Current Management of Ventricular Tachycardia:Approaches and Timing
Roy M. John, MBBS, PhD and William Stevenson, MD
Introduction
Ventricular tachycardia (VT) can be monomorphic, with each QRS the same as the preceding and following QRS, or polymorphic, with a continually changing QRS. The latter, when sustained, usually results in syncope or cardiac arrest. Sustained monomorphic VT (SMVT) may be hemodynamically stable or unstable based on the rate of the tachycardia,underlying ventricular function and volume status of the patient. Thus the arrhythmia can have various manifestations. In patients with structural heart disease SMVT is associated with a risk of sudden death as the arrhythmia can degenerate to ventricular fibrillation. Alternatively, VT may cause syncope, or, if the rate of the VT is slow, patients may present with progressive heart failure. The most common presentation now is in patients who will have received an implanted cardioverter de fibrillator (ICD) due to prior VT or a known risk for sudden death. These patients present with repeated ICD therapies due to VT. The term “VT storm” describes three of more episodes of VT that occur within a 24-hour period and warrants immediate attention as it is associated with a high mortality [1, 2].
A small proportion of SMVT is due to idiopathic VT, occurring in patients with no identi fiable structural heart disease. Patients with idiopathic VT generally have a good prognosis and sudden death is rare [3]. Thus, they are managed differently from those with structural heart disease. The distinction between VT associated with structural heart disease and idiopathic VT is therefore crucial in formulating management strategies. This review will focus on recognition of VT mechanisms, current therapeutic options and timing of interventions such as catheter ablation in the management of VT.
Types of VTs and Related Mechanisms
The approach and outcomes of treatment are dependent on the nature of the arrhythmia substrate. The presenting arrhythmia often suggests the arrhythmia mechanism and location of the arrhythmia substrate. Monomorphic PVCs or VT have the same ventricular activation sequence from beat to beat.The QRS morphology is a clue to its origin. The majority of sustained monomorphic VTs (MVT) is due to scar related re-entry. The scars can remodel over time producing recurrent VT after years of stability. A single large ventricular scar can support multiple VT circuits, causing VTs with different QRS morphologies and rates. Once VT occurs, up to 40-50% of patients will experience a recurrence within 2 years [4].
In approximately 8-10% of patients, MVT originates from the Purkinje system due to automaticity or re-entry [5]. A specific form of VT due to bundle branch reentry is seen typically in patients with structural heart disease, and inter-ventricular conduction delay or left bundle branch block on the sinus rhythm ECG. The re-entry wavefront propagates antegradely down the right bundle, then through the septum and retrogradely up the left bundle to complete the reentrant circuit. VT has a left bundle branch block-like configuration, but the circuit can also revolve in the reverse direction, creating a VT with right bundle branch block pattern.Although catheter ablation of the right bundle is curative, most patients have other scar-related VTs as well [6].
Idiopathic VT often manifests as monomorphic PVCs or repetitive MVT and most commonly has a focal origin. These VTs are typically provoked by adrenergic stimulation and triggered automaticity is the likely mechanism. The right and left ventricular out flow tracts are the most common origins followed by regions adjacent to an AV valve annulus or within a papillary muscle [3]. Frequent idiopathic PVCs and repetitive VT may lead to LV dysfunction. However, it can be difficult to know if the arrhythmia is idiopathic and causing ventricular dysfunction, or is a consequence of ventricular disease. A specific form of idiopathic LV VT due to re-entry within the fascicles is responsive to verapamil (Belhassen’s VT) and amenable to catheter ablation.
Polymorphic VT is characterized by beat-to-beat variation in QRS morphology due to varying ventricular activation sequences and frequently degenerates into ventricular fibrillation. These arrhythmias are usually triggered by acute myocardial ischemia, electrolyte disturbances, or repolarization abnormalities such as QT prolongation induced by drugs (e.g. class 3 antiarrhythmic drugs), hypokalemia, chronic bradycardia and combinations of these factors. Polymorphic VTs are also encountered in ventricular hypertrophy and heart failure.Patchy areas of myocardial fibrosis, common in the non-ischemic cardiomyopathies, can theoretically predispose to spiral wave break up of a re-entrant VT producing a polymorphic VT.
Rarely, monomorphic PVCs of Purkinje origin can trigger ventricular fibrillation (VF) often presenting with electrical storms. Although initially described in patients without structural heart disease and termed “idiopathic VF”, it is recognized that PVC triggered VF can also occur in patients with heart failure, prior myocardial infarction, and cardiomyopathies, including amyloidosis [7, 8].
Management of Ventricular Tachycardia
Initial management after termination of the acute arrhythmia is directed toward identi fication of structural heart disease and exclusion of potential predisposing factors such as electrolyte abnormalities and myocardial ischemia. When a patient with no prior known cardiac history presents with a sustained VT,coronary angiography, and echocardiography are common early investigative steps. Cardiac magnetic resonance imaging with gadolinium contrast injection is helpful in de fining ventricular morphology,function and the presence of myocardial scarring,in filtration or in flammation, which may help clinch specific diagnoses such as arrhythmogenic RV cardiomyopathy and amyloidosis [9].
VT storm that is hemodynamically unstable and fails to be suppressed by beta-blockers, amiodarone and lidocaine may warrant deep sedation including the use of general anesthesia to reduce sympathetic drive. Inability to control unstable VT should prompt consideration of ventricular assist devices and urgent catheter ablation in an experienced center (see below).
Unless a reversible cause is identi fied, a sustained ventricular tachycardia in association with structural heart disease usually warrants consideration of implantable cardioverter de fibrillator (ICD)placement (Figure 1).
Implantable Cardioverter De fibrillators (ICDs)
ICDs are highly effective for termination of ventricular fibrillation or tachycardia and improve mortality in cardiac arrest survivors and in patients at risk for sudden death due to structural heart diseases [10]. In all cases ICDs are recommended only if there is also expectation for survival of at least a year with acceptable functional capacity (Figure 1).The exception is in cases of patients with end-stage heart disease who are awaiting cardiac transplantation outside the hospital, or who have left bundle branch block QRS prolongation such that they are likely to have improvement in ventricular function with cardiac re-synchronization therapy from a biventricular ICD.
