Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia
Nor Adlin Yusoff, Mun Fei Yam, Hooi Kheng Beh, Khairul Niza Abdul Razak, Tri Widyawati,3, Roziahanim Mahmud, Mariam Ahmad, Mohd Zaini Asmawi
1School of Pharmaceutical Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia
2Integrative Medicine Cluster, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Penang, Malaysia
3Pharmacology Department, Medical Faculty, University of Sumatera Utara, 20155 Medan, Indonesia
1. Introduction
Diabetes mellitus is a complicated metabolic disorder of the endocrine system, characterized by hyperglycaemia due to defects in insulin secretion, insulin action, or both[1]. This metabolic disorder leads to multiple macro- and micro-vascular complications which affect several organs, including the kidneys, heart, skin and brain.Oxidative stress has been postulated as a factor which plays a central role in the pathoetiology and progression of diabetes and its vascular complications[2]. Evidence supports the notion that hyperglycaemic conditions cause oxidative stress due to increased production of the mitochondrial reactive oxygen species which have been implicated in the pathogenesis of β–cell dysfunction and insulin resistance,as well as the development of diabetic complications[3]. Classic antioxidants, vitamins E and C, though controversial, have been proven to have antioxidant protective effects against reactive oxygen species[4]and have been shown to prevent the development of diabetic microvascular complications in diabetic rat models[5]. This raises the hypothesis that supplementation with antioxidants may be of utility in providing chemoprotection to counter the pathogenesis of diabetes and its complications[6].
Vinegar is commonly used as a food condiment, and as a traditional medicine to treat various diseases, such as diabetes, fever, microbial infections, and gastrointestinal problems[7]. The consumption of vinegar as a home remedy to manage high blood glucose levels has been recorded before the advent of today’s antidiabetic drugs[8]. A review of the literature on vinegar showed various pharmacological effects have been reported in association with its use, which included antihypertensive[9], antioxidant[10], antibacterial[11], and antidiabetic[12]effects. Nypa fruticans Wurmb. vinegar locally known as nipa palm vinegar (NPV)is one of the traditional vinegar produced by fermentation of ‘nira’, a nipa palm (Nypa fruticans Wurmb.)sap. It is predominatly consumed by people throughout the East Asian region. Unlike some types of vinegar, namely apple cider and balsamic vinegars, which have been studied extensively, to the best of our knowledge, there is not much information regarding the antidiabetic activity of NPV, nor is there a report exploring the relationship between antidiabetic activity and the antioxidants found in the vinegar. Hence, the present study was performed to inspect the scientific rationale of using NPV to ameliorate hyperglycaemia, and to investigate its potential antioxidant activity.
2. Materials and methods
2.1. Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azinobis-3-ethylbenzothiozoline-6-sulfonic acid (ABTS), D-Glucose,Folin–Ciocalteau reagent, gallic acid, butylated hydroxytoluene,ferric chloride, potassium persulfate, potassium ferricyanide,quercetin, sodium phosphate monobasic, sodium phosphate dibasic,streptozotocin, and trichloroacetic acid were purchased from Sigma Aldrich (St. Louis, MO, USA), whereas 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox)was obtained from Calbiochem (Merck KGaA, Darmstadt, German). Insulin ELISA kit was obtained from Crystal Chem, Inc. (Downers Grove, IL, USA).All other chemicals and solvents used were of reagent grade.
2.2. Vinegar preparation and extraction
NPV used in the study was supplied by a local producer from Titi Bakong, Yan, Kedah, Malaysia (5°48' 9.42? N, 100°22' 35.32? E).Several parts of nipa palm were authenticated by Dr Rahmad Zakaria from Herbarium Unit, School of Biological Sciences, Universiti Sains Malaysia and the voucher specimens were deposited at the same unit with the voucher number of USM.Herbarium 11541.
NPV was extracted using liquid-liquid extraction method, as described by Qiu and collegues[13]with minor modifications. Ethyl acetate and water were consecutively used to extract the phenolic compounds of NPV. NPV (500 mL)was concentrated to a final volume of 250 mL at 37 ℃ using a vacuum rotary evaporator (Buchi Labortechnik, AG CH-9230 Flawil, Switzerland). Concentrated NPV was first extracted with ethyl acetate in a ratio of 1:1 using a separatory funnel. The ethyl acetate layer (upper layer)was then separated; and the residue was collected and considered as aqueous layer. Both of the two collected extracts were concentrated at 40 ℃using a vacuum rotary evaporator, and lyophilized using a freeze–drier (Labconco Corporation, Kansas City, Missouri, USA). Finally,the extracts were stored in the freezer at –4 ℃ until further use in the designated experiment.
2.3. Experimental animals
Adult male Sprague-Dawley rats (200 to 250 g), aged 8 to 10 weeks obtained from the Animal Research and Service Centre, Universiti Sains Malaysia, were used in the study. All animals were housed and acclimatized in a well-ventilated animal transit room (12 h light/12 h dark cycle)at the School of Pharmaceutical Sciences, Universiti Sains Malaysia. Throughout the experiment, the rats had ad libitum access to water and food pellets (Gold Coin Feedmills, Malaysia).The study protocol was approved by the Animal Ethic Committee at Universiti Sains Malaysia [Approval number: USM/ Animal Ethics Approval/ 2011/ (71)(326)].
