the different catheter based mitral repair devices, Cardioband appears to have the greatest effectiveness at reducing MR severity.
Direct Annuloplasty with Mitralign
The Mitralign Percutaneous Annuloplasty System(Mitralign, Tewksbury, MA, USA) represents the third mitral annuloplasty approach with a significant number of treated patients. The approach is based on surgical annular suture plication [15] (Figure 7).A deflectable retrograde transaortic catheter is advanced into the LV and used to deliver pledgeted anchors through the posterior mitral annulus. These anchors can be tethered together resulting in shortening of the annulus circumference up to 17 mm. In 15 patients treated in a phase one trial, the 1-year results demonstrated at least 1+ MR reduction that was associated with improved NYHA function class and reduction in tenting area and depth. A CE approval registry trial has been completed and submitted for evaluation by the notified body. Results from 64 patients with FMR and a mean ejection fraction of 35% have been reported. There was a 5.9% 1 month mortality, which is similar to the rate observed in other trials with this population. Significant reductions in LV chamber dimensions were seen at 30 days. The LVED volume decreased from 186.4±44 to 169.0±38mL, a decrease of 20% (P<0.001), and the LVES volume decreased from 122.8±44 to 110.5±38 mL, a decrease of 13% (P<0.008). Anteroposterior and septal-lateral annular dimensions decreased 39% and 26% respectively, both P<0.001.NYHA class and MR grade improved [16].
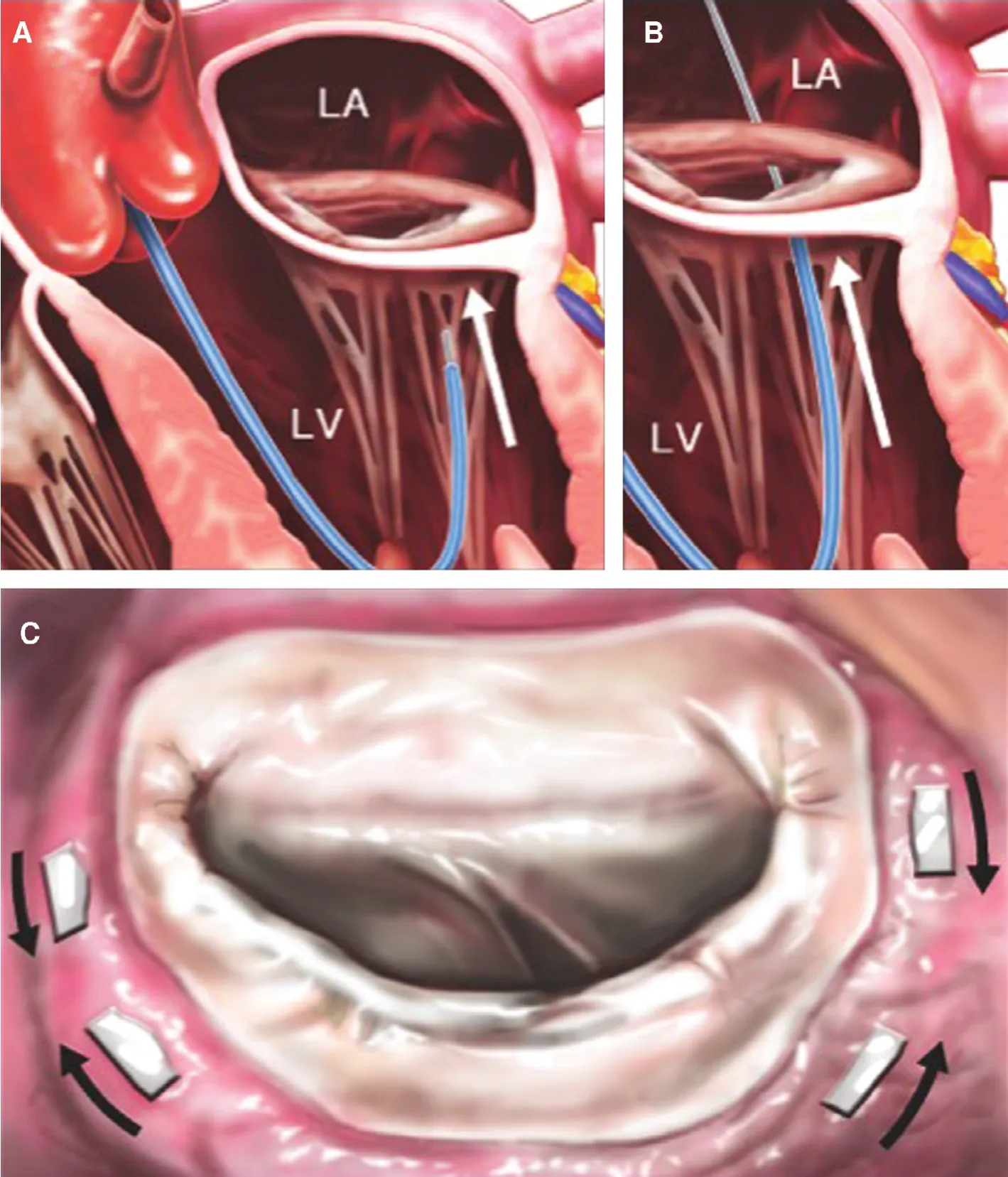
Figure 7 Mitralign Annular Plication.Panel (A) shows the retrograde guide catheter in the LV, with the distal catheter tip under the mitral annulus, behind the posterior leaflet (arrow). A wire has been passed from the left ventricle (LV) through the annulus and into the left atrium (LA) in panel (B). Two pairs of wires are used to place pledgets near both commissures, shown from the left atrial side in panel (C).The pledgets are drawn together (arrows) to decrease the mitral annular circumference. From Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol 2014;63:2057–68. Artwork by Craig Skaggs.
Recently the Mitralign device has been used to treat tricuspid regurgitation. This is based on the established surgical “Kaye” procedure of tricuspid plication in which one of the tricuspid leaflets is plicated to create a bicuspid valve [17]. Surgical suture bicuspidization is performed by placement of pledget-supported mattress sutures from the anteroposterior to the posteroseptal commissures along the posterior annulus. The Mitralign pledget system is well suited for this procedure. A first in human use of the Mitralign with highly successful reduction of tricuspid regurgitation has been reported and several cases have further been successfully performed [18].
Transcatheter Mitral Valve Replacement (TMVR)