Despite the high efficacy of ICDs for termination of ventricular arrhythmias, there is considerable morbidity associated with their use. ICD shocks are painful, associated with increased mortality, mostly from deteriorating heart failure and may result in post traumatic stress disorder [11]. Inappropriate or unnecessary shocks due to sinus tachycardia, atrial fibrillation, non-sustained VT or lead malfunction occur at an annual rate of approximately 3.5% [12].In addition, unnecessary right ventricular pacing can aggravate ventricular dysfunction from pacing induced dys-synchrony and should be avoided[13]. Appropriate programming of ICD arrhythmia detection criteria and anti-tachycardia pacing therapy to terminate VT can reduce the odds of necessary and unnecessary shocks without compromising safety [14].
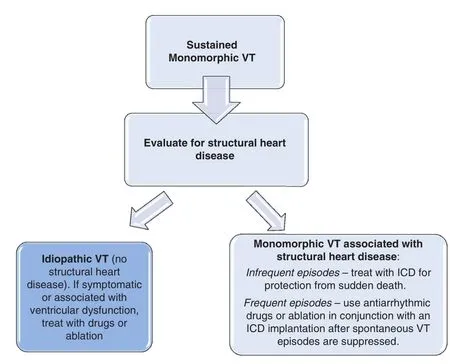
Figure 1 Algorithm for Management of Sustained Monomorphic Ventricular Tachycardia.
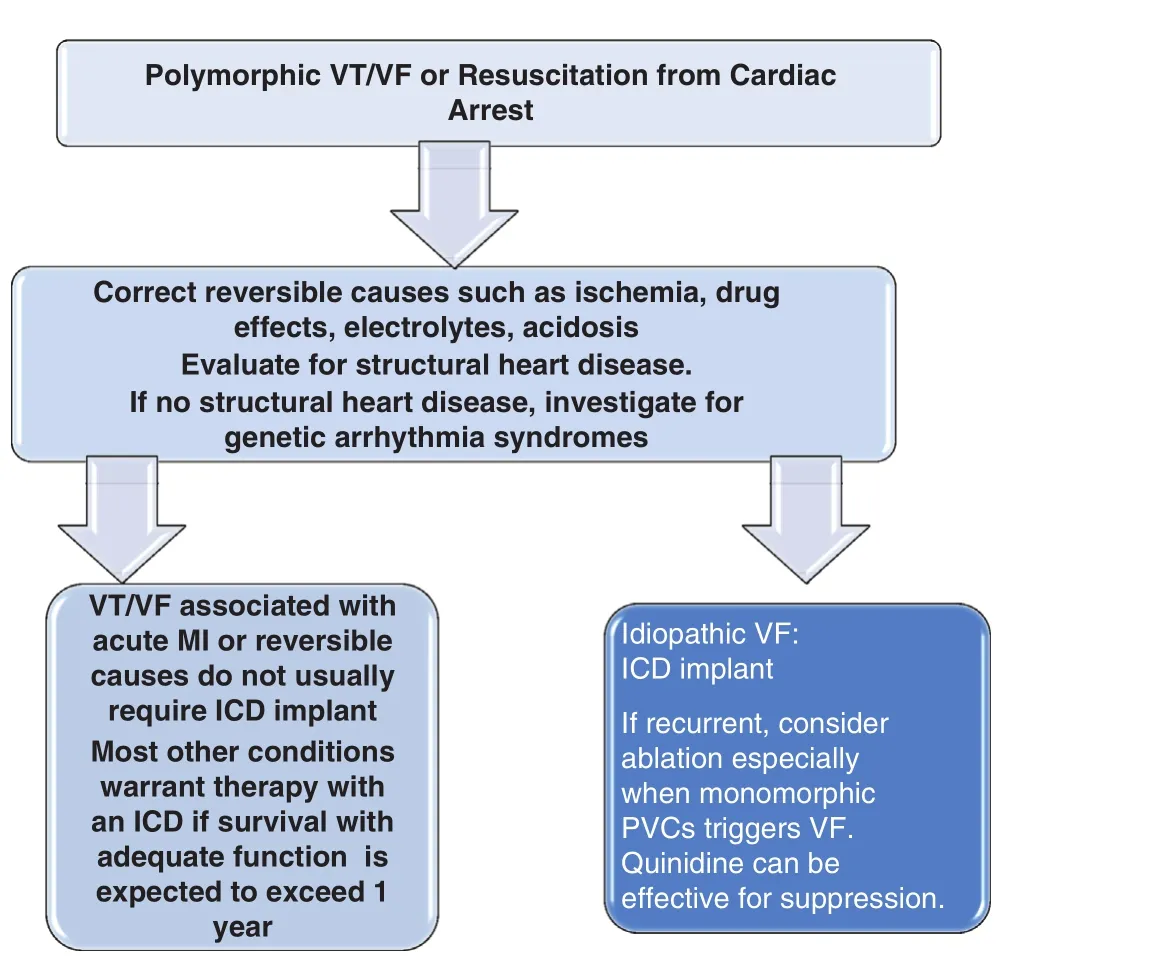
Figure 2 Algorithm for Management of Polymorphic Ventricular Tachycardia or Fibrillation.VT, Ventricular tachycardia; VF, ventricular fibrillation; MI,myocardial infarction; ICD, implantable cardioverter de fibrillator.
ICD implantation has a 3% risk of complications, including pneumothorax, cardiac perforation,bleeding, and heart failure decompensation, but the procedure related mortality is less than 1% [15].ICD infections require device and lead removal,with a 0.25% procedural mortality in experienced centers. Patients receiving ICDs require careful follow-up to detect arrhythmias that are predictive of heart failure deterioration and monitoring for device malfunction, and assessment of battery life.Remote monitoring systems that allow automated and patient triggered transmissions of ICD recordings has simpli fied follow-up and enhanced early detection of arrhythmias and ICD problems [16].
