Development,characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug
Poovi Ganesan*,Rajpriyadarsini Soundararajan,Uma Shanmugam, Vinothini Ramu
Department of Pharmaceutics,College of Pharmacy,Mother Theresa Post Graduate and Research Institute of Health Sciences(A Government of Puducherry Institution),Puducherry 605006,India
Development,characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug
Poovi Ganesan*,Rajpriyadarsini Soundararajan,Uma Shanmugam, Vinothini Ramu
Department of Pharmaceutics,College of Pharmacy,Mother Theresa Post Graduate and Research Institute of Health Sciences(A Government of Puducherry Institution),Puducherry 605006,India
ARTICLEINFO
Article history:
Received 19 March 2015
Received in revised form 11 May 2015
Accepted 19 May 2015
Available online 14 July 2015
Hydrochlorothiazide
Ethyl cellulose
Hydroxypropyl methylcellulose
Second generation solid dispersion
Microsphere
Solvent evaporation method
The poor dissolution characteristics of water-insoluble drugs are a major challenge for pharmaceutical scientists.Reduction of the particle size/increase in the surface area of the drug is a widely used and relatively simple method for increasing dissolution rates.The objective of this study was to improve solubility,release and comparability of dissolution of a poorly soluble drug using two different types of formulations(solid dispersions and microspheres).Hydrochlorothiazide was used as a model drug.The solid dispersions and microspheres were prepared by solvent evaporation method using ethyl cellulose, hydroxypropyl methylcellulose in different drug-to-carrier ratios(1:1,1:2 w:w).The prepared formulations were evaluated for interaction study by Fourier transform infrared spectroscopy,differential scanning calorimetry,percentage of practical yield,drug loading, surface morphology by scanning electron microscopy,optical microscopy and in-vitro release studies.The results showed no interaction between the drug and polymer,amorphous state of solid dispersions and microspheres,percentage yield of 42.53%to 78.10%,drug content of 99.60%to 99.64%,good spherical appearance in formulation VI and significant increase in the dissolution rate.
?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Among all newly discovered chemical entities about 40%of drugs are lipophilic and fail to reach the market due to their poor aqueous solubility[1].For orally administered drugs solubility is one of the rate limiting parameters to achieve their desired concentration in the systemic circulation in pharmacological response[2].According to the equation of Noyes and Whitney,this may be achieved by reduction of the particle size/ increase in the surface area of the drug which is accessible for the dissolution medium and an enhancement of its solubility in addition to a relatively simple method for increasing dissolution rates[3].However,altering the drug particle itself carries obvious limitations which are inadequate for enhancement of bioavailability.Therefore,additional physical changes, including control of drug release from their formulations should be taken into consideration[4].Moreover,there are two key strategies to alter the release and subsequent absorption of drugs:one is based on a modification of the drug,and the other is based on a modification of the dosage form as a new drug delivery system[5].
The new drug delivery systems are having an edge over conventional ones in terms of many biopharmaceutical parameters;among such drug delivery systems are controlled/ prolonged release solid dispersion[6,7]and micro particles/ microsphere[8,9].These systems can achieve therapeutically effective concentration of the drug in the systemic circulation over an extended period of time with better patient compliance[10,11].Water insoluble carriers are generally used to produce a controlled release formulation.The properties of the carriers have major influences on the release profile of the dispersed drug,specifically the second generation carriers.These carriers include ethyl cellulose,hydroxypropyl cellulose,hydroxypropyl methylcellulose,cellulose acetate phthalate,ethyl acetate,Chitosan,and methacrylic acid copolymers[12].