The great technical and clinical success of transcatheter aortic valve replacement (TAVR) has naturally led to tremendous interest in developing transcatheter mitral valve replacement devices [1]. The first steps toward mitral replacement were accomplished by the use of TAVR devices for treating mitral bioprostheitc or mitral annuloplasty ring failures [19,20]. Percutaneous repair devices frequently leave some residual MR. Replacement devices promise to eliminate MR. The complex geometry, large dimensions, an coupling to the left ventricle make TMVR a vastly more complex effort. Several TMVR devices have been successfully implanted in patients [21–27](Figures 8 and 9). Investments in these devices have rapidly catapulted into the billions of dollars in only the last year. The total number of patients treated with these devices at the time of this writing is only around 100 cases. At least one device has stopped development, due to findings of thrombus on the device despite oral anticoagulation therapy [28]. The challenges to development of TMVR are amplified by the focus on patients with late stage LV failure who are severely ill with multiple comorbidities. Thus,some of the early mortality seen with TMVR is due to LV failure and other coexisting illnesses despite technical success with device implantation. Another challenge is device delivery into the mitral annulus due to the large dimensions of the mitral valve and thus the bulk of the devices. Transseptal delivery has been accomplished but this route is made especially difficult by the challenge of achieving coaxial alignment of the TMVR device. Thus, surgical exposure for apical delivery has been the most commonly used implantation approach. Among patients with pre-existing LV dysfunction, the apical incision adds an additional incremental insult. Another important challenge is the relationship of the mitral inflow to the LV outflow tract (LVOT). In many cases, especially with associated LV hypertrophy or a small LV cavity,the TMVR implant protrudes into the LVOT causing LVOT obstruction, which can be severe and difficult to manage. To address many of these challenges,extensive assessment prior to patient selection and device implantation is critical. The use of computed tomographic angiographic scans has become the standard for measurement of mitral annular dimensions and assessment of the potential for LVOT obstruction. Software modeling of the appearance of the valve implant has become a common part of preprocedure assessment. Three-dimensional printing from the CT scan evaluations is used as well.
TMVR is developing rapidly. In addition to the technical challenges, there remains a significant uncertainty regarding the ideal target populations for these devices. The frequent association of MR and LV dysfunction will require significant further trial evaluation to assess the utility of TMVR, and to define when LV failure is likely to improve with MR treatment.