Following an overnight fast, experimental diabetes was induced in healthy Sprague-Dawley rats with a single intraperitoneal injection of streptozotocin at a dose of 55 mg/kg b.w. The streptozotocin solution was prepared fresh by dissolving streptozotocin in 0.9%normal saline at 4 ℃. For the first 24 h following the streptozotocin injection, a 5 % glucose solution was given in place of the drinking water to avoid the onset of drug-induced hypoglycaemia[14]. Three days after injection, fasting blood glucose levels were measured using an Accu–Check glucometer (Roche, Mexico City)based on the glucose-oxidase method. The animals whose fasting blood glucose levels were within the range of 11-20 mmol/L were selected for the study.
2.4. Antidiabetic activity
Assessment of the antidiabetic activities of NPV and its aqueous and ethyl acetate extracts were carried out using normal, glucoseloaded, and streptozotocin-induced diabetic rats.
2.4.1. Acute hypoglycaemic test in normal rats
In this test, a total of 30 normal rats were used. The rats were randomly divided into 5 groups of 6 rats each and treated orally as follows: Group Ⅰ served as the negative control and received the vehicle only (distilled water, 10 mL/kg b.w.). Group Ⅱ served as the positive control and received glibenclamide (10 mg/kg b.w.). GroupⅢ, Ⅳ and Ⅴ received NPV, the aqueous extract, and the ethyl acetate extract, respectively, at a dose of 1 g/kg b.w. Blood samples were drawn from the tail vein prior to treatment (0 h)and 1, 2, 3, 5 and 7 h after treatment. Fasting blood glucose levels were measured using the glucose-oxidase method (Accu-Check glucometer, Roche,Mexico City)[15].
2.4.2. Intraperitoneal glucose tolerance test in glucoseloaded rats
Thirty normal rats were divided into 5 groups of 6 rats each and treated orally as follows: GroupⅠserved as the negative control and received the vehicle only (distilled water, 10 mL/kg b.w.). Group Ⅱserved as the positive control and received metformin (500 mg/kg b.w.). Group Ⅲ received NPV (1 g/kg b.w.). Group Ⅳ received the aqueous extract (1 g/kg b.w.). Group Ⅴ received the ethyl acetate extract (1 g/kg b.w.). After an hour of oral dosing, the rats were injected intraperitoneally (ip.)with a glucose solution at a dose of 1 g/kg b.w. Blood samples were collected via the tail vein at time 0 h(prior to oral administration)and 15, 30, 45, 60, 90 and 120 min after glucose administration. Blood glucose measurements were carried out as previously mentioned.
2.4.3. Acute anti-hyperglycaemic test in diabetic rats
The test procedure was similar to the one applied in the acute hypoglycaemic test in normal rats (2.4.1), except for the use of streptozotocin-induced diabetic rats in place of the normal rats,and glibenclamide being substituted for by metformin (500 mg/kg b.w.)as the positive control. The assigned groups were as follows:Group Ⅰ: Normal control + distilled water (10 mL/kg b.w), GroupⅡ: Diabetic + distilled water (10 mL/kg b.w.), Group Ⅲ: Diabetic+ Metformin (500 mg/kg b.w.), Group Ⅳ: Diabetic + NPV (1 g/kg b.w.), Group Ⅴ: Diabetic + Aqueous extract (1 g/kg b.w.)and GroupⅥ: Diabetic + Ethyl acetate extract (1 g/kg b.w.).
2.4.4. Sub-chronic (12 days)antihyperglycaemic test in diabetic rats
For a sub-chronic study, the same groups of diabetic animals described in section 2.4.3 continued to receive the same oral treatment, twice daily, with a 12-h dosing interval for a period of 12 d. Blood glucose levels were recorded on days 0, 3, 6, 9 and 12,following an overnight fast. At the end of the study, the overnight fasted rats were euthanized, and blood samples were collected via cardiac puncture for glucose and insulin analyses. The insulin analysis was performed using an ELISA kit (Ultra Sensitive Rat Insulin, Crystal Chem, Inc., IL 60515, USA).
2.5. Antioxidant properties
For antioxidant assays, NPV and its extracts were prepared by the same procedure chosen for the antidiabetic assessments.
2.5.1. DPPH radical-scavenging assay
The DPPH radical-scavenging activities of NPV and its extracts were determined according to Yam et al[16]with minor modifications.Briefly, different sample concentrations (0.78, 1.56, 3.13, 6.25,12.50, and 25.00 mg/mL), and 0.1 mM DPPH radical solution,were prepared in methanol. A 100 μL aliquot of each sample was mixed with 200 μL of the DPPH radical solution in a 96-well plate and incubated in the dark at room temperature for 30 min. In the blank assay, distilled water substituted for the sample. Quercetin and butylated hydroxytoluene served as the positive controls. The assay was performed in triplicate. Absorbance was measured at 517 nm using a microplate reader (Power Wave×340, USA)and the percentage of DPPH radical scavenging activity was calculated as follows:
where Ab and As denoted the absorbance of the blank and the sample, respectively. The inhibitory concentration that scavenged 50% of the DPPH free radical activity (IC50)was obtained by interpolation from linear regression analysis.
