The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
?
The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
Agustina Setiawati*, Handika Immanuel, Mery Tri Utami
Department of Drug Design and Discovery, Faculty of Pharmacy, Sanata Dharma University, Yogyakarta 55281, Indonesia
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.012
Tel: +62 274 883037
Fax: +62 274 886529
E-mail: nina@usd.ac.id
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 19 Oct 2015
Received in revised form 30 Nov 2015
Accepted 7 Dec 2015
Available online 31 Dec 2015
Keywords:
Typhonium flagelliforme Colon cancer
WiDr
Cyclooxygenase 2
ABSTRACT
Objective: To determine the inhibition activity of Typhonium flagelliforme Lodd. Blume (T.flagelliforme) leaf extract on cyclooxygenase 2 (COX-2) expression of colon cancer cells.
Methods: T.flagelliforme leaf extract was prepared to macerate in ethyl acetate. In vitro anticancer activity was assayed by MTT method on WiDr colon cancer cells. This study applied apoptosis induction assay to investigate the mechanism of cell death using double staining method. COX-2 expression was stained by immunocytochemistry.
Results: T.flagelliforme showed anticancer activity and induced apoptosis on WiDr cells through inhibition of COX-2 expression with IC5070 mg/mL.
Conclusions: This study showed that T.flagelliforme is a promising chemopreventive agent for colon cancer through COX-2 inhibition.
1. Introduction
Colon cancer is one of the leading causes of cancer related death in developed countries, and it is a frequently diagnosed cancer in both of male and female[1]. It was also ranked as the third most commonly diagnosed cancer worldwide, especially in the Southeast Asian Nations [2,3]. Colon cancer is known as a disease in industrialized countries, but the pattern is economically changing nowadays. Since its incidence rapidly increases [4,5], many attempts are employed to prevent and cure colon cancer. There are many possible clinical managements of colon cancer treatment, such as surgery, chemotherapy, radiotherapy and adjuvant chemotherapy [5,6]. Many studies had been designed to develop colon cancer treatment by attacking specific targets which is a key strategy to cure colon cancer without endangering normal cells[7-9].
Colon cancer overexpresses cyclooxygenase 2 (COX-2) in both carcinoma and adenoma[10-12]. COX-2, an enzyme that is responsible for converting arachidonic acid to prostaglandins [13], plays an important role in cell proliferation and apoptosis regulation of colon cancer [14]. Prostaglandin E2, a type of COX-2 product, promotes angiogenesis and stimulates colon cancer growth by preventing apoptosis [15,16]. Thus, COX-2 inhibitors had shown to successfully prevent colon cancer growth and polyp formation [16-21]. Moreover, COX-2 is a specific molecular target for anticancer screenings on colon cancer.
Many studies investigated a natural product activity on colon cancer [22-25], especially addressing COX-2 as molecular target [10,25]. Curcumin, a yellow pigment isolated from turmeric, successfully inhibited COX-2 on WiDr colon cancer cells [21]. Another promising natural product which potentially exhibits anticancer activity on colon cancer is Typhonium flagelliforme Lodd. Blume (T.flagelliforme). Its leaves contain glycoside flavonoid, isovitexin, as well as alkaloids [26,27]. Previous studies showed that T.flagelliforme revealed cytotoxic activity on MCF-7 breast cancer cells [28], and induced apoptosis on murine leukemia WEHI-3 cells [29] and lymphocyte CEM-SS [30]. However, the cytotoxic activity of T.flagelliforme on colon cancer cells with COX-2 as the molecular target remains delusive. Therefore, this study investigated cytotoxic activity ofT.flagelliforme leaf extract on WiDr colon cancer cells that highly express COX-2.
2. Materials and methods
2.1. Plant materials
The leaves of T.flagelliforme were harvested from Malang (East Java, Indonesia) in June 2014, and sun-dried after thorough washing. The plant materials were identified in Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada (Reference No. BF/275/Ident/Det/VI/2014).
2.2. Cytotoxic, apoptosis and immunocytochemistry assay materials
WiDr cells were collected from Parasitology Department, Faculty of Medicine, Universitas Gadjah Mada. The cells were maintained in Roswell Park Memorial Institute medium (Gibco) containing fetal bovine serum 10% (v/v) (Gibco) and penicillinstreptomycin 1% (v/v) (Gibco). Dimethyl sulfoxide was used to dissolve stock solution of extract and celecoxib was purchased from Merck. Celecoxib, as a positive control, was prepared from Celebrex?. Cytotoxic assay was measured by MTT (Sigma) method, while the apoptosis assay was examined by ethidium bromide and acridine orange (Sigma) staining. Immunocytochemistry used COX-2 primary antibody purchased from Thermo Scientific Lab Vision, while the universal secondary antibody was derived from Starr Trek Universal HRP Detection System No.901-STUHRP700-090314. All culture plates used in this study were Iwaki?and all tips and microtubes were supplied by Biologix?.