In order to investigate the effect of second generation polymers on the dissolution release mechanism of poorly soluble drugs from solid dispersions(SD)and microspheres (MS),hydrochlorothiazide[(6-chloro-1,1-dioxo-3,4-dihydro-2H-1,2,4-benzothiadiazine-7 sulfonamide)(HCT),a poorly water soluble drug(0.7 mg/ml)]was used as a model drug for these purposes.The HCT is a potent diuretic which inhibits the kidney’s ability to retain water.It is widely used in the management of hypertension in combination with cardiovascular drugs.It is a white or nearly white,almost odorless, crystalline powder and has a slightly bitter taste.Hydrochlorothiazide is considered as a class IV drug according to the BCS.It has low and variable oral bioavailability which is attributed to poor solubility,slow dissolution and poor membrane permeability[13].Hydrochlorothiazide is absorbed from the GI tract and apparently not metabolized and excreted unchanged in urine.At least 61%of the drug is reportedly eliminated from the body when excretion is essentially completed within 24 h post administration.The oral bioavailability of the drug was reported to be 60-80%of the administered dose[14].In this study,two different types of formulations such as solid dispersions and microspheres were prepared by solvent evaporation method using ethyl cellulose(EC)and hydroxylpropyl methylcellulose(HPMC)in different drug-to-carrier ratios(1:1,1:2 w:w).These preparations may release the maximum amount of the drug for controlling/prolonged period of time and it may also increase the residence time;this in turn may increase the bioavailability when compared to conventional drug multiple dosing regimen.
2. Materials and methods
2.1. Materials
Hydrochlorothiazide was obtained from IPCA Laboratories Ltd.(Mumbai,India).Hyroxypropyl methylcellulose was purchased from Colorcon,Mumbai.Ethylcellulose and Poly vinyl alcohol(PVA)were procured from Sigma-Aldrich, Germany.All other chemicals were of analytical reagent grade.
2.2. Preparations of solid dispersions by solvent evaporation method
The physical mixture of the drug and water soluble carrier were dissolved in 20 ml of common solvent(5%acetic acid for FI, FII and Acetone for FIII,FIV)and the resulting clear solution is rapidly heated for evaporating the solvent and to get a glassy solid mass.The obtained solid mass was transferred onto aluminum plates and the solvent was left to evaporate in open air for 2 days.After complete removal of the solvent the solid dispersions were granulated and stored at 25°C in desiccators[11,15].
2.3. Preparations of microspheres by emulsion solvent evaporation method
In this technique the drug is dissolved in a polymer which was previously dissolved in 50 ml of solvent and the resulting solution is added to aqueous phase containing 5 ml of 1%PVA as stabilizing agent.The above mixture was agitated at 500 rpm, then the drug and polymer(EC&HPMC)were transformed into fine droplet which solidified into rigid microspheres by solvent evaporation and then collected by filtration and washed with de-mineralized water and desiccated at room temperature for 24 h[16].
Different concentrations and ratios of polymers used in the formulation of solid dispersion and microspheres are mentioned in Table 1.
2.4. Analytical method for drug concentration measurements(UV method)
The ultraviolet spectrophotometric method was selected in the present study for the estimation of hydrochlorothiazide.The drug solution[20μl/ml in 0.1M HCl]was scanned in between the wavelength of 400-200 nm.The wavelength of 273 nm was selected and utilized for further quantitative analysis.
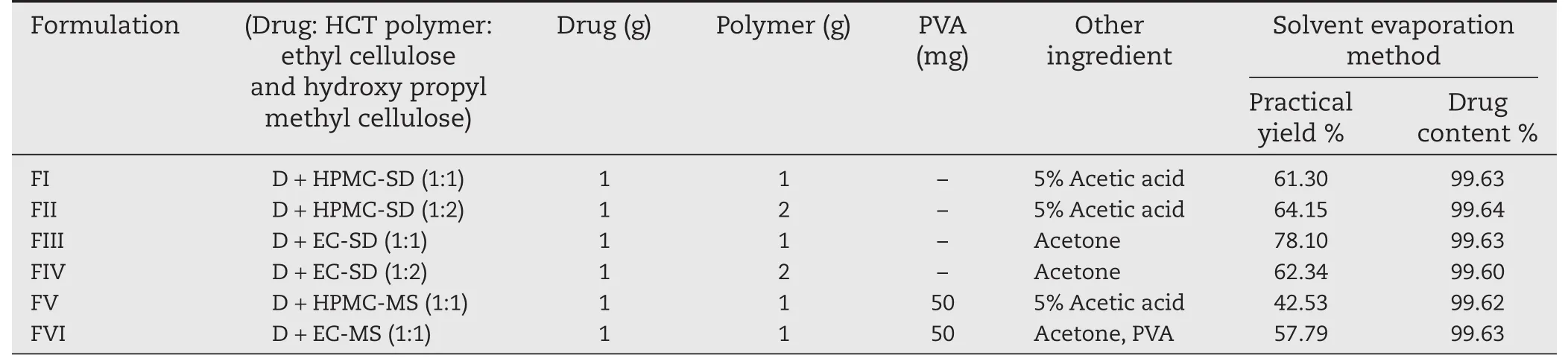
Table 1-Composition of formulation and effect of percentage of recovery,drug content.