Figure 8 Transcatheter Mitral Replacement Devices.Left, Edwards Lifesciences CardiAQ. This porcine pericardial mitral boiprosthesis has a self expanding nitinol frame and uses fixation anchors for stability in the mitral annulus. It is covered with a polytetrafluoroethylene membrane to minimize paravalvular leakage. The frame design features two sets of opposing left ventricular anchors and allows for annular attachment without radial force. The frame engages and preserves the native subvalvular apparatus. Right, Abbott Tendyne. The Tendyne valve has three porcine pericardial tissue leaflets sewn onto a circular nitinol inner frame. The inner frame is sutured to an outer nitinol frame that is covered in porcine pericardium with a polyethylene terephthalate fabric cuff that provides the sealing surface with the native annulus. The outer frame is D-shaped, which helps seat the valve in the mitral annulus. Together the valve inner and outer form a self-expanding prosthetic valve, which is connected to a braided fiber tether made of polyethylene. The tether passes through the left ventricular myocardium near the apex, where it is fastened to a pad on the epicardium. During implantation, the tether is used to adjust tension and optimize the position of the valve within the mitral annulus.
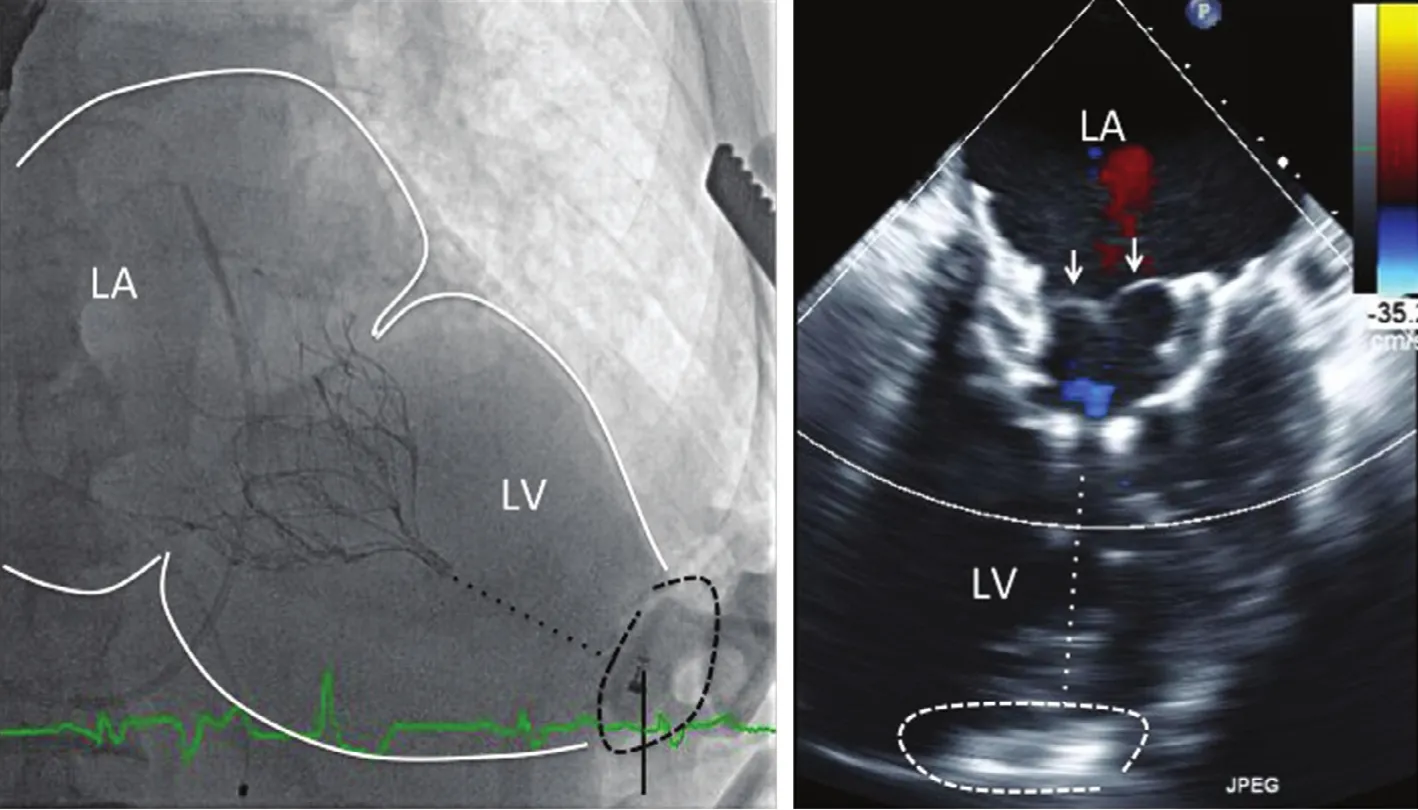
Figure 9 The Left Panel Shows a Fluoroscopic Image of a Tendyne Valve After Transapical Implantation.The nitinol frame is anchored in the mitral annulus between the left atrium (LA) and the left ventricle (LV). The dotted line from the ventricular tip of the prosthesis to the apex represents the apical tether, which is connected to an apical fixation button,encircled by the black dashed line. The right panel is a systolic frame on TEE. The closed mitral leaflets can be clearly seen(arrows). There is no MR. The dotted white line shows the location of the apical tether, and the dashed line encircles the apical fixation or anchoring button.