2.3. Extract preparation
The extract was prepared by using maceration method. Dried leaves of T.flagelliforme were ground and soaked in ethyl acetate (1:10) for 24 h until all substances were extracted. The liquid extract was slowly evaporated to discard the residual solvent, until viscous extract was obtained.
2.4. MTT cytotoxic assay
Cytotoxic assay was designed based on several previous studies[31-33]. WiDr colon cancer cells were cultured in culture tissue flask until 70%-80% confluent, and then 5×103cells were seeded into 96-well plate. The plate was incubated at 37°C and under 5% CO2for 24 h. The medium was removed and the cells were rinsed twice by using phosphate buffer solution (PBS). The stock solution of extract and celecoxib were prepared to dissolve them into dimethyl sulfoxide and dilute them with the medium into various concentrations. Each concentration (100 mL/well) was added into 96-well plate and measured in triplicates. Later, the plate was incubated at 37°C and under 5% CO2for 24 h. The medium was then removed and 10% MTT containing medium was added into each well. The reaction between MTT and succinate hydrogenase of cells to form formazan needed 4 h. At the end of incubation time, 100 mL sodium dodecyl sulfate was added to each well to dissolve formazan crystals. The plate was incubated in dark room for 12-24 h and formazan crystals were measured by using ELISA reader at a wavelength of 595 nm.
2.5. Apoptosis induction assay
WiDr cells were cultured into coverslips in 24-well plate (5×104/well). The cells were adapted at 37°C and 5% CO2for 24 h. The medium was removed and rinsed twice by using PBS. The extract (100 mg/mL) and celecoxib at IC50concentration (68 mmol/L) were added into the plate and incubated in the same condition as previously described. The medium was removed from the cells and rinsed by using PBS. The coverslips were transferred to object glasses, then acridine orange/ethidium bromide was dropped into the coverslips. The cells were immediately observed under fluorescence microscope.
2.6. Immunocytochemistry assay
WiDr cells were seeded in 6-well plate and incubated under 5% CO2and 37°°C for 24 h. The extract at 100 mg/mL and celecoxib at IC5068 mmol/L were added to the cells and incubated for further 24 h. At the end of incubation time, cells were harvested and washed by PBS. The cells were suspended in medium, placed and fixed in object glass for 5 min. Hydrogen peroxidase was dropped into the object glass and incubated at room temperature for 10-15 min. The cells were washed twice with PBS and monoclonal antibody of COX-2 was added into the cells and incubated at least for 1 h at room temperature. The cells were washed three times with PBS and added with secondary antibody, incubated at room temperature for 10 min, and washed four times with PBS. The solution of 3,3′-diaminobenzidine, as chromogen, was added to the cells and incubated for 3-8 min. Finally, the cells were washed with distilled water and added with hematoxylin solution followed by 3-4 min incubation. The expression of COX-2 was observed under inverted microscope.
2.7. Data analysis
Cell viability was calculated from MTT data using equation:
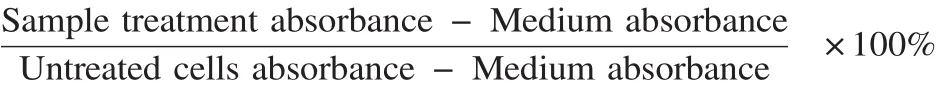
Cell viability data were analyzed by using linear regressions at four linier points to calculate IC50of extract and celecoxib. Apoptosis induction semiquantitatively counted the number of apoptotic, necrotic and living cells in three different areas of an object glass. Each treatment was performed in triplicate. The number of COX-2 expressing cells was analyzed by t-test using Microsoft Excel 2013.
3. Results
This study investigated the cytotoxic effect of T.flagelliforme leaf extract on WiDr colon cancer cells by targeting COX-2. Celecoxib, a selective COX-2 inhibitor, was used as a positive control. Dixon et al. reported celecoxib was a prospective anticancer on colon cancer [10]. Cytotoxic effect, assessed withMTT assays, measured the absorbance of formazan complex at 595 nm that equaled to the number of living cells. As presented in Figures 1 and 2, T.flagelliforme leaf extract (R2= 0.995) and celecoxib (R2= 0.954) showed dose dependent cytotoxic activity on WiDr colon cancer cells with IC5070 mg/mL and 68 mmol/L, respectively.