Drug Therapy for Ventricular Arrhythmias
Drugs have an important role in reducing the frequency of recurrent symptomatic arrhythmias.Beta-adrenergic blockers are the most commonly used drugs because many arrhythmias are provoked by sympathetic stimulation, and they have a favorable safety pro file [10]. However, they have limited efficacy for suppressing re-entrant arrhythmias associated with structural heart disease.
Membrane active antiarrhythmic drugs that block cardiac ion channels have little role in preventing sudden death in patients with structural heart disease,but are useful for reducing symptomatic arrhythmias. The sodium channel blocking drugs such as flecainide and propafenone are occasionally considered for idiopathic VTs, but these agents should be avoided in patients with heart disease. They have negative inotropic effects, and flecainide increased mortality when given to survivors of myocardial infarction [10]. Quinidine is better tolerated hemodynamically due to its effect on action potential duration and its vasodilatory properties that tend to offset any negative inotropy from sodium channel blockade. However, all these drugs have signi ficant proarrhythmic effects such that use of sodium channel blockers in patients with structural heart disease is largely limited to those with an ICD. Drugs that predominantly block potassium channels (sotalol,dofetilide) prolong repolarization and are better tolerated in patients with structural heart disease.However, these drugs can cause torsade de pointes VT, and the risk is likely increased in heart failure due to electrophysiologic changes that accompany myocardial hypertrophy and impaired excretion of many drugs. Careful monitoring for excessive QT prolongation during initiation is required.
Both oral amiodarone and sotalol reduce ventricular and atrial arrhythmias that can lead to ICD shocks [17]. Amiodarone is most effective for control of ventricular arrhythmias but extra-cardiac toxicities (prominently thyroid, lung, liver, and neurologic) prevent long-term use in more than 20%of patients. In a recent trial in patients with LVEF< 35%, amiodarone had no effect on mortality in NYHA class II heart failure, but was associated with increased mortality in class III heart failure[18]. Nevertheless, amiodarone is a reasonable consideration for patients who have had spontaneous sustained VT or VF, but who have a contraindication to or refuse to have an ICD. Newer antiarrhythmic drugs including analogues of amiodarone such as dronadarone, have been disappointing because of limited efficacy and increased mortality in patients with heart failure [19].
Catheter Ablation for Ventricular Arrhythmias
General Considerations
Catheter ablation has emerged as an important therapeutic option for most ventricular arrhythmias.Recent advances in imaging, ablation techniques and hemodynamic support have allowed for safe and effective ablation even in unstable patients.Hence, ablation should be an early consideration in the course of recurrent VT that trigger symptoms or ICD shocks. However, patient selection should consider careful balancing of risks and bene fits.Appropriate expertise and facilities including cardiac surgical back up is needed for optimal success while minimizing procedural risks [6].
Prior to endocardial ablation, ventricular thrombus should be excluded by echocardiography in patients with structural heart disease. The presence of concomitant atrial fibrillation without a sufficient period (>4 consecutive weeks) of therapeutic anticoagulation, must prompt consideration of transesophageal echocardiogram to exclude a left atrial appendage thrombus, as cardioversion of VT during ablation may revert the patient to sinus rhythm with heightened risk of thromboembolism. General anesthesia is increasingly used in our laboratory during ablations for scar-related VT. It has the advantage of keeping the patient still dur-ing long procedures, which facilitates the use of electro-anatomic mapping systems and is preferred when pericardial access is necessary for epicardial mapping and ablation [20].
Hemodynamic support using percutaneous Left Ventricular Assist Devices (pLVAD) with an Impella Recover 2.5 (Abiomed, Inc.), a Tandem Heart Device (CardiacAssist, Inc.) or extracorporeal membrane oxygenators (ECMO) may be considered for selected high risk patients to allow extended mapping during VT. Recent small retrospective series have shown that pLVAD support is feasible during ablation [21]. VTs could be mapped for relatively longer periods of time and were terminated by RFA more often compared to historical control groups without hemodynamic support or with an intra-aortic balloon pump. However there was no difference in acute procedural success or VT recurrence rates during follow up. In addition the magnetic motor of the Impella system can interfere with electroanatomic mapping systems, especially during mapping in the ventricular out flow tracts.Extracorporeal membrane oxygenation (ECMO)offers biventricular support and does not interfere with the mapping systems. ECMO does not unload the LV as compared to pLVAD but offers better hemodynamic support (4-6 L/min).
As most patients with heart disease undergoing ablation have an ICD, the majority of VTs are documented on intracardiac electrograms (EGMs)recorded from the pacing and de fibrillating electrodes rather than a 12-lead electrocardiogram.These EGMs can provide clues to the nature of the arrhythmia and their assessment is important to exclude the possibility of unnecessary ICD therapy due to atrial fibrillation or sinus tachycardia at rates above the VT detection rate. Long-short intervals from some pacing algorithms, such as the minimal ventricular pacing mode, initiate VT in some patients and can be corrected by reprogramming.The presence of a uniform PVC initiating VF can be a clue to triggers from the Purkinje fibers [8]. Intracardiac EGMs also allow documentation of the VT cycle length and the response to anti-tachycardia pacing that may be helpful in assessing the nature of the arrhythmia.
Whenever possible, a 12 lead ECG recording of spontaneous VT (termed a clinical VT) should be obtained as the QRS morphology suggests the VT origin, which facilitates planning of the initial mapping approach. A right bundle branch block-like configuration in lead V1indicates likely LV origin,and a left bundle branch block-like configuration indicates a septal or RV origin. Superior and inferior frontal plane axes indicate exit from the inferior or anterior walls respectively. Dominant R or S waves in V3/V4indicate a basal or apical origin respectively. These guidelines are not always reliable, particularly in the presence of extensive scars.In non-ischemic cardiomyopathy, QS complexes in lead I during VT often indicates that VT arises from a circuit in the sub-epicardium of the basal lateral LV. These criteria are not reliable in ischemic heart disease [6, 22, 23].