TMVR for Calcific Mitral Stenosis
A subset of patients with mitral valve disease have mitral stenosis or mixed stenosis and regurgitation from mitral annular calcification (MAC). Surgical valve replacement is limited by the difficulty caused by sewing into the heavily calcified mitral annulus and the high risk of complications. Therefore, this group of patients has limited options for therapy.Balloon-expandable TAVR devices have been used to treat patients with severe calcific disease [29,30]. There is a growing registry experience internationally. The results of the TMVR in MAC Global Registry were presented in 2015 [31–33]. The most recent analysis includes 64 patients treated between September of 2012 and July of 2015 with the compassionate use of balloon-expandable valves. Most of these patients (93.5%) had severe mitral stenosis and 6.5% had predominantly mitral regurgitation.Mean age was 73±13 years, 66% were females, and mean STS score was 14.4±9.5%. The mean mitral gradient in patients mitral stenosis was 11.45±4.4 mmHg and the mean mitral area was 1.18±0.5 cm2.SAPIEN (Edwards Lifesciences, Irvine, CA,USA) valves were used in 7.8%, SAPIEN XT in 59.4%, SAPIEN 3 in 28.1% and Inovare (Braile Biomedica, Brasil) in 4.7%. Access was transatrial in 15.6%, transapical in 43.8%, and transseptal in 40.6%. Technical success according to MVARC criteria [34] was achieved in 46 patients (72%),primarily limited by the need for a second valve in 11 (17.2%). Mean mitral gradient post-procedure was 4±2.2 mmHg, paravalvular regurgitation was mild or absent in all. Important cardiac complications included valve embolization in four patients(6.25%) and left ventricular tract obstruction with hemodynamic compromise was seen in 6 (9.3%).Thirty-day all-cause mortality was 29.7%. Most of the deaths were non cardiac (17.2%) and cardiovascular in 12.5%. The high non-cardiac mortality observed was attributed to the extremely ill patient population treated in whom the benefit or successful TMVR was diminished by advanced multiple comorbidities similar to the findings observed in the early experience with dedicated TMVR devices.Even though complications were observed, 84% of the 25 patients with 30-day follow-up data were in NYHA class I or II. This registry represents the early experience of TMVR with balloon expandable valves in patients with severe MAC using a device not designed for use in the mitral position,In the United States a FDA investigational device exemption multicenter trial is underway to prospectively evaluate the use of the Edwards TAVR device for this disease [35]. The challenges of transseptal versus apical delivery, valve sizing, and the potential for LVOT obstruction are the issues that require careful evaluation in this prospective trial.
Repair versus Replacement as the Main Strategy for MR Therapy
There is a debate regarding the best general approach to MR therapy [36]. The attraction of mitral replacement is the complete elimination of MR. The attraction of repair is safety and simplicity. To date the replacement technologies remain entirely investigational and a substantial amount of development will be needed to achieve full potential. For replacement,the potential for device thrombosis in the low-flow left atrial chamber, paravalvular leak resulting from the circular mitral orifice, LVOT obstruction, and the challenges of transatrial and transseptal delivery all need to be addressed and refined. For repair, less than complete resolution of MR and uncertain durability must be balanced against the relative safety.