2.5.2. ABTS radical-scavenging activity/ Trolox equivalent antioxidant capacity
The ABTS assay is based on the inhibition of the absorbance of ABTS radical cation at 734 nm when it is exposed to an antioxidant[17]. To 10 μL of either sample (0.25, 0.5, 1, 2 and 4 mg/mL)or Trolox standard solution, 2 mL of ABTS radical solution was added. The mixture, protected from the light, was then incubated for 6 min at room temperature and the absorbance was measured at 734 nm using HITACHI U-2800 spectrophotometer. Phosphate buffer saline, in place of sample was used as the blank, and the assay was conducted in triplicate. Trolox equivalent antioxidant capacity values of samples were calculated from the Trolox standard curve and expressed as mmol of Trolox equivalent per g of plant extract. The percentage of ABTS radical scavenged was calculated as follows:
where Ab and As denoted the absorbance of the blank and the sample, respectively.
2.5.3. Reducing power assay
The reducing power assay was performed following the method described by Atangwho et al[18]. Briefly, dissolved in distilled water,1 mL of the sample (0.13-4.00 mg/mL)was mixed with 2.5 mL of phosphate buffer saline (0.2 M, pH 7.2)and 2.5 mL of 1%potassium ferricyanide. Then, the mixture was incubated in a water bath at 50 ℃ for 20 min. After adding 2.5 mL of 10% trichloroacetic acid, the mixture was centrifuged (Eppendorf 5403, Engelsdorf,Germany)at 3 000×g for 10 min. Subsequently, 2.5 mL of the supernatant layer were transferred into a clean tube and mixed with 2.5 mL of distilled water and 1 mL of 0.1% ferric chloride; and the absorbance was measured at 700 nm using a HITACHI U – 2800 spectrophotometer. Higher absorbance values indicated greater reducing power. Quercetin and butylated hydroxytoluene were used as controls and treated same as the sample. All assays were performed in triplicate.
2.6. Total phenolics and flavanoids assays
The total phenolic content of NPV, and that of each of its extracts,was measured using a 96 well plate reader (Power Wave×340,USA)as described by Brimson et al[19]. Briefly, 50 μL of Folin–Ciocalteau reagent, diluted with distilled water in a ratio of 1:10 beforehand, were incubated with 50 μL of each of the tested samples at various concentrations (6.3, 12.5, 25.0 and 50.0 mg/mL)for 20 min in the dark. Afterwards, 35 μL of sodium carbonate were added.Following another 20-min incubation, the absorbance was read at 750 nm. Gallic acid (0-200 μg/mL)was used as a standard for the calibration curve. The total phenolic content was reported as mg gallic acid equivalent/g of the dry extract using the following linear equation derived from the calibration curve:
The total flavonoid content of NPV, and that of each of its extracts,was determined according to the method of Brimson et al[19]. On a 96-well plate, 50 μL of each of the tested samples at various concentrations (6.3, 12.5, 25.0 and 50.0 mg/mL)were mixed with 5 μL of 10 % aluminium chloride hexahydrate solution, 5 μL of 1 M potassium acetate solution and 140 μL of distilled water, and incubated for 40 min in the dark. After incubation, the absorbance was measured at 415 nm using a microplate reader (Power Wave×340, USA). Rutin (0-200 μg/mL)was used as a standard for the calibration curve. The total flavonoid content was represented as mg rutin equivalent/g of dry extract using the following linear equation derived from the calibration curve:
2.7. Chemical profiling: studying the active aqueous extract
A HP 6890N gas chromatograph coupled with a HP 59731 mass spectrometer (Agilent Technologies Inc. (NYSE:A)Palo Alto, CA,USA)was used for GC-MS analysis. Separations were accomplished with a HP – INNOWax capillary column (0.25 mm i.d.×30 m specification length×0.25 μm film thickness, Agilent 19091N-133). The analysis conditions were as follows: maximum column temperature was programmed at 270 ℃; GC was performed in the splitless mode; initial inlet temperature and the detector were set at 250 ℃; oven temperature was initiated at 60 ℃ for 2 min,then raised to 250 ℃ at a rate of 20 ℃/min and held for 20 min;run time was 31.50 min; flow rate of helium carrier gas was 1 mL/min; injection volume was 1 μL solution of extract (5 mg/mL); scan parameter was performed in full scan mode within the range of 35-650 m/z. The individual compounds were identified by comparing their acquired mass spectra and retention times with ChemStation data base (NIST02).
2.8. Statistical analysis
For the antidiabetic study, data was expressed as the mean±standard error of the mean (SEM). The results were analysed using the one way analysis of variance (ANOVA)followed by Dunnett’s comparison test. For the antioxidant study, all measurements were performed in triplicate and expressed as the mean±SEM. The results were analysed using one-way ANOVA, and Tukey’s multiple comparison test. The results were statistically significant at P<0.05.
3. Results
3.1. Acute effects of NPV and its extracts on normal and diabetic rats
A single oral administration of NPV and its aqueous and ethyl acetate extracts at the dose of 1 g/kg b.w. did not significantly affect the blood glucose levels of euglycaemic rats within the 7-h test duration. As shown in Figure 1A, only the reference drug,glibenclamide (10 mg/kg b.w.)reduced blood glucose to markedly lower levels, bordering on hypoglycaemic shock in the euglycaemic rats.