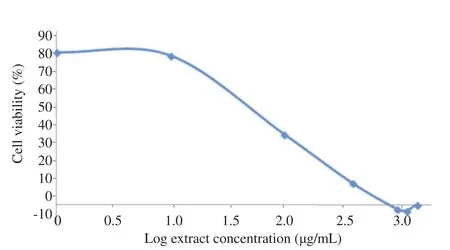
Figure 1. Effect of T.flagelliforme leaf extract in various concentrations on WiDr cells viability.
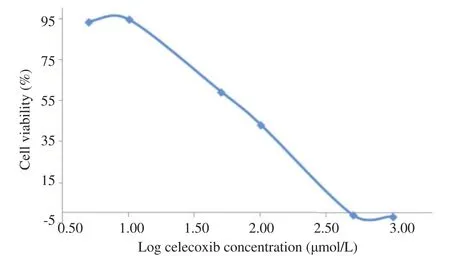
Figure 2. Effect of celecoxib in various concentrations on WiDr cells viability.
Double staining method was employed to determine the mechanism of cells death. T.flagelliforme leaf extract induced apoptosis [(65.27±1.27)%] on WiDr cells as well as celecoxib [(88.67±1.23)%] (Table 1). This method also successfully identified apoptosis stage and resulted in insignificant difference to flow cytometry method[34]. In early apoptotic cells, the nuclei showed condensed yellow-green fluorescence by acridine orange. On the other hand, the nuclei of late apoptotic cells showed condensed orange fluorescence by ethidium bromide (Figure 3).
Molecular mechanism of T.flagelliforme leaf extract was specifically observed by immunocytochemistry method. COX-2 expressing cells showed brown color while non-expressing cells showed purple color (Figure 4). WiDr cells were stained as intense brown color, but T.flagelliforme leaf extract and celecoxib treated cells were stained as purple color. This result indicated that WiDr highly expressed COX-2 but T.flagelliforme leaf extract and celecoxib inhibited COX-2 expression. Semi-quantitative analysis of this result confirmed that T.flagelliforme leaf extract and celecoxib significantly suppressed COX-2 expression on WiDr cells (P<0.05) (Table 2). This data suggested that cytotoxic activity of T.flagelliforme leaf extract on WiDr cells was through COX-2 downregulation.
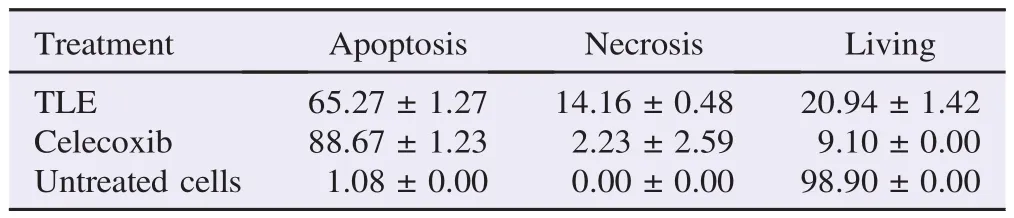
Table 1 Cell distribution after double staining. %.
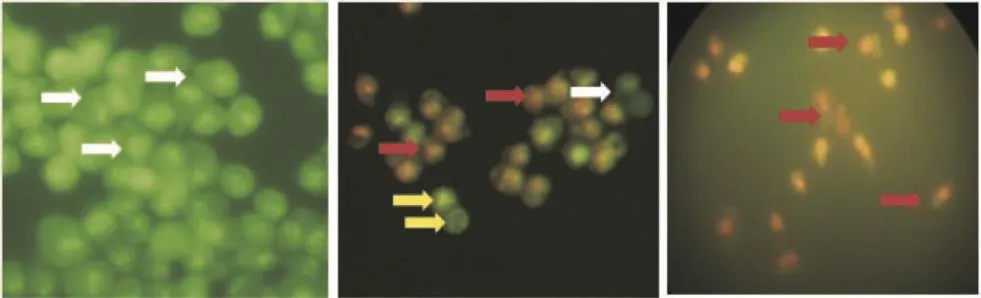
Figure 3. The observation of apoptotic cells on double staining method under fluorescence microscope using 400×magnification.A: Untreated cells; B: Cells treated 100 mg/mL T.flagelliforme leaf extract; C: Cells treated 68 mmol/L celecoxib; White arrow: Living cell; Yellow arrow: Early apoptotic cell; Red arrow: Late apoptotic cell.