Approaches to Ablation in Structural Heart Disease
Programmed ventricular stimulation should be performed first to induce the arrhythmias to unequivocally con firm the diagnosis, assess the QRS morphology, and de fine an endpoint for ablation. If non-inducible or the induced arrhythmia is hemodynamically unstable, a substrate based mapping and ablation approach is generally used. If the VT is inducible and hemodynamically tolerated, the reentrant circuits can be characterized using mapping techniques and ablation applied to terminate VT, providing con firmation that the ablation target is causing the VT.
In substrate-based approaches to mapping and ablation, scar regions are de fined during sinus or paced rhythm by creating voltage maps of the area of interest. There is a close correlation of endocardial and epicardial scar with low EGM voltage(bipolar EGM less than 1.5 mV on the endocardium and 1.0 mV for the epicardium) [24]. Endocardial bipolar EGMs do not reliably identify intramural or epicardial scar, but these can often be detected from analysis of un filtered or minimally filtered unipolar EGMs, as these have a broader “ field of view” [22,23, 25].
In addition to de fining the area of low voltage, specific features of recorded EGMs allow de finition of the reentry substrate. Multicomponent, fractionated EGMs, split or late potentials (occurring after the QRS complex) indicate asynchronous activation of myocyte bundles with intervening fibrosis causing slow conduction [18, 26, 27]. Pacing in the area of interest identi fies excitable tissue in the scar, areas of slow conduction characterized by long stimulus to QRS intervals, and a paced QRS morphology that approximates the morphology of the VT suggests close proximity to the VT circuit exit. Ablation is performed during stable sinus or paced rhythm, targeting these abnormal areas. Our approach is to render the tissue electrically un-excitable to pacing to attempt to insure that an adequate ablation lesion is created [28, 29].
In approximately 5-15% of patients with coronary artery disease, and more than a third of patients with non-ischemic cardiomyopathy, scar-related VT circuits are located in the sub-epicardium and cannot be ablated from the endocardium. In the absence of pericardial adhesions from prior cardiac surgery or pericarditis the epicardium can be accessed percutaneously for mapping and ablation. Epicardial ablation is usually preceded by coronary angiography to make sure a coronary vessel does not over lie the ablation target, with risk of coronary occlusion due to ablation [30, 31].
If a clinical VT, de fined as one that has occurred spontaneously, is inducible at the beginning of the procedure, non-inducibility of such VT is the minimum procedural endpoint that is sought. Abolition of all inducible VTs has been associated with a lower risk of VT recurrence and cardiac mortality in some, but not all studies [32, 33]. Location of arrhythmia substrate (usually scar) and the underlying disease state affects the likelihood of success. Ablation is most successful in conditions where potential re-entry channels can be de fined on the endocardium or epicardium (e.g. myocardial infarction, arrhythmogenic right ventricular cardio myopathy) but is less successful for intramural circuits or epicardial circuits in close proximity to coronary arteries [34, 35]. Ablation failure is often to due to inability to create transmural, durable lesions or failure to reach intramural substrate(e.g. intraventricular septum), or protection of the VT substrate by close proximity to coronary arteries or the left phrenic nerve, or by epicardial fat.
Transcoronary ethanol ablation has been used in selected patients when catheter ablation fails. A coronary vessel supplying the VT substrate is identi fied for administration of absolute ethanol. Limitations include failure to identify a coronary target, potential for damage to large areas of myocardium and administration of intravenous contrast load in HF patients who may have pre-existing renal impairment [36]. Surgical cryoablation is also an option when catheter ablation fails and may allow separation of an overlying coronary artery from epicardial substrate and dissection through epicardial fat allowing successful ablation [37].
VT Ablation in specific Substrates
Ischemic Cardiomyopathy
Ventricular scar is present from prior myocardial infarction, with ongoing remodeling, creating the anatomic substrate for scar-related, often multiple, re-entrant VTs [6]. Challenges for post infarct VT ablation include: (i) inducibility of multiple VTs (on average 3/patient due to separate reentry circuits or a shared area of slow conduction with variable exits); (ii) likelihood of a broad reentry circuit isthmus (>2-3 cm) [38]; (iii) hemodynamically unstable VTs [6, 39]. Hence substrate mapping is increasingly preferred to mapping during VT.
Multicenter studies of catheter ablation in ischemic cardiomyopathy show that at least one VT is abolished in 72-96% of patients and all inducible VTs eliminated in 38-72% of patients with 50-88%of patients remaining VT free over a mean follow up of >12 months and 30-100% continuing on previously ineffective AADs [34, 40]. VT episodes are markedly reduced in the majority of patients [40].Procedural mortality is approximately 3% with 1 year mortality of 9-18% with most deaths attributed to heart failure [6, 19, 40]. In prospective multicenter trials approximately 50% of patients have at least one recurrence of VT, but the frequency of VT episodes is reduced. Patients who fail ablation and have recurrent VT have a higher mortality than those who remain in sinus rhythm [41].
Non-ischemic Cardiomyopathy
Non-ischemic cardiomyopathy (NICM) is a heterogeneous group of diseases including idiopathic cardiomyopathy, genetic LV cardiomyopathies (lamin A/C, Titin mutations), arrhythmogenic RV cardiomyopathies (ARVC), in flammatory disease (sarcoid heart disease, myocarditis) and hypertrophic cardiomyopathy (HCM). Sustained monomorphic VT is due to scar-mediated re-entry in over 80%of patients with the remainder being focal in origin or due to bundle branch re-entry. Key differences compared to ischemic cardiomyopathy include: (i)generally smaller scars with multiple re-entrant VTs despite small scar; (ii) predilection for anatomical location around the valve annuli or septum; (iii) less frequent transmural scars; (iv) more frequent intramural scars; (v) extensive epicardial scarring that may occur in the presence of normal endocardium or extend beyond the region of endocardial scarring [6, 42, 43]. A recent study found basal anteroseptal or inferolateral scar accounting for 89% of arrhythmogenic substrate in patients with NICM and sustained MVT [42]. The former can be targeted endocardially whereas the latter frequently require an epicardial approach.