Meanwhile, the fasting blood glucose levels of the streptozotocininduced diabetic rats were significantly higher when compared with those of the normal rats (Figure 1B). A single oral administration of NPV and its aqueous and ethyl acetate extracts at a dose of 1 g/kg b.w. did not significantly affect the fasting blood glucose levels of streptozotocin-induced diabetic rats within the 7-h test duration.However, compared to the diabetic control, the reference drug,metformin, administered at a dose of 500 mg/kg b.w., significantly reduced the blood glucose levels from the 1st until the 7th hour of the study (P<0.05).
3.2. Effects of NPV and its extracts on intraperitoneal glucose tolerance test
Figure 2 shows the effects of NPV and its ethyl acetate and aqueous extracts, as well as metformin, on the blood glucose levels of normal rats following an intra-peritoneal glucose challenge. Compared to the control group, unlike metformin, none of the sample-treated groups produced significant reduction (P<0.05)in the rise of the fasting blood glucose levels measured 15 min after glucose loading.Metformin elicited a 40.8% reduction of the initial value. Up to 90 min after glucose loading, NPV and its aqueous extract exerted significant reduction in the blood glucose levels as compared to the control group (P<0.05). The reduction pattern caused by the tested samples was sustained in tandem with metformin for all the time points of measurement.
3.3. Subchronic effects of NPV and its extracts on glucose and insulin levels in streptozotocin-induced diabetic rats
Figure 3 shows the effect of repeated administration of NPV and its extracts on fasting blood glucose and serum insulin levels of normal rats, untreated and treated diabetic rats. As shown in Figure 3A, an intraperitoneal injection of streptozotocin to normal rats induced a significant increase in fasting blood glucose levels (76.3%; P<0.01)throughout the study period. Administered twice daily for 12 d,NPV, its aqueous and ethyl acetate extracts, and metformin caused significant reduction of blood glucose levels at the end of the study when compared to the diabetic control (P<0.05). The reduction was 12.0%, 56.6%, 28.7% and 46.2%, respectively, compared to the initial values. However, only the aqueous extract was able to significantly lower blood glucose levels, starting from day 9, by 31.7% (P<0.01); and sustain the effect throughout day 12.
Unparallel with its effect on blood glucose levels, streptozotocin caused significant decreases in serum insulin levels as compared to the normal rats (94.7%; P<0.001)(Figure 3B). Only the aqueous extract and metformin were found to cause significant improvement to serum insulin concentrations (79.8% and 91.2%, respectively)when compared to the diabetic control at the end of the study. Of all the three tested samples, the aqueous extract was considered the most potent antidiabetic preparation.
3.4. Antioxidant activities of NPV and its extracts
Figure 4A shows the DPPH radical scavenging activities of NPV and its ethyl acetate and aqueous extracts at various concentrations(0.8-25.0 mg/mL). All the tested samples scavenged the DPPH radical in a concentration–dependent manner, with the activity of the less polar sample being significantly higher than that of the polar sample. The ethyl acetate extract exhibited the highest scavenging activity, followed by NPV and the aqueous extract, with the IC50values being (2.770±0.012)mg/mL, (7.780±0.021)mg/mL,and (17.120±0.053)mg/mL, respectively. Lower IC50values indicated greater scavenging capacities and vice versa. However,the DPPH scavenging activity of the ethyl acetate extract was not comparable to, and was significantly lower than those of the reference compounds, quercetin and butylated hydroxytoluene [IC50= (0.007±0.001)mg/mL, and (0.200±0.007)mg/mL, respectively].
Another effective method to determine an extract’s radical scavenging activity is the ABTS radical cation decolorisation assay. The antioxidant activity was expressed as trolox equivalent antioxidant capacity. Trolox equivalent antioxidant capacity measures the antioxidant strength of a sample based on the standard compound, trolox. Similar to the DPPH assay, all the tested samples exhibited concentration–dependent radical scavenging activities at a concentration range of 0.25 to 4 mg/mL (Figure 4B). The ethyl acetate extract had the highest trolox equivalent antioxidant capacity value [(2.15±0.07)mM], below which came the values of NPV[(0.53±0.03)mM]and the aqueous extract [(0.230±0.001)mM].
As shown in Figure 4C, the reducing power assay showed a pattern of antioxidant strengths identical to the ones revealed by the DPPH and ABTS assays. The trend was as follows: the ethyl acetate extract> NPV > the aqueous extract. The reducing power of NPV and the reducing power of its aqueous extract increased as the concentration of the sample increased (0.25, 0.5, 1, 2 and 4 mg/mL). On the other hand, the ethyl acetate extract, at a concentration of 0.25 mg/mL, had a reducing power (absorbance=0.25)comparable to a concentration of 6.25 mg/mL of quercetin (absorbance=0.27)and higher than that of butylated hydroxytoluene (absorbance = 0.19).