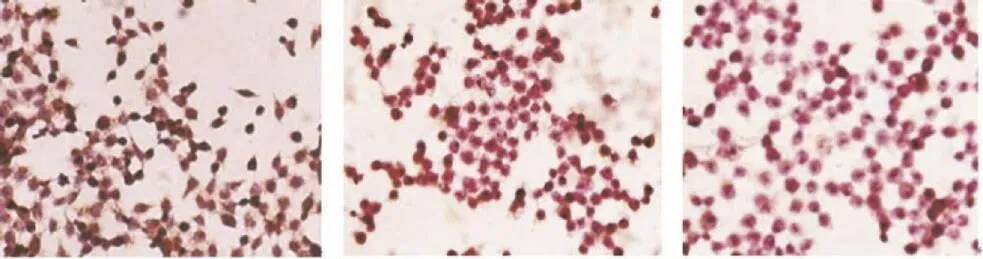
Figure 4. The observation of COX-2 expression cells on immunocytochemistry method under light microscope using 400×magnification.A: Untreated cells; B: Cells treated 100 mg/mL T.flagelliforme leaf extract; C: Cells treated 68 mmol/L celecoxib; Brown color: COX-2 expression.
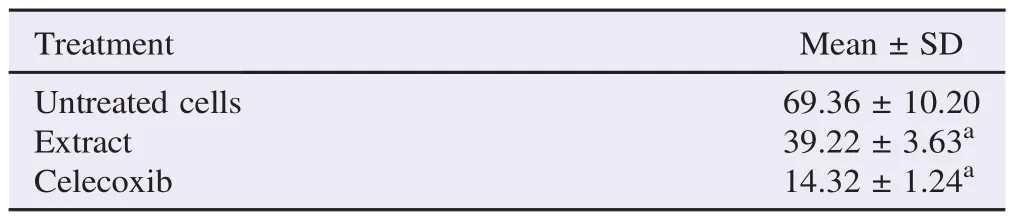
Table 2 Number of cells that expressed COX-2.
4. Discussion
This study evaluated the anticancer activity of T.flagelliforme leaf extract on WiDr colon cancer cells through COX-2 expression. COX-2 produced prostaglandin E2that increased proliferation and prevented cells from apoptosis[14,15]. Addressing COX-2 is an effective strategy to screen chemopreventive agents on colon cancer.
Our results concluded that T.flagelliforme leaf extract, as well as celecoxib, performed anticancer activity on WiDr cells by inhibiting COX-2 expression. These results have also opened interesting question on whether the inhibition of COX-2 expression of T.flagelliforme leaf extract was possible due to the presence of antioxidant compounds. It is well known that the colon is prone to oxidative condition and antioxidant deficiency [35]. Under oxidative stress, COX-2 was highly produced to modulate inflammation and induce carcinogenesis [36]. Prooxidant stress factor such as cigarette smoking, a diet high inn-6 polyunsaturated fatty acid, and alcohol consumption may increase genotoxic damage of intestine [37].
On the other hand, the presence of antioxidant suppresses reactive oxygen species (ROS) formation and inhibits cancer cell proliferation [38,39]. A study by La Vecchia et al. showed an inverse correlation between antioxidant intakes and the risk of colorectal cancer [40]. Another study reported that low antioxidant status could support cancer development and therefore antioxidant supplement may be beneficial for cancer patient[41].
Isovitexin, isolated compound from ethyl acetate extract of T.flagelliforme leaves [26], showed antioxidant activity protecting cells from ROS [42]. This compound was reported to be responsible for the cytotoxic activity and COX-2 inhibition activity of T.flagelliforme leaf extract on WiDr cells. Previous studies established that antioxidants reduced the risk of colorectal cancer by COX-2 downregulation [43,44]. However, the mechanism of anticancer activity through antioxidant activity still needs to be further investigated since the role of ROS on carcinogenesis has dual role in either induction or inhibition of carcinogenesis [40,45]. Carcinogenesis can be induced by ROS formation in higher concentration, while ROS could also be possible to induce apoptosis on cells [40,46].
To conclude, T.flagelliforme leaf extract offers a new promising chemopreventive agent on colon cancer. Our data showed that T.flagelliforme leaf extract inhibits COX-2 expression. Nevertheless, details on molecular mechanism of these benefits remain to be established in the future study.