Catheter ablation for VT in the non ischemic cardiomyopathies is less well studied and VT recurrence rates are higher than in the ischemic patients,occurring in 50-60% in short term follow up (up to 1 year), although VT burden can be signi ficantly reduced. Complete non-inducibility can be achieved in 38-67% of patients [30, 42, 43].
In ARVC, fibrofatty replacement progresses from the subepicardial layer to the endocardium particularly affecting the free wall of the RV along the tricuspid annulus and in the out flow tract. Epicardial scar area is often larger than endocardial scar [44]. Thus epicardial ablation is frequently required either as a primary target or after failed endocardial ablation procedure [45]. With the use of epicardial ablation freedom from VT is achieved in 77-89% of patients, but late recurrences can occur due to the progressive nature of the disease[6, 44-46].
SMVT is rare in patients with HCM, and usually due to scar-mediated re-entry [38]. Combined epicardial and endocardial mapping and ablation is feasible and can reduce spontaneous VTs but data on outcome is limited [47, 48].
Timing of VT Ablation
Increasing experience and technical advances warrant consideration of catheter ablation for most patients who have recurrent symptomatic VT episodes, without waiting to exhaust allpharmacological options, in the hope of avoiding ICD shocks and antiarrhythmic drug toxicities.Table 1 summarizes current recommendations for the use of catheter ablation according to the 2009 EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias [6].
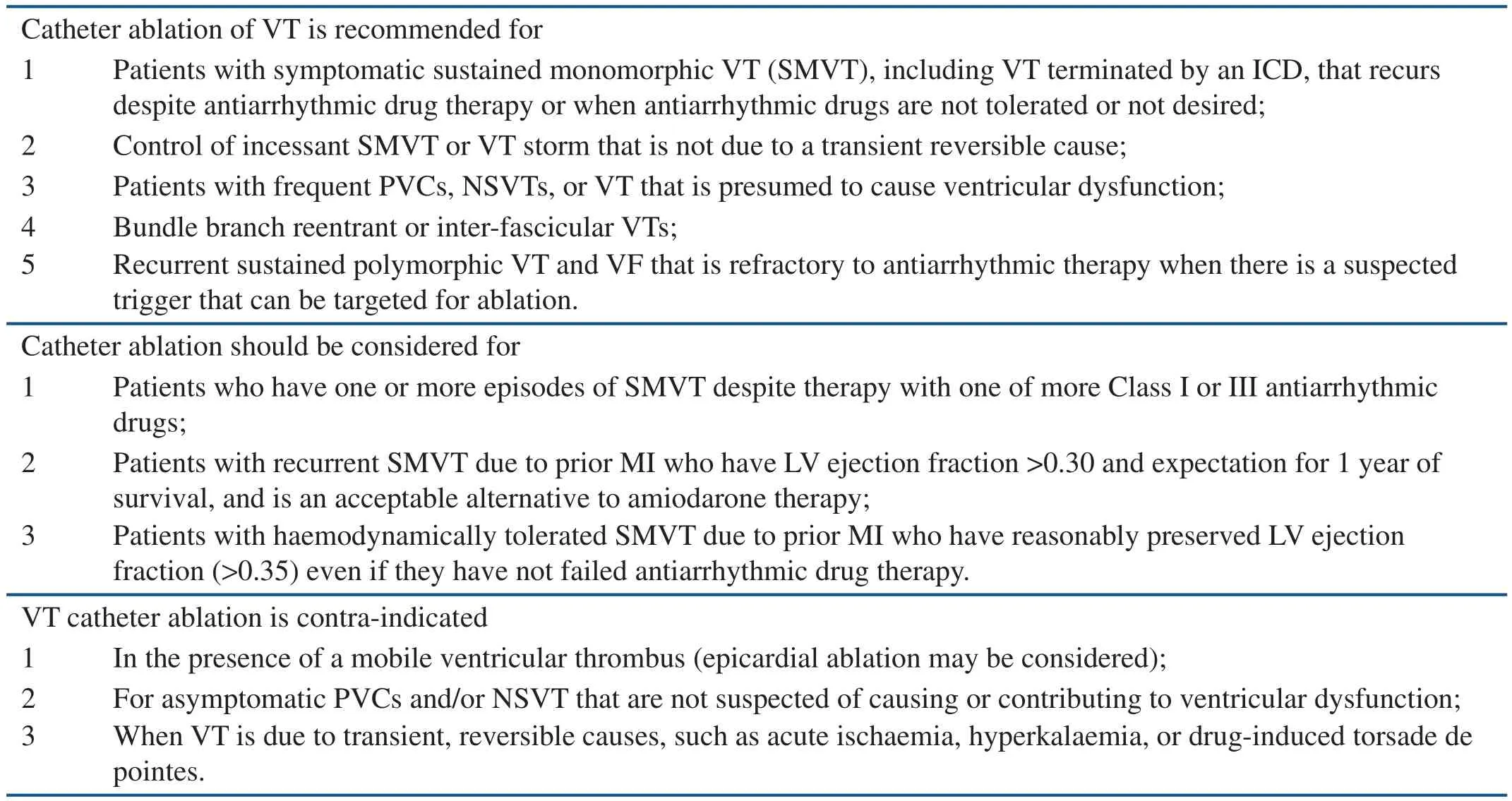
Table 1 Indications for Catheter Ablation of Ventricular Tachycardia in Patients with Structural Heart Disease(Adapted from Reference [6]).