3.5. Phytochemical contents of NPV and its extracts
The total phenolic contents were calculated from the linear regression equation of gallic acid standard calibration curve(y=0.002 2x+0.121 4, R2=0.994 8)and expressed in gallic acid equivalents, whereas total flavonoids were determined using the linear regression equation of rutin standard calibration curve(y=0.004 8x+0.004 7, R2=0.995 6)and expressed in rutin equivalents.Results showed that ethyl acetate extract possessed higher concentration of total phenolics [(21.61±1.34)mg GAE/g extract)and flavonoids [(2.75±0.16)mg RE/g extract]than NPV [phenolics:(7.24±0.82)mg GAE/g extract, flavonoids: (1.53±0.11)mg RE/g extract]and aqueous extract [phenolics: (3.32±0.05)mg GAE/g extract, flavonoids: (0.55±0.08)mg RE/g extract].
The correlation coefficiency (r)between in vitro antioxidant activities (DPPH, ABTS and reducing power)and phenolic compounds of NPV and its extracts was determined. There was a linear positive correlation between these antioxidant parameters(Table 1). Correlation between total phenolic content and antioxidant activities obtained from DPPH, ABTS and reducing power assay were 0.962 4, 0.999 9 and 0.913 9, respectively. Meanwhile for the flavonoid content, the correlation with antioxidant activities assayed via DPPH, ABTS and reducing power tests were 0.999 1, 0.975 3 and 0.795 6, respectively.

Table 1Correlation coefficiencies (r)between different antioxidant activity parameters (DPPH, ABTS, reducing power actitivity)and total phenolic contents or total flavanoid contents of NPV and its extracts.
3.6. Chemical profiling analysis of the active aqueous extract
Aqueous extract which exhibited the strongest anti-diabetic activity was subjected to the chemical profiling analysis using GC-MS. The listed compounds in Table 2 are the compounds with match quality above 90% as compared with NIST02 mass spectral database. The analysis revealed approximately five compounds, three of which were identified as non-phenolic compounds, namely acetic acid at the highest percentage of 35.25%, followed by 2,3 butanediol and 1-(2-butoxyethoxy)ethanol at the respective percentage of 0.75%and 3.86%. In addition, two other compounds were further identified as 5-bromo-2-hydroxybenzaldehyde (0.99%)and 4-aminophenylphenylmethanone (9.74%).
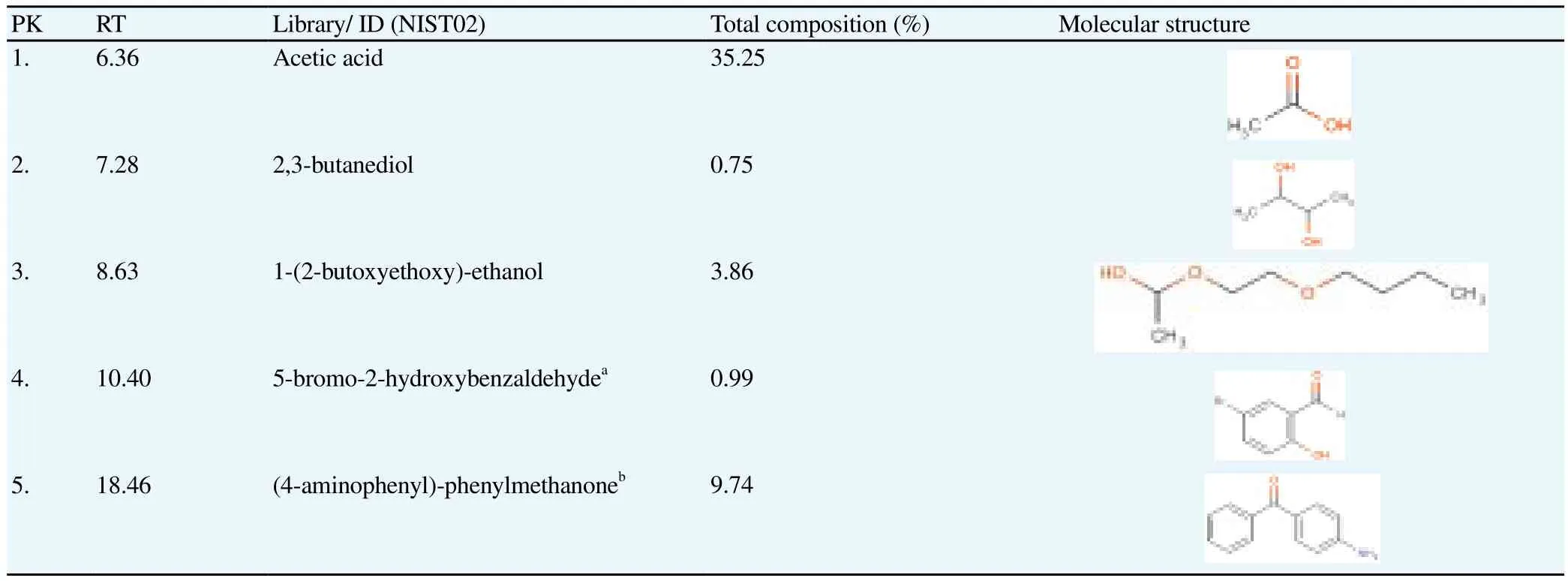
Table 2Chemical profile of the active aqueous extract of NPV.