Conclusion
Will percutaneous replacement ultimately supplant repair? At this stage of development, replacement has been used in only around 100 patients in total, with a variety of devices and a high rate of complications. There are many challenges to transitioning from open apical surgery to percutaneous transseptal replacement. MitraClip repair has been clearly established as remarkably safe and effective for symptom improvement, and has been implanted in over 30,000 patients. Several other repair approaches are developing rapidly. Thus at the very least repair will have an important role for the foreseeable future, while replacement will take years to mature.
Conflict of Interest
Declaration of interest: the corresponding author confirms grants and personal fees from Abbott,BSC and Edwards.
REFERENCES
1. Feldman T, Leon MB. Prospects for percutaneous valve therapies.Circulation 2007;11:2866–77.
2. Feldman T, Wasserman HS,Herrmann HC, Gray W, Block PC,Whiltlow PL, et al. Percutaneous mitral valve repair using the edge-to-edge technique: 6 month results of the EVEREST phase iclinical trial. J Am Coll Cardiol 2005;46:2134–40.
3. Feldman T, Foster E, Glower D,Kar S, Rinaldi MJ, Fail PS, et al.For the EVEREST II investigators:percutaneous repair or surgery for mitral regurgitation. New Engl J Med 2011;364:1395–406.
4. Feldman T, Kar S, Elmariah S,Smart SC, Trento A, Siegel RJ,et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation 5-year results of EVEREST II (Endovascular Valve Edge-to-Edge Repair Study). J Am Coll Cardiol 2015;66:2844–54.
5. Whitlow P, Feldman T, Pedersen W, Lim S, Kipperman R, Smalling R, et al. The EVEREST II high risk study: acute and 12 month results with catheter based mitral valve leaflet repair. J Am Coll Cardiol 2012;59:130–9.
6. Glower D, Kar S, Lim DS, Bajwa T, Quesada R, Whitlow P, et al.Percutaneous MitraClip device therapy for mitral regurgitation in 351 patients - high risk subset of the EVEREST II study. J Am Coll Cardiol 2014;64:172–81.
7. Philip F, Athappan G, Tuzcu EM, Svensson LG, Kapadia SR.MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic review. Catheter Cardiovasc Interv 2014;84:581–90.
8. D’ascenzo F, Morettic, Marra WG, Montefusco A, Omede P, Taha S, et al. Meta-analysis of the usefulness of Mitraclip in patients with functional mitral regurgitation. Am J Cardiol 2015;116:325–31.
9. Munkholm-Larsen S, Wan B, Tian DH, Kearney K, Rahnavardi M,Dixen U, et al. A systematic review on the safety and efficacy of percutaneous edge-to-edge mitral valve repair with the MitraClip system for high surgical risk candidates.Heart 2014;100:473–8.
10. Lim DS, Reynolds MR, Feldman T, Kar S, Herrmann HC, Wang A,et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation following transcatheter mitral valve repair with the MitraClip system. J Am Coll Cardiol 2014;64:182–92.
11. Schofer J, Siminiak T, Haude M,Herrman JP, Vainer J, Wu JC,et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009;120:326–33.
12. Siminiak T, Wu JC, Haude M,Hoppe UC, Sadowski J, Lipiecki J, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail 2012;14:931–8.
13. Maisano F, Vanermen H, Seeburger J, Mack M, Falk V, Denti P, et al.Direct access transcatheter mitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur J Cardiothorac Surg.2012;42:524–9.
14. Maisano F, Taramasso M, Nickenig G, Hammerstingl C, Vahanian A, Messika-Zeitoun D, et al.Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J.2015;36:1651–9.
15. Mandinov L, Bullesfeld L, Kuck KH, Grube E. Early insight into Mitralign direct annuloplasty for treatment of functional mitral regurgitation. Interv Cardiol Rev 2011;6:170–2.
16. Nickenig G, Hammerstingl C.The mitralign transcatheter direct mitral valve annuloplasty system.EuroIntervention 2015;11(Suppl W):W62–3.
17. Ghanta RK, Chen R, Narayanasamy N, McGurk S, Lipsitz S, Chen FY, et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: midterm results of 237 consecutive patients. J Thorac Cardiovasc Surg 2007;133:117–26.
18. Schofer J, Bijuklic K, Tiburtius C, Hansen L, Groothuis A, Hahn RT. First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J Am Coll Cardiol 2015;65:1190–5.