2.5. Standard plot of hydrochlorothiazide in 0.1 M hydrochloric acid buffer
Weighed quantity of hydrochlorothiazide(25 mg)was dissolved and the volume made up to 25 ml with 0.1M HCl buffer pH 1.2 to give a concentration of 1000μg/ml.From this stock solution different volumes were transferred into 10 ml volumetric flasks and volume was made up to 10 ml with 0.1M HCl buffer pH 1.2 to get different concentrations ranging from 1 to 15μg/ml concentrations.The absorbance was measured at 273 nm against a blank using UV spectrophotometer.
2.6. Percentage practical yield
Percentage practical yield is calculated to determine percent yield or efficiency of any method,thus it is helpful in the selection of appropriate methods of production.Solid dispersions/ microspheres were collected and weighed to determine practical yield(PY)from the following equation[17]:
2.7. FT-infrared spectroscopy
Infrared spectroscopy was conducted using an Avatac 320-FT IR spectrophotometer and the spectrum was recorded in the region of 4000-400 cm-1.The procedure consisted of dispersing a sample(drug,solid dispersions and microspheres)in KBR (200-400 mg)and compressing into the discs by applying a pressure of 5 tons for 5 min in a hydraulic press.The pellet was placed in the light path and the spectrum was obtained.
2.8. Differential scanning calorimetry
Differential scanning calorimetry(DSC)measurements were carried out on a Modulated DSC V1.1A TA instrument 2000 (Japan)equipped with a thermal analysis data system(TA instrument).The instrument was calibrated using indium(156°C), tin(232°C)and zinc(419.5°C)as internal standards.Samples of 2-10 mg were placed in aluminum pans(Al-Crucibles,40 Al) and sealed.The probes were heated from 25 to 400°C at a rate of 10 K/min under nitrogen atmosphere.
2.9. Drug content
Solid dispersions and microspheres equivalent to 10 mg of hydrochlorothiazide were weighed accurately and dissolved in 10 ml of methanol.The solution was filtered,diluted suitably and drug content was analyzed at 273 nm by UV spectrophotometer[18].The actual drug content was calculated using the following equation as follows:
2.10. Scanning electron microscopy
The shape and surface morphology of the microspheres was examined using Scanning Electron Microscopy(SEM)(JSMT20,Tokyo,Japan).An appropriate sample of microspheres was mounted on metal stubs,using double-sided adhesive tapes. Samples were gold coated and observed for morphology,at an acceleration voltage of 15 kV.
2.11. Optical microscopy
The growth of CBZ crystals in water from the various solid dispersions was observed using a light microscope(Nikon Inc., Melville,NY)and the photos were captured by digital camera (Sony Electronics Inc.,Japan).
2.12. In vitro release study
The dissolution studies on pure drug,tablets and solid dispersions/microspheres(equivalent to 10 mg of drug)were performed using dissolution test apparatus.The condition of dissolution test was as follows:medium-900 ml 0.1M HCl (pH 1.2);speed-100 rpm;temperature-37±0.5°C;apparatus-USP type II rotating paddle.During the dissolution study, 10 ml aliquot was withdrawn at different time intervals from 5 to 110 min and replaced with an equal volume of fresh medium.The withdrawn samples were filtered through Whatman filter paper no.42 and absorbance was measured at 273 nm against 0.1M HCl blank.
3. Results and discussion
3.1. Standard plot of hydrochlorothiazide in 0.1 M hydrochloric acid buffer
The Standard plot of HCT was prepared in 0.1 M Hydrochloric acid buffer,pH 1.2 and the ultraviolet spectrophotometric method was used to analyze HCT at the wavelength of 273 nm which revealed good linearity in the solution systems in the concentration range of 1-15μg/ml(R2=0.9999)(Fig.1).