4. Discussion
Diabetes is strongly co-associated with oxidative stress induction.The use of antioxidants in both the treatment and prevention of diabetes was scrutinized in several studies. However, recent studies reported contrasting findings regarding the benefits of antioxidant therapeutics in the management of diabetes. To aid in unravelling such an uncertainty, the present study was conducted to evaluate the hypoglycaemic activities of NPV and its extracts in normal, glucoseloaded, and streptozotocin-induced diabetic rats. The antioxidant activities of NPV and its extracts were also investigated to examine the possible role of the present antioxidants in the observed antidiabetic activity. In light of the present results, this study showed that single administration of NPV and its extracts to normal rats did not significantly affect fasting blood glucose levels up to 7 h post–dosing, unlike the control drug, glibenclamide. The results suggest that NPV and its extracts are less likely to induce hypoglycaemia in an acute condition, which is a desirable feature for an oral hypoglycaemic drug, especially the one that is consumed as part of the daily dietary intake. Glibenclamide, at a dose of 10 mg/kg b.w.,reduced blood glucose levels to markedly lower levels–bordering on a hypoglycaemic shock after two hours of its administration.Sulfonylureas have been reported to induce hypoglycaemia in euglycaemic rats due to their inherent ability to stimulate the pancreatic β–cells to release insulin[20]. The results suggest that the mechanism of the antidiabetic action of NPV and its extracts may be different from that of sulfonylureas. Similar results were found in the acute study (single administration)in which streptozotocin-induced diabetic rats were used. Except for the control drug metformin, none of the tested samples exerted significant blood glucose lowering effects upon acute intervention. However, in glucose-loaded rats,NPV and its aqueous extract significantly inhibited the rise of blood glucose levels 90 min after glucose loading. It is known that glucose tolerance test indices insulin sensitivity. The results gave a preliminary indicator for the antihyperglycaemic potency of the aqueous extract of NPV. Several medicinal plants have been reported to exert positive effects against diabetes by improving insulin sensitivity; examples of such plants are Aloe vera, Cinnamomum cassia and Mangifera indica[21].
Considering that none of the samples were able to lower the blood glucose levels of the treated groups in the acute study (up to 7 h observation), the administration of NPV, its extracts, and metformin was continued for 12 d to study the effects of their repeated oral administration on blood glucose levels and serum insulin concentrations in streptozotocin-induced diabetic rats. Streptozotocin significantly produced hyperglycaemia, which was accompanied by hypoinsulinemia, in the induced rats. Of all the three tested samples, the aqueous extract caused a significant decrease in blood glucose levels and successfully improved insulin secretion throughout the course of study. These effects were comparable to those of the positive control, metformin. The results suggest that the aqueous extract of NPV could contain hypoglycaemic compounds with the capacity to act long-term, wherein the effectiveness of the compounds may depend on the accumulative effect of the active principles. Similar findings were reported by Peungvicha et al[22]and Jouad et al[23], who reported significant blood glucose lowering effects of Piper sarmentosum and Spergularia purpurea after repeated oral administration. The potential mechanism by which NPV’s aqueous extract could exert its antidiabetic effect could be the stimulation of insulin secretion from existing β–cells within the pancreas[1]and/or enhancing the proliferation or regeneration of pancreatic β–cells following their destruction by streptozotocin[24],which should subsequently increase the secretion of insulin. This hypothesis is strengthened by the significant improvement in the insulin concentration, observed within the rats treated with the aqueous extract as shown in Figure 3b. Improvement in the insulin production may further enhance the glucose uptake and utilization peripherally, as evidenced by the comparable blood glucose lowering effect of aqueous extract to that of metformin. It is established that metformin acts mainly on peripheral glucose utilization[25]and a presence of insulin is needed for this drug’s therapeutic efficacy[26].In order to investigate the contribution of the present antioxidant compounds in the antidiabetic activity of NPV and its extracts, three in vitro antioxidant assays were conducted; DPPH and ABTS free radical scavenging activity assays, and the reducing power assay.All three in vitro antioxidant assays recorded similar antioxidant strengths of the samples, whereby the ethyl acetate extract recorded the most potent antioxidant capacity. A distinctive trend was observed whereby the antioxidant activity of the ethyl acetate extract exceeded that of NPV, which was in turn superior to that of the aqueous extract. Also, the ethyl acetate extract recorded the greatest proportion of phenolic and flavonoid compounds among the tested samples. There have been several reports on the antioxidant activity of vinegars particularly their ethyl acetate extracts[27,28]. Additionally,a number of phenolic compounds have been isolated from the ethyl acetate extracts of some vinegars, namely dihydroferulic acid,dihydrosinapic acid and caffeic acid ethyl ester[29]. Thus, it can be concluded that the major anti-oxidative components are relatively less polar, which appears to be partitioned in the ethyl acetate extract.