Anticancer activity of T.flagelliforme leaf extract and celecoxib on WiDr colon cancer cells was mediated by COX-2 inhibition, however the molecular mechanism needs to be further investigated.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are grateful to Adam Hermawan, PhD., Pharm. and Kholid Nur Alfan, Pharm for helping to prepare this manuscript.
References
[1] Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87-108.
[2] World Health Organization. Latest world cancer statistic. Geneva: World Health Organization; 2013. [Online] Available from: https:// www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf [Accessed on 27th August, 2015]
[3] Kimman M, Norman R, Jan S, Kingston D, Woodward M. The burden of cancer in member countries of the Association of Southeast Asian Nations (ASEAN). Asian Pac J Cancer Prev 2012; 13: 411-20.
[4] Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19(8): 1893-907.
[5] Labianca R, Nordinger B, Beretta GD, Brouquet A, Cervantes A, ESMO Guidelines Working Group. Primary colon cancer: ESMO clinical practical guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010; 21: v70-7.
[6] The Association of Coloproctology of Great Britain and Ireland. Guidelines for the management of colorectal cancer. London: The Association of Coloproctology of Great Britain and Ireland; 2007. [Online] Available from: http://www.uhb.nhs.uk/Downloads/pdf/ CancerPbManagementColorectalCancer.pdf [Accessed on 27th August, 2015]
[7] Jiang Y, Zhang C, Chen K, Chen Z, Sun Z, Zhang Z, et al. The clinical significance of DC-SIGN and DC-SIGNR, which are novel markers expressed in human colon cancer. PLoS One 2014; 9: e114748.
[8] Wang XW, Zhang YJ. Targeting mTOR network in colorectal cancer therapy. World J Gastroenterol 2014; 20(15): 4178-88.
[9] Mishra J, Dromund J, Quazi SH, Karanki SS, Shaw JJ, Chen B, et al. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol 2013; 86(3): 232-50.
[10] Dixon DA, Blanco FF, Bruno A, Patrignani P. Mechanistic aspects of COX-2 expression in colorectal neoplasia. Recent Results Cancer Res 2013; 191: 7-37.
[11] Qi J, Dong Z, Liu J, Zhang JT. EIF3i promotes colon oncogenesis by regulating COX-2 protein synthesis and b-catenin activation. Oncogene 2014; 33(32): 4156-63.
[12] Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol 2014; 14: 1.
[13] Park J, Conteas CN. Anti-carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol 2010; 2(4): 169-76.
[14] Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010; 2010: 215158.
[15] Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2in inflammation and cancer. Semin Immunopathol 2013; 35(2): 123-37.
[16] Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancerstimulated mesenchymal stem cells create a carcinoma stem-cell niche via prostaglandin E2signaling. Cancer Discov 2012; 2(9): 840-55.
[17] Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep 2010; 62: 233-44.
[18] Rahman M, Selvarajan K, Hasan MR, Chan AP, Jin C, Kim J, et al. Inhibition of COX-2 in colon cancer modulate tumor growth and MDR-1 expression to enhance tumor regression in therapyrefractory cancers in vivo. Neoplasia 2012; 14(7): 624-33.
[19] Kodela R, Chattopadhyay M, Goswami S, Gan ZY, Rao PP, Nia KV, et al. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: differences in mode of cyclooxygenase inhibition. J Pharmacol Exp Ther 2013; 345: 85-94.
[20] Makar KW, Poole EM, Resler AJ, Seufer B, Curtin K, Kleinstein SE, et al. COX-1 (PTGS1) and COX-2 (PTGS2) polymorphisms, NSAID interactions, and risk of colon and rectal cancer in two independent populations. Cancer Causes Control 2013; 24(12): 2059-75.
[21] Rosas C, Sinning M, Ferreira A, Fuenzalida M, Lemus D. Celecoxib decreases growth and angiogenesis and promotes apoptosis in a tumor cell line resistant to chemotherapy. Biol Res 2014; 47: 27.
[22] Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol 2014; 20(16): 4618-25.
[23] Amado NG, Predes D, Moreno MM, Carvalho IO, Mendes FA, Abreu JG. Flavonoids and Wnt/b-catenin signaling: potential role in colorectal cancer therapies. Int J Mol Sci 2014; 15: 12094-106.
[24] Nassar ZD, Aisha AF, Idris N, Khadeer Ahamed MB, Ismail Z, Abu-Salah KM, et al. Koetjapic acid, a natural triterpenoid, induces apoptosis in colon cancer cells. Oncol Rep 2012; 27: 727-33.