3.2. FT-infrared spectroscopy
The FTIR spectrum of pure HCT exhibited presence of characteristic peaks which included peaks at 3356.05 cm-1, 3261.43 cm-1,3161.75 cm-1for NH-stretching,peak at 1596.04 cm-1for stretching of the C=C aromatic ring,peak at 1317.12 cm-1for C=N stretching,peak at 1239.93 cm-1for SO2stretching.The FTIR study did not show any additional peak, significant shift and disappearance of characteristic peaks in all the formulations but most of the peaks of drug were present. This confirms the absence of any physical interaction between drugs and polymers(Fig.2).The differences in transmittance may be due to varying concentration of drug.
3.3. Differential scanning calorimetry
Fig.3 showed the DSC thermogram of pure drug,polymer (HPMC,EC)and different dosage form of solid dispersions, microspheres.The DSC thermogram of HCT exhibited two thermal events,one at about 270°C and the second in 340°C that could be associated to the HCT melting point and thermal decomposition,respectively[19].There was no peak detected in the HCT dispersed in HPMC and EC solid dispersions(FII, FIII).These results suggested that the crystallinity of HCT in solid dispersions disappeared.The lack of a peak in the HCT encapsulated in HPMC and EC microspheres indicated that the drug was present in a more amorphous state than in crystalline form.From the result it was confirmed that HCT was crystalline but solid dispersions and microspheres were noncrystalline and amorphous in the state.
3.4. Percentage practical yield and drug content
In all formulations,the drug content was found to be between 99.60%and 99.64%and the practical yield was found to be between 42.53%and 78.10%.All the formulation of different ratio showed the presence of high drug content and low practical yield(Table 1)which indicates that the drug is uniformly dispersed in the powder and well loaded in the sphere formulation.The high drug content or drug loading is the function of the characteristics of polymer,drug,surfactant and crosslinking agent,etc.Since the drug is hydrophobic in nature,there was less chance of diffusion of drug away from the polymer network during preparation[20,21].The product yield depended upon the agglomeration and sticking of polymer to blades of stirrer and to the wall of the beaker during microsphere formation.The product yield was also found to be dependent on the choice of the polymer and its viscosity. The decrease in yield of the microspheres containing HPMC polymer than EC polymer may be due to migration of HPMC into continuous phase forming agglomerates accompanied with sticking of the polymer to the stirrer blade,beaker surface as well as during filtration of microspheres[22].In addition during evaporation of solvent,the drug may diffuse out of microsphere together with volatile nature of solvent before the droplet of microsphere solidification,leading to a low product yield,and also to either low drug content or low entrapment efficiency which depends on the choice of the polymer,solvent,drug,processing parameter,etc.[23,24],.Therefore,the method solvent evaporation used in this study appears to be suitable for formulation III when compared to all other formulations.
3.5. Morphological characterization of polymeric microspheres
Fig.4a and 4b,showed the morphology of HPMC and EC microspheres.The surfaces of microparticles depends on(1) a saturated solution of polymer producing smooth and high yield microparticles.The undissolved polymer produced irregular and rod shaped particles.(2)The diffusion rate of solvent is too fast and the solvent may diffuse into the aqueous phase before stable microparticles are developed and formed,causing the aggregation of microparticle preparation[25,26].Among the two polymers,the HPMC possessed sparing soluble characteristics.There was less chance of formation of smooth and high yield microspheres using the HPMC polymer,because a portion of the HPMC polymer solution aggregated in a fiberlike structure,as it solidified prior to forming microspheres. Hence,in FV the SEM pictures showed relatively smooth spherical shaped and fiber-like structure(Fig.4a).EC possessed good solubility property in acetone.There was a high chance of formation of smooth and high yield microspheres using the EC polymer[10].Due to a saturated solution of polymer and fast diffusion rate of solvent it showed the smooth spherical shape and high yield microspheres(Fig.4b).A similar finding was reported by Dhanalekshmi et al.[8,10].Due to the solubility and diffusion rate among the natural polymers,the FVI showed good spherical appearance.
Fig.5a-e shows the morphology of pure drug,HPMC and EC solid dispersion,examined by optical microscopy.The pure drug appeared in the form of irregular crystal particles whereas in the case of all the solid dispersion formulations of HCT particles were in almost amorphous form,which indicates a reduction in particle size.These observations provide the evidence of solid dispersion formation.