As discussed above, the aqueous extract exerted the most potent antidiabetic activity, whilst the ethyl acetate extract showed the most potent antioxidant activity with the highest total phenolic and flavonoid contents. A significant correlation was observed between the antioxidant parameters. These findings further suggested that the antioxidants namely phenolic compounds contained in NPV may not contribute significantly towards the antidiabetic activity of this vinegar. Hence, in agreement with the recent findings, it can be concluded that the observed antidiabetic activity of NPV’s aqueous extract might be due to the role played by the nonphenolic compounds present in the sample. It is unlikely that those antidiabetic bioactives should be identical to those responsible for the in vitro antioxidant activity. Even though previous studies presented evidence that showed phenolic compounds like tannins and flavonoids were responsible for the hypoglycaemic activities of medicinal plants[30,24], the significant findings in the present study indicated that this may not be applicable to all medicinal plants and their biologically diverse products. The findings are consistent with earlier reports which demonstrated that samples with high phenolic contents did not always confer high antidiabetic activities[31].
Interestingly, further chemical profiling analysis of the active aqueous extract of NPV revealed the presence of non-phenolic constituent, namely acetic acid (35.25%), which appeared to be the main active constituent of NPV’s aqueous extract, together with 2,3-butanediol and 5-bromo-2-hydroxybenzaldehyde, which respectively accounted for 0.75% and 0.99% of the total composition of the extract. Acetic acid has been demonstrated to play a significant role in the observed antidiabetic activity of many types of vinegars by altering specific metabolic processes in the liver and the gastrointestinal tract[9,32]. A considerable number of studies have focused on butanediol, mainly due to its role as a hypoglycaemic agent[33]. 5-bromo-2-hydroxybenzaldehyde (0.99%)also known as 5-bromosalicylaldehyde, a derivative of vanillin, has been proven to have promising therapeutic effects in the treatment of diabetes due to its ability to inhibit glucose hepatic production and to slow down digestion and absorption of dietary carbohydrates by inhibiting the activity of -glucosidase[34,35]. From the chemical profile of NPV’s aqueous extract, it can be concluded that its observed antidiabetic activity is due to the synergistic action of those compounds. Yet, the prominent role is attributable to acetic acid.
Conclusively, the study reported herein has shown that aqueous extract of NPV possessed antihyperglycaemic activities comparable to the standard antidiabetic drug, metformin, while the ethyl acetate extract precipitated significant antioxidant effects attributable to its high phenolic content. These findings suggest that the antioxidant compounds of NPV do not contribute much towards the overall observed antidiabetic effect. This undermines the general presumption of antioxidant compounds, in other medical plants, playing major physiological roles. Further pharmacological investigation is underway to elucidate the mechanisms underlying the antidiabetic activity of NPV’s aqueous extract.
Conflict of interest statement
We declare that we have no conflict of interest.
This work is financially supported by Research University Grant of Universiti Sains Malaysia (1001/PFARMASI/815080).
[1]Arunachalam K, Parimelazhagan T. Antidiabetic activity of Ficus amplissima Smith. bark extract in streptozotocin induced diabetic rats. J Ethnopharmacol 2013; 147(2): 302-310.
[2]Sarma AD, Mallick AR, Ghosh A. Free radicals and their role in different clinical conditions: an overview. Int J Pharm Sci Res 2010; 1(13): 185-192.
[3]Pitocco D, Martini F, Zaccardi F, Ghirlanda G, Bagchi D, Sreejayan N.Chapter 24 - antioxidants, healthy diet, and diabetes: Oxidative stress and nutrition. In: Diabetes nutritional and therapeutic interventions for diabetes and metabolic syndrome. San Diego: Academic Press; 2012, p. 299-313.
[4]Garg MC, Bansal DD. Protective antioxidant effect of vitamins C and E in streptozotocin induced diabetic rats. Indian J Exp Biol 2000; 38(2):101-104.
[5]Bursell S-E, King GL. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res Clin Pr 1999; 45(2): 169-182.
[6]Logani M, Davies R. Lipid oxidation: biologic effects and antioxidants –a review. Lipids 1980; 15(6): 485-495.
[7]Myers RL. The 100 most important chemical compounds: a reference guide.1st ed. CT: Greenwood Press; 2007.
[8]O' Keefe JH, Gheewala NM, O' Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol 2008; 51(3): 249-255.
[9]Kondo S, Tayama K, Tsukamoto Y, Ikeda K, Yamori Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats.Biosci, Biotechnol, Biochem 2001; 65(12): 2690-2694.
[10]Budak HN, Guzel-Seydim ZB. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J Sci Food Agric 2010; 90(12): 2021-2026.
[11]Tsujihata S, Entani E, Asai M, Tsukamoto Y, Ohta M. Mathematical modeling to predict the bactericidal effect of processed vinegar on Escherichia coli O157: H7. Int J Food Microbiol 1998; 43(1): 135-138.
[12]Lim S, Yoon JW, Choi SH, Cho BJ, Kim JT, Chang HS, et al. Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin-resistant rat model. Metabolism 2009; 58(1): 8-15.
[13]Qiu J, Ren C, Fan J, Li Z. Antioxidant activities of aged oat vinegar in vitro and in mouse serum and liver. J Sci Food Agric 2010; 90(11): 1951-1958.
[14]Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM)rats. J Ethnopharmacol 2012; 142(1):65-71.