19. Wilbring M, Alexiou K, Tugtekin SM, Arzt S, Ibrahim K, Matschke K, et al. Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve,valve-in-ring, and valve-in-nativering. J Thorac Cardiovasc Surg 2014;147:210–9.
20. Webb JG, Wood DA, Ye J, Gurvitch R, Masson JB, Rodes-Cabau J,et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848–57.
21. Sondergaard L, De Backer O, Franzen OW, Holme SJ,Ihlemann N, Vejlstrup NG, et al.First-in-human case of transfemoral CardiAQ mitral valve implantation. Circ Cardiovasc Interv 2015;8:e002135.
22. Sondergaard L, Brooks M,Ihlemann N, Jonsson A, Holme S,Tang M, et al. Transcatheter mitral valve implantation via transapical approach: an early experience.Eur J Cardiothorac Surg 2015. pii:ezu546. [Epub ahead of print].
23. Ussia GP, Quadri A, Cammalleri V, DeVico P, Muscoli S, Marchei M, et al. Percutaneous transfemoral-transseptal implantation of a second-generation CardiAQ?mitral valve bioprosthesis: first procedure description and 30-day follow-up. EuroIntervention 2015.Available from: http://www.pcronline.com/eurointervention/ahead_of_print/201509-01#sthash.YVVCh9oV.dpuf.
24. Lutter G, Lozonschi L, Ebner A,Gallo S, Marin y Kall C, Missov E, et al. First-in-human off-pump transcatheter mitral valve replacement. JACC Cardiovasc Interv 2014;7:1077–8.
25. Moat N, Duncan A, Lindsay A,Quarto C, Blanke P, Leipsic J,et al. Transcatheter mitral valve replacement for the treatment of mitral regurgitation: in-hospital outcomes of an apically tethered device. J Am Coll Cardiol 2015;65:2352–3.
26. Cheung A, Stub D, Moss R,Boone RH, Leipsic J, Verheye S,et al. Transcatheter mitral valve implantation with tiara bioprosthesis. EuroIntervention 2014;10(Suppl U):U115–9.
27. Abdul-Jawad Altisent O, Dumont E, Dagenais F, Senechal M, Bernier M, O’Connor K, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015;66:1011–9.
28. Bapat V, Buellesfeld L, Peterson MD,Hancock J, Reineke D, Buller C, et al.Transcatheter mitral valve implantation (TMVI) using the Edwards Fortis device. EuroIntervention 2014;10(Suppl U):U120–8.
29. Guerrero M, Greenbaum A, O’Neill W. First in human percutaneous implantation of a balloon expandable transcatheter heart valve in a severely stenosed native mitral valve. Catheter Cardiovasc Interv 2014;83:E287–91.
30. Hasan R, Mahadevan VS, Schneider H, Clarke B. First in human transapical implantation of an inverted transcatheter aortic valve prosthesis to treat native mitral valve stenosis.Circulation 2013;128:e74–6.
31. Fassa AA, Himbert D, Brochet E,Depoix JP, Cheong AP, Alkhoder S, et al. Transseptal transcatheter mitral valve implantation for severely calcified mitral stenosis. JACC Cardiovasc Interv 2014;7:696–7.
32. Himbert D, Bouletic, Iung B,Nejjari M, Brochet E, Depoix JP,et al. Transcatheter valve replacement in patients with severe mitral valve disease and annular calcification. J Am Coll Cardiol 2014;64:2557–8.
33. Guerrero M, Dvir D, Himbert D,Urena M, Eleid M, Mahadevan V,et al. Transcatheter mitral valve replacement with balloon expandable valves in native mitral valve disease due to severe mitral annular calcification: results from the first global registry. J Am Coll Cardiol 2015;66:B291–2.
34. Stone GW, Adams DH, Abraham WT, Kappetein AP, Genereux P,Vranckx P, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol 2015;66:308–21.
35. Available from: www.clinicaltrials.gov/ct2/show/NCT02370511?term=MITRAL&rank=1, accessed September 6, 2015.
36. Maisano F, Alfieri O, Banai S,Buchbinder M, Colombo A, Falk V, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs replacement? Eur Heart J 2015;36:1651–9.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
- Identification and Management of Iatrogenic Aortocoronary Dissection
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Coronary Artery Chronic Total Occlusion
- Carotid Artery Stenting: 2016 and Beyond
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