[25] Patel VB, Misra S, Patel BB, Majumdar AP. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr Cancer 2010; 62(7): 958-67.
[26] Farida Y, Wahyudi PS, Wahono S, Hanafi M. Flavonoid glycoside from the ethyl acetate extract of keladi tikus Typhonium flagelliforme (Lodd) Blume leaves. Asian J Nat Appl Sci 2012; 1(4): 16-21.
[27] Mankaran S, Dinesh K, Deepak S, Gurmeet S. Typhonium flagelliforme: a multipurpose plant. Int Res J Pharm 2013; 4(3): 45-8.
[28] Nobakht GM, Kadir MA, Stanslas J, Charng CW. Cytotoxic effect of Typhonium flagelliforme extract. J Med Plant Res 2014; 8(31): 1021-4.
[29] Mohan S, Abdul AB, Abdelwahab SI, Al-Zubairi AS, Aspollah Sukarid M, Abdullah R, et al. Typhonium flagelliforme inhibits the proliferation of murine leukemia WEHI-3 cells in vitro and induces apoptosis in vivo. Leuk Res 2010; 34(11): 1483-92.
[30] Mohan S, Bustamam A, Abdelwahab SI, Al Zubairi AS, Aspollah M, Abdullah R, et al. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: its activation coopled with G0/G1 phase cell cycle arrest. J Ethnopharmacol 2010; 131(3): 592-600.
[31] Yuliani SH, Anggraeni CD, Sekarjati W, Panjalu A, Istyastono EP, Setiawati A. Cytotoxic activity of Anredera cordifolia leaf extract on hela cervical cancer cells through p53-independent pathway. Asian J Pharm Clin Res 2015; 8(2): 328-31.
[32] Setiawati A, Riswanto FO, Yuliani SH, Istyastono EP. Anticancer activity of mangosteen pericarp dry extract against MCF-7 breast cancer cell line through estrogen receptor-a. Indonesian J Pharm 2014; 25: 119-24.
[33] Astuti P, Wahyono, Nuryastuti T, Purwantini I, Purwanto. Antimicrobial and cytotoxic activities of endophytic fungi isolated from Artemisia annua L. J Appl Pharm Sci 2014; 4(10): 47-50.
[34] Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res 2015; 21: 15-20.
[35]¨Ozg¨onul A, Aksoy N, Dilmec F, Uzunk¨oy A, Aksoy S?. Measurement of total antioxidant response in colorectal cancer using a novel automated method. Turk J Med Sci 2009; 39(4): 503-6.
[36] Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010; 49(11): 1603-16.
[37] Stone WL, Krishnan K, Campbell SE, Palau VE. The role of antioxidants and pro-oxidants in colon cancer. World J Gastrointest Oncol 2014; 6(3): 55-66.
[38] Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 2010; 38(1): 96-109.
[39] Diaconeasa Z, Leopold L, Rugin a D, Ayvaz H, Socaciu C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int J Mol Sci 2015; 16: 2352-65.
[40] La Vecchia C, Decarli A, Serafini M, Parpinel M, Belloco R, Galeone C, et al. Dietary total antioxidant capacity and colorectal cancer: a large case-control study in Italy. Int J Cancer 2013; 133: 1447-51.
[41] Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 2012; 16: 1295-322.
[42] Cao D, Li H, Yi J, Zhang J, Che H, Cao J, et al. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS One 2011; 6: e21071.
[43] Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, et al. Antioxidant and DNA methylation-related nutrients and risk of distal colorectal cancer. Cancer Causes Control 2010; 21(8): 1171-81.
[44] Wang L, Gao S, Jiang W, Luo C, Xu M, Bohlin L, et al. Antioxidative dietary compounds modulate gene expression associated with apoptosis, DNA repair, inhibition of cell proliferation and migration. Int J Mol Sci 2014; 15: 16226-45.
[45] Afanas'ev I. Reactive oxygen species signaling in cancer: comparison with aging. Aging Dis 2011; 2(3): 219-30.
[46] Vullanueva C, Kross RD. Antioxidant-induced stress. Int J Mol Sci 2012; 13: 2091-109.
*Corresponding author:Agustina Setiawati, Department of Drug Design and Discovery, Faculty of Pharmacy, Sanata Dharma University, Yogyakarta 55281, Indonesia.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- Evaluations of cytotoxicity of Smilax myosotiflora and its effects on sexual hormone levels and testicular histology in male rats