Two randomized trials (VTACH and SMASHVT) explored the early (after the initial episode of VT) use of catheter ablation in ICD recipients with prior myocardial infarction, VT and impaired left ventricular (LV) function [4, 49]. Both studies found a signi ficant reduction in VT recurrences during follow up of approximately 2 years.However, neither of these trials was sufficiently powered to examine mortality. In a retrospective study, Bunch et al. reported a lower risk of death and heart failure hospitalizations in patients treated with VT ablation after an ICD shock compared to patients managed medically only [50, 51]. Recent publications support an early invasive approach.In a single center, observational study, Dinov et al. reported better acute and long-term success if catheter ablation of scar-related VT was performed within 30 days after the first documented VT [34]. Once again, a mortality bene fit was not evident in patients who underwent early VT ablation compared to those who had their ablation late(> 1 year) after their first presentation with VT. The bene fits of early catheter ablation as compared to medical therapy are the subject of several ongoing randomized clinical trials.
Management of VT Storm
Electrical or VT storm is associated with a high mortality. A meta-analysis found a mortality of 17% during a little over 1 year follow up with heart failure accounting for ~2/3rdof all deaths [1].Catheter ablation controls VT in >90% of patients with 74-92% remaining free of incessant VT or recurrent VT storm [1, 39]. Single episodes of VT recurrences occur in a third of patients [6].Sympathetic denervation or renal artery denervation are emerging potential therapies [52]. In rare patients, recurrent VT is provoked by unifocal PVCs from the Purkinje fibers, out flow tracts, or papillary muscles that can be targeted for ablation(see above) [7, 8].
Idiopathic PVCs or VT
Idiopathic VT is a diagnosis of exclusion and often require the use of sophisticated imaging techniques such as cardiac MRI or positron emission tomography to exclude areas of scar or in flammation that may not be evident by echocardiography. Most idiopathic PVCs originate from the ventricular out flow tracts and tend to be monomorphic [3]. In some patients the arrhythmia arises from an extension of myocardium for a variable distance above the aortic or pulmonic valves. An autopsy study showed that extensions are most common in the right coronary cusp (55%) than the left coronary cusp (24%) and non-coronary cusp (<1%). In contrast, myocardial extensions above the pulmonic valves are evenly distributed (45-60%) [53]. Other sites for idiopathic PVCs include the papillary muscles, myocardium adjacent to the AV valves, and from the fascicles of the conduction system. An epicardial peri-venous focus has been identi fied in some patients and may need mapping in the great cardiac vein or the anterior inter-ventricular vein [54].
Very frequent PVCs or non-sustained VT can induce a cardiomyopathy that can be reversed with either pharmacological suppression or catheter ablation [55-57]. Such arrhythmias may also exacerbate pre-existing left ventricular function and is a cause of loss of effective biventricular pacing in patients dependent on cardiac re-synchronization therapy [58]. The diagnosis of PVC-induced cardiomyopathy is one of exclusion and often retrospective based on recovery of LV function after control of the arrhythmia.
The exact mechanism of PVC-induced cardiomyopathy is unclear. Ventricular dyssynchrony,alterations in intracellular calcium handling,changes in heart rate dynamics, hemodynamic parameters as well as changes in myocardial and peripheral autonomic function have been postulated [59]. The frequency of PVCs correlates with the severity of left ventricular dysfunction at the time of initial presentation [55, 56]. However, in some patients, a high PVC burden does not impair LV function whereas in others a lower PVC burden may do so. The lowest PVC burden resulting in a reversible cardiomyopathy was noted to be 10% in one study [55]. This relationship is likely to be complex however, not appreciated by a single “cut off” value. PVC frequency can also vary substantially from day to day. PVC duration(≥140 ms to 150 ms), an epicardial site of origin,PVC coupling intervals ≤ 600 ms, PVC interpolation, a long history of palpitations (>60 months),and the complete absence of symptoms have all been associated with higher likelihood of LV dysfunction [59-63].
Beta-blockers and calcium channel blockers have only a modest effect in suppressing PVCs, but are commonly used for idiopathic PVCs because of their safety. More potent antiarrhythmic drugs such as flecainide, mexilitine and amiodarone are more effective but long term use is limited by side effects and flecainide is avoided in the presence of heart disease or depressed ventricular function [64]. Hence,catheter ablation is an attractive option for many patients. A recent randomized study of patients with frequent PVCs from the RV out flow tract found a greater decrease in burden of PVCs following ablation compared to drug therapy although LV function improved in both groups [65]. Whether PVC ablation is superior to drugs in reversing LV dysfunction is unknown.
The ECG can help localize site of arrhythmia origin. Most PVCs or VTs having a left bundle branch morphology with precordial transition at V3and inferiorly directed axis, have a RV out flow tract origin. A prominent R wave in V1or early transition before V3with inferior axis suggests a left ventricular out flow tract origin. However, the complex anatomy of the out flow tract and variable distance of myocardial extensions above the valves precludes precise localization from ECG criteria [53]. Systematic mapping of the RV outflow tract pulmonary artery, great cardiac/anterior inter-ventricular vein via the coronary sinus followed by the aortic root and cusps, and LV out flow tract is often required. Papillary muscle VTs tend to have a broader QRS (typically greater than 150 milliseconds), usually have a monophasic R or qR in V1, and Q waves tend to be absent. The frontal plane axis is superiorly directed for those originating from the posterior LV papillary muscle and inferiorly directed when the origin is the anterior papillary muscle [66].
Catheter ablation is successful in achieving greater than 80% reduction in ectopic activity in 70-90% of patients, with highest success rates for arrhythmias arising from the RV out flow tract[57-59, 65]. The efficacy of ablation for the less common foci such as the LV out flow tract, are not well characterized. A common reason for failure is the inability to induce the arrhythmia for adequate mapping.
There is limited data on the time course of recovery of LV function after ablation of PVCs. One study showed that 68% of patients recover within 4 months with 32% requiring mean of 12±9 months(range 5-45 months) for recovery [60]. Early improvement (within 1 week) predicted near complete reversibility [67].