3.6. In-vitro release study
In this study the effect of the release profile of poorly water soluble drug like HCT from a different dosage form of solid dispersion,microsphere,tablet and pure drug was studied using EC and HPMC as polymers for the formulations.Dissolution profiles of pure drug and different dosage form of solid dispersion,microsphere and tablet after 110 min is shown in Fig.6. It revealed that all formulations underwent an initial linear release phase followed by equilibration.In addition,it can be clearly observed that the dissolution rate of pure drug was low because 53.4%of drug dissolved in 110 min and there was no change observed in its solubility and drug release when increasing time which revealed its intrinsic solubility property.
In the case of other formulations,there was a marked increase in the dissolution rate of HCT,when compared to pure HCT which exhibited that molecular dispersion and size reduction of coarse particle into colloidal particle increase the surface area and wettability,thereby increasing drug release. The dissolution rate of drug after 110 min for formulation T, FI,FII,FIII,FIV,FV and FVI was found to be 62.77%,99.03%, 83.37%,80.59%,97.62%,72.25%and 76.52%respectively.The decrease in drug release in the tablet when compared to formulation FI,FII,FIII,FIV,FV and FVI may be due to no reduction in particle size by their preparation technique and added excipients didn’t have much influence in their solubility which showed the relatively same release profile of pure drug.But the slight increase in drug release in tablet than the pure drug may be due to the addition of a wetting agent such as sodium lauryl sulfate,sodium di-isobutyl sulfosuccinate.
In the case of FI and FII,the rapid release of the drug was shown in FI and controlled release was shown in FII.The results revealed that the release rate decreased as the concentration of HPMC increased.At higher polymer loading,the viscosity of the gel matrix is increased which results in a decrease in the effective diffusion coefficient of the drug[27].This indicates that the drug/polymer ratio is an important factor affecting the rate of release of drugs from HPMC matrices. Factors that may contribute to differences in drug dissolution profile as a function of changes in the total polymerconcentration include differences in water penetration rate, water absorption capacity and polymer swelling[15]. Moreover,this may be due to increase in viscosity which will increase the particle size and decrease the surface area.Increase in viscosity may also increase the diffusional path length, which might also be the reason for reduction in drug release. In contrast,in the case of FIII and FIV,the increase in drug release was shown in FIV which may attribute to increased penetration of the solvent molecules in the presence of the hydrophobic polymer,leading to increased diffusion of the drug from the matrix,showing a complete polymer saturation solution.
In the case of FV and FVI,the increased and controlled release was observed in FVI which may be due to the presence of suitability of carrier(EC,PVA)and the percentage of PVA which may prevent aggregation of fine drug particles, thereby providing a larger surface area for dissolution.The wetting properties are also greatly increased due to the surfactant property of the polymer(PVA),resulting in decreased interfacial tension between the medium and the drug,hence the higher dissolution rates.The presence of PVA polymers also inhibits crystal growth of the drug which facilitates faster dissolution.From the SEM image result,it was evident that the addition of PVA is a suitable stabilizer to prevent aggregation and keep the product uniform in size and shape in FVI,thereby providing a larger surface area for dissolution. But in the case of FV,the concentration of polymer(HPMC) and the percentage of PVA are not suitable for controlling the release of the drug and the SEM image itself shows irregular shape and size which may affect the viscosity,thereby retarding drug release.From this release study,it was evident that the PVA stabilizer even possesses good inherent properties, when combined with two different natures of polymer(HPMC -natural polymer,EC-semi synthetic polymer)in microsphere preparation which alters drug release.In addition,due to the high viscous nature of HPMC polymer than EC polymer, it increases the diffusion path length,which might also be the reason for reduction in drug release.Hence FV showed controlled low percentage of drug release and FVI showed controlled high percentage of drug release.Besides,it can be clearly observed that the dissolution/release rate of drug was higher in all the solid dispersion formulations(FI,FII,FIII,FIV was 99.03%,83.37%,80.59%and 97.62%respectively)than microsphere formulations(FV,FVI was 72.25%and 76.52% respectively).