[15]Dimo T, Ngueguim F, Kamtchouing P, Dongo E, Tan P. Glucose lowering efficacy of the aqueous stem bark extract of Trema orientalis (Linn)Blume in normal and streptozotocin diabetic rats. Pharmazie - Int J Pharm Sci 2006; 61(3): 233-236.
[16]Yam M, Sadikun A, Asmawi M. Antioxidant potential of Gynura procumbens. Pharm Biol 2008; 46(9): 616-625.
[17]Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9): 1231-1237.
[18]Atangwho IJ, Egbung GE, Ahmad M, Yam MF, Asmawi MZ. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del.growing in Malaysia. Food Chem 2013; 141(4): 3428-3234.
[19]Brimson JM, Brimson SJ, Brimson CA, Rakkhitawatthana V, Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-? neurotoxicity in HT-22 Mouse hippocampal cells: possible active compounds include lupeol, stigmasterol and ? -sitosterol. Int J Mol Sci 2012; 13(4): 5074-5097.
[20]Pepato M, Keller E, Baviera A, Kettelhut I, Vendramini R, Brunetti I.Anti-diabetic activity of Bauhinia forficata decoction in streptozotocindiabetic rats. J Ethnopharmacol 2002; 81(2): 191-197.
[21]Eddouks M, Bidi A, El Bouhali B, Hajji L, Zeggwagh NA. Antidiabetic plants improving insulin sensitivity. J Pharm Pharmacol 2014. 66(9);1197-1214.
[22]Peungvicha P, S Thirawarapan S, Temsiririrkkul R, Watanabe H, Kumar Prasain J, Kadota S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J Ethnopharmacol 1998; 60(1): 27-32.
[23]Jouad H, Eddouks M, Lacaille-Dubois MA, Lyoussi B. Hypoglycaemic effect of Spergularia purpurea in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol 2000; 71(1-2): 169-177.
[24]Florence NT, Benoit MZ, Jonas K, Alexandra T, Desire DDP, Pierre K, et al. Antidiabetic and antioxidant effects of Annona muricata(Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 2013; 151(2):784-790.
[25]Klip A, Leiter LA. Cellular mechanism of action of metformin. Diabetes care 1990; 13(6): 696-704.
[26]Bailey CJ. Antidiabetic Drugs other than Insulin. In: Offermanns S,Rosenthal W, editors. Encyclopedia of molecular pharmacology. 2nd ed.New York: Springer; 2008, p. 116-125.
[27]Kawano K, Morimura S, Mori E, Matsushita H, Ohta H, Kida K.Isolation and identification by cytoprotection assay of antioxidative compound contained in vinegar produced from sweet potato-shochu postdistillation slurry. Food Sci Tech Res 2010; 16(4): 327-332.
[28]Nishidai S, Nakamura Y, Torikai K, Yamamoto M, Ishihara N, Mori H, et al. Kurosu, a traditional vinegar produced from unpolished rice,suppresses lipid peroxidation in vitro and in mouse skin. Biosci Biotechnol Biochem 2000; 64(9):1909-1914.
[29]Shimoji Y, Tamura Y, Nakamura Y, Nanda K, Nishidai S, Nishikawa Y, et al. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar). J Agric Food Chem 2002;50(22): 6501-6503.
[30]Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol 2002;81(2): 155-160.
[31]Mccue P, Kwon YI, Shetty K. Anti-amylase, anti-glucosidase and antiangiotensin I-converting enzyme potential of selected foods. J Food Biochem 2005; 29(3): 278-294.
[32]Johnston CS. Chapter 22 - Medicinal uses of vinegar. In: Watson RR,editor. Complementary and alternative therapies and the aging population.San Diego: Academic Press; 2009, p. 433-443.
[33]Meenakshi C, Latha Kumari K, Shyamala Devi C. Biochemical Studies on the effects of S-1, 3-butanediol of diabetes induced rats. Indian J Physiol Pharmacol 1995; 39(2): 145-148.
[34]Hashimoto J, Motohashi K, Sakamoto K, Hashimoto S, Yamanouchi M,Tanaka H, et al. Screening and evaluation of new inhibitors of hepatic glucose production. J Antibiot 2009; 62(11): 625-629.
[35]Misra S, Pandeya KB, Tiwari AK, Ali AZ, Saradamani T, Agawane SB,et al. Antihyperglycemic, alpha-glucosidase inhibitory and DPPH free radical scavenging activity of 5-bromosalicylaldehyde and schiff bases.Med Chem Res 2011; 20(9): 1431-1437.
 Asian Pacific Journal of Tropical Medicine2015年8期
Asian Pacific Journal of Tropical Medicine2015年8期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of Yupingfeng granules on HA and Foxp3+ Treg expression in patients with nasopharyngeal carcinoma
- Rifabutin reduces systemic exposure of an antimalarial drug 97/78 upon co- administration in rats: an in-vivo & in-vitro analysis
- Analysis of good practice of Public Health Emergency Operations Centers
- Effect of Yupingfeng granules on HA and Foxp3+ Treg expression in patients with nasopharyngeal carcinoma
- Antitumor effect of recombinant human endostatin combined with cisplatin on rats with transplanted Lewis lung cancer
- Late cardioprotection of exercise preconditioning against exhaustive exercise-induced myocardial injury by up-regulatation of connexin 43 expression in rat hearts