In patients with heart disease PVCs may be idiopathic or related to an abnormal substrate, such as within an infarct scar or at its border, and the site of origin may also correspond to the exit site of an inducible reentrant VT [68].
Conclusion and Take Home Message
Ventricular arrhythmias are an important cause of morbidity and mortality in patients with structural heart disease.
AADs are limited in their efficacy and catheter ablation has emerged as a useful therapy. HF remains the major cause of mortality after VT ablation, consistent with the concern that VT is an indication of disease progression in patients with impaired ventricular function, warranting attention to heart failure therapies.
The efficacy of catheter ablation for monomorphic VT varies with the underlying disease etiology,the pattern and distribution of scar, proximity of scar to critical epicardial structures and accessibility of scar areas for catheter ablation.
Novel technologies are hoped to improve future outcomes.
PVC-induced cardiomyopathy is important to recognize as a reversible condition. Further work is needed to elucidate the mechanism of PVC-induced cardiomyopathy and the relationship between PVC burden and development of cardiomyopathy.
Conflict of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Nayyar S, Ganesan AN, Brooks AG, et al. Venturing into ventricular arrhythmia storm: a systematic review and meta-analysis. Eur Heart J 2013;34:560.
2. Exner DV, Pinski SL, Wyse DG,et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable de fibrillators (AVID) trial. Circulation 2001;103(16):2066-71.
3. Prystowsky EN, Padanilam BJ,Joshi S, Fogel RI. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol 2012;59:1733-44.
4. Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before de fibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31-40.
5. Lopera G, Stevenson WG, Soejima K, et al. Identi fication and ablation of three types of ventricular tachycardia involving the his-purkinje system in patients with heart disease. J Cardiovasc Electrophysiol 2004;15:52-8.
6. Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA).Europace 2009;11:771-817.
7. Marrouche NF, Verma A, Wazni O, et al. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol 2004;43:1715-20.
8. Haissaguerre M, Shah DC, Jais P,et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 2002;359:677-8.
9. Penugonda N. Cardiac MRI in in filtrative disorders: a concise review. Curr Cardiol Rev 2010;6(2):134-6.
10. Priori SG, Biomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guideline fro the management of patients with ventricular arrhythmias and the prevention fo sudden cardiac death. Eur Heart J 2015;36:2793-867.
11. Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA.Implantable cardioverter de fibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study.Europace 2003;5:381-9.
12. van Rees JB, Borleffs CJ, de Bie MK, et al. Inappropriate implantable cardioverter-de fibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol 2011;57:556-62.
13. Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable de fi-brillator: the Dual Chamber and VVI Implantable De fibrillator(DAVID) Trial. J Am Med Assoc 2002;288:3115-23.
14. Koneru JN, Swerdlow CD, Wood MA, Ellenbogen KA. Minimizing inappropriate or “unnecessary”implantable cardioverter-de fibrillator shocks: appropriate programming. Circ Arrhythm Electrophysiol 2012;4:778-90.
15. Lee DS, Krahn AD, Healey JS,et al. Evaluation of early complications related to De Novo cardioverter de fibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol 2010;55:774-82.
16. Saxon LA, Hayes DL, Gilliam FR,et al. Long-term outcome after ICD and CRT implantation and in fluence of remote device follow-up:the ALTITUDE survival study. Circulation 2010;122:2359-67.
17. Connolly SJ, Dorian P, Roberts RS,et al. Comparison of beta-blockers,amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter de fibrillators: the OPTIC Study: a randomized trial. J Am Med Assoc 2006;295:165-71.
18. Stevenson WG, Friedman PL,Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol 1997;29:1180-9.
19. Connolly AJ, Camm JA, Halperin JA, et al. Dronadarone in high risk permanent atrial fibrillation. N Engl J Med 2011;365:2268-76.
20. Wissner E, Stevenson WG, Kuck KH. Catheter ablation of ventricular tachycardia in ischaemic and non-ischaemic cardiomyopathy:where are we today? A clinical review. Eur Heart J 2012;33:1440-50.
21. Miller MA, Dukkipati SR,Mittnacht AJ, et al. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol 2011;58:1363-71.
22. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288-96.
23. Hutchinson MD, Gerstenfeld EP,Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:49-55.
24. Hsia HH, Lin D, Sauer WH, Callans DJ, Marchlinski FE. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identi fication of endocardial conducting channels. Heart Rhythm 2006;3:503-12.
25. Chopra N, Tokuda M, Ng J,et al. Relation of the unipolar low-voltage penumbra surrounding the endocardial low-voltage scar to ventricular tachycardia circuit sites and ablation outcomes in ischemic cardiomyopathy. J Cardiovasc Electrophyiol 2014;25:602-8.
26. de Bakker JM, Wittkampf FH. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol 2010;3:204-13.
27. Nayyar S, Wilson L, Ganesan AN,et al. High-density mapping of ventricular scar: a comparison of ventricular tachycardia (VT) supporting channels with channels that do not support VT. Circ Arrhythm Electrophysiol 2014;7:90-8.
28. Soejima K, Stevenson WG, Maisel WH, Sapp JL, Epstein LM.Electrically unexcitable scar mapping based on pacing threshold for identi fication of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation.Circulation 2002;106:1678-83.
29. Soejima K, Suzuki M, Maisel WH,et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation 2001;104:664-9.
30. Tokuda M, Tedrow UB, Kojodjojo P, et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol 2012;5:992-1000.
31. Sarkozy A, Tokuda M, Tedrow UB,et al. Epicardial ablation of ventricular tachycardia in ischemic heart disease. Circ Arrhythm Electrophysiol 2013;6:1115-22.
32. Ghanbari H, Baser K, Yokokawa M, et al. Noninducibility in postinfarction ventricular tachycardia as an end point for ventricular tachycardia ablation and its effects on outcomes: a meta-analysis.Circ Arrhythm Electrophysiol 2014;7:677-83.
33. Santangeli P, Frankel DS, Marchlinski FE. End points for ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol 2014;7:949-60.