Solid dispersions and microspheres were highly in amorphous form(Figs.3-5),in contrast to pure HCT,which contributed to an increase of dissolution/release of drug in a prolonged and controlled manner,not in the immediate manner.Prolonged and controlled release of HCT was achieved with the preparation of solid dispersions and microsphere using a solvent evaporation method in the FII,FIII,FV,and FVI at the 20 min onwards,which may be due to using of second generation polymer(EC and HPMC),but which cannot be seen using first generation polymer where rapid release of the drug occurs when increasing time.Besides,the immediate and prolonged release profiles of solid dispersions can be attributed to the dispersion of drug in the polymer matrices of FI and FIV which may be due to increased wettability,improved dispersibility of drug particles,and existence of the drug in amorphous form with improved solubility and absence of aggregation of drug particles.But in the case of FV and FVI,the second generation polymer was actively involved in the controlled release of drug from the microsphere which may be due to the presence of the drug as core within the rate controlling polymer(reservoir type),wherein the solid dispersion drug is homogeneously dispersed in the rate controlling polymer (monolithic).
Kim et al.and Vilhelmsen et al.reported that the second generation solid dispersions were made using amorphous carriers,which are mostly polymers[4,28],whereas the first generation solid dispersions were made using crystalline carriers.These form thermodynamically stable crystalline solid dispersions[16].In the 1960s,it was reported that amorphous solid dispersions were more effective than crystalline solid dispersions due to their thermodynamic stability [29,30].Also Lloyd et al.and Pokharkar et al.demonstrated that drugs with low water solubility have higher solubility when they are in amorphous state rather than in crystalline state [31,32].Theoretically,a certain amount of energy is demanded for breaking up the crystal lattice during the dissolution process if the drug is in its crystal state[4].However,amorphous drugs do not need such energy[33],making the drug more easily released[34].This improved drug release rate ultimately promotes drug’s bioavailability,making solid dispersions more ideal for administrating hydrophobic oral drugs[4].
From the result,it was evident that using second generation polymer and its different percentage of concentration in solid dispersion is an advanced approach for immediate and prolonged release of poorly soluble drug than by using first generation polymer in solid dispersion which has immediate release.Moreover,in optimized condition/using third generation polymer(which include additional surface active properties e.g.inulin,inutec SP1,compritol 888 ATO,gelucire 44/14, poloxamer 407,etc.,)in the preparation of solid dispersion may be an advance technology for controlled release of poorly soluble drug than microsphere.Also,due to its easy preparation,solid dispersion would be one of the exciting frontiers of controlled release drug delivery systems[18,35].Kim et al. reported some commercial applications of solid dispersion formulation using second generation polymer which is shown in Table 2[4].
4. Conclusion
The present study was conducted to improve and compare the dissolution of HCT using two different types of formulations (solid dispersions and microspheres)by a solvent evaporation method with different ratios of HPMC and EC.From the result,it was clear and evident that even the solid dispersion (SD)technique and microsphere(MS)technique had improved the dissolution rate of drug to a great extent,the percentage of the dissolution/release rate was higher in solid dispersion formulation than microsphere formulation.Finally, it could be concluded that with future development of this technology,the solid dispersions have tremendous potential in the area of controlled release dosage form design such asmicrospheres because of the wide availability of a variety of carriers and it would continue to enable novel applications in drug delivery and solve problems associated with the delivery of poorly soluble drugs.
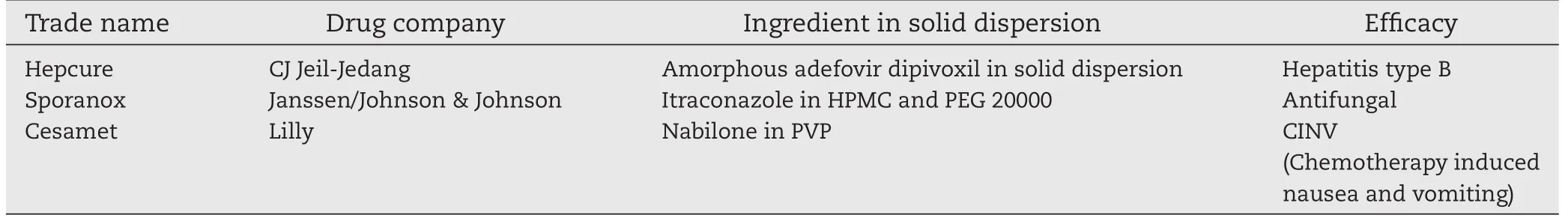
Table 2-Examples of commercial applications of solid dispersion formulation.