34. Dinov B, Fiedler L, Schonbauer R,et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the prospective heart centre of leipzig VT (HELP-VT) study. Circulation 2014;129:728-36.
35. Verma A, Kilicaslan F, Schweikert RA, et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation 2005;111:3209-16.
36. Tokuda M, Sobieszczyk P, Eisenhauer AC, et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update.Circ Arrhythm Electrophysiol 2011;4:889-96.
37. Anter E, Hutchinson MD, Deo R,et al. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:494-500.
38. Ueda A, Fukamizu S, Soejima K,et al. Clinical and electrophysiological characteristics in patients with sustained monomorphic reentrant ventricular tachycardia associated with dilated-phase hypertrophic cardiomyopathy. Europace 2012;14:734-40.
39. Della Bella P, Riva S, Fassini G,et al. Incidence and signi ficance of pleomorphism in patients with postmyocardial infarction ventricular tachycardia. Acute and longterm outcome of radiofrequency catheter ablation. Eur Heart J 2004;25:1127-38.
40. Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;118:2773-82.
41. Tung R, Vaseghi M, Frankel DS,et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm 2015;12:1997-2007.
42. Piers SR, Leong DP, van Huls van Taxis CF, et al. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy: the impact of noninducibility. Circ Arrhythm Electrophysiol 2013;6:513-21.
43. Desjardins B, Yokokawa M, Good E, et al. Characteristics of intramural scar in patients with nonischemic cardiomyopathy and relation to intramural ventricular arrhythmias. Circ Arrhythm Electrophysiol 2013;6:891-7.
44. Haqqani HM, Tschabrunn CM,Betensky BP, et al. Layered activation of epicardial scar in arrhythmogenic right ventricular dysplasia:possible substrate for con fined epicardial circuits. Circ Arrhythm Electrophysiol 2012;5:796-803.
45. Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366-75.
46. Marchlinski FE, Zado E, Dixit S,et al. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation 2004;110:2293-8.
47. Santangeli P, Di Biase L, Lakkireddy D, et al. Radiofrequency catheter ablation of ventricular arrhythmias in patients with hypertrophic cardiomyopathy: safety and feasibility. Heart Rhythm 2010;7:1036-42.
48. Inada K, Seiler J, Roberts-Thomson KC, et al. Substrate characterization and catheter ablation for monomorphic ventricular tachycar-dia in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2011;22:41-8.
49. Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of de fi-brillator therapy. N Engl J Med 2007;357:2657-65.
50. Bunch TJ, Weiss JP, Crandall BG,et al. Patients treated with catheter ablation for ventricular tachycardia after an ICD shock have lower long-term rates of death and heart failure hospitalization than do patients treated with medical management only. Heart Rhythm 2014;11(4):533-40.
51. Dinov B, Arya A, Bertagnolli L,et al. Early referral for ablation of scar-related ventricular tachycardia is associated with improved acute and long-term outcomes: results from the heart center of Leipzig ventricular tachycardia registry.Circ Arrhythm Electrophysiol 2014;7(6):1144-51.
52. Vaseghi M, Gima J, Kanaan C,et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm 2014;11:360-6.
53. Gami AS, Noheria A, Lachman N,et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011;30:5-15.
54. Daniels DV, Lu YY, Morton JB,et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identi fication from the 12-lead electrocardiogram. Circulation 2006;113:1659-66.
55. Baman TS, Lange DC, Ilg KJ, et al.Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865-9.
56. Del Carpio Munoz F, Syed FF,Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval,morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011;22:791-8.
57. Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular out flow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259-65.
58. Lakkireddy D, Di Biase L, Ryschon K, et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol 2012;60:1531-9.
59. Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition.Circ Arrhythm Electrophysiol 2012;5:229-36.
60. Yokokawa M, Kim HM, Good E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm 2012;9:1460-4.
61. Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy.Heart Rhythm 2011;8:1046-9.
62. Yokokawa M, Kim HM, Good E, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm 2012;9:92-5.
63. Niwano S, Wakisaka Y, Niwano H, et al. Prognostic signi ficance of frequent premature ventricular contractions originating from the ventricular out flow tract in patients with normal left ventricular function. Heart 2009;95:1230-7.
64. Capucci A, Di Pasquale G, Boriani G, et al. A double-blind crossover comparison of flecainide and slowrelease mexiletine in the treatment of stable premature ventricular complexes. Int J Clin Pharmacol Res 1991;11:23-33.
65. Ling Z, Liu Z, Su L, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular out flow tract:prospective randomized study.Circ Arrhythm Electrophysiol 2014;7:237-43.
66. Yamada T, Doppalapudi H,McElderry HT, et al. Idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: prevalence, electrocardiographic and electrophysiological characteristics, and results of the radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2010;21:62-9.
67. Hasdemir C, Kartal Y, Simsek E,Yavuzgil O, Aydin M, Can LH.Time course of recovery of left ventricular systolic dysfunction in patients with premature ventricular contraction-induced cardiomyopathy. Pacing Clin Electrophysiol 2013;36:612-7.
68. Bogun F, Crawford T, Chalfoun N, et al. Relationship of frequent postinfarction premature ventricular complexes to the reentry circuit of scar-related ventricular tachycardia. Heart Rhythm 2008;5:367-74.
 Cardiovascular Innovations and Applications2016年1期
Cardiovascular Innovations and Applications2016年1期
- Cardiovascular Innovations and Applications的其它文章
- Implantable Cardiac De fibrillators: Who Needs Them and Who Does Not?
- The Subcutaneous Implantable Cardioverter-De fibrillator: A Practical Review and Real-World Use and Application
- Syncope and Early Repolarization: A Benign or Dangerous ECG Finding?
- Changing the Way We “See” Scar:How Multimodality Imaging Fits in the Electrophysiology Laboratory
- Stroke Prevention in Atrial Fibrillation:Current Strategies and Recommendations
- Principles of Arrhythmia Management During Pregnancy