REFERENCES
[1]Lipinski CA,Lombardo F,Dominy BW,et al.Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001;46:3-26.
[2]Mahesh I Limbachiya NT.Techniques solubility enhancement poorly soluble drugs:a review.IJPRD 2012;4(04):71-86.
[3]Nora AU,Bernhard CL.Solid dispersions of nimodipine and polyethylene glycol 2000:dissolution properties and physico-chemical characterization.Eur J Pharm Biopharm 2005;59:107-118.
[4]Kim KT,Lee JY,Lee MY,et al.Solid dispersions as a drug delivery system.J Pharm Investig 2011;41(3):125-142.
[5]Lackman L.Teoria e pratica na industria farmaceutica. 1st ed.Lisboa:Funda?ao Calouste Gulbenkian;2001.p.737-738.
[6]Tran HTT,Park JB,Hong KH,et al.Preparation and characterization of pH-independent sustained release tablet containing solid dispersion granules of a poorly water soluble drug.Int J Pharm 2011;415(1-2):83-88.
[7]Kim HJ,Lee SH,Lim EA,et al.Formulation optimization of solid dispersion of mosapride hydrochloride.Arch Pharm Res 2011;34(9):1467-1475.
[8]Dhanalekshmi UM,Poovi G,Kishore N,et al.In-vitro characterization and in-vivo toxicity study of repaglinide loaded poly(methyl methacrylate)nanoparticles.Int J Pharm 2010;396:194-203.
[9]Vasconcelos T.Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs.Drug Discov Today 2007;12:23-25.
[10]Dhanalekshmi UM,Poovi G,Reddy NP.In-vitro observation of repaglinide engineered polymeric nanoparticles.Dig J Nanomater Bios 2012;7(1):1-18.
[11]Poovi G,Dhanalekshmi UM,Narayanan N,et al.Preparation and characterization of repaglinide loaded chitosan polymeric nanoparticles.Res J Nanosci Nanotechnol 2011;1(1):12-24.
[12]Giri TK,Kumar K,Alexander A,et al.A novel and alternative approach to controlled release drug delivery system based on solid dispersion technique.Bull Fac Pharm Cairo Univ 2012;50:147-159.
[13]Patel RB,Patel UR,Rogge MC,et al.Bioavailability of hydrochlorothiazide from tablets and suspensions.J Pharm Sci 1984;73:35-361.
[14]Sietsema WK.The absolute oral bioavailability of selected drugs.Int J Clin Pharmcol Ther Toxicol 1989;27:179-211.
[15]Wan LS,Heng PW,Wong LF.Relationship between swelling and drug release in a hydrophilic matrix.Drug Dev Ind Pharm 1993;19:1201-1210.
[16]Nighute AB,Bhise SB.Preparation and evaluation of rifabutin loaded polymeric microspheres.Res J Pharm Technol 2009;2(2):371-374.
[17]Konno H,Handa T,Alonzo DE,et al.Effect of polymer type on the dissolution profile of amorphous solid dispersion containing felodipine.Eur J Pharm Biopharm 2008;70:493-499.
[18]Vasconcelos TF,Sarmento B,Costa P.Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs.Drug Discov Today 2007;12:1068-1075.
[19]Menon D,El-Ries M,Alexander KS,et al.A thermal analysis study of the decomposition of hydrochlorothiazide.Instrum Sci Technol 2002;30:329-340.
[20]Dhanaraju MD,Elizabeth S,Poovi G.Dexamethasone release from glutaraldehyde cross-linking chitosan microspheres:in vitro/in vivo studies and non-clinical parameters response in rat arthritic model.J Pharm Investig 2011;41(5):279-288.
[21]Blanco MD,Gomez C,Olmo R,et al.Chitosan microspheres in PLG films as devices for cytarabine release.Int J Pharm 2000;202:29-39.
[22]Jagtap YM,Bhujbal RK,Ranade AN,et al.Effect of various polymers concentrations on physicochemical properties of floating microspheres.Indian J Pharm Sci 2012;74(6):512-520.
[23]Phutane P,Shidhaye S,Lotlikar V,et al.In vitro evaluation of novel sustained release microspheres of glipizide prepared by the emulsion solvent diffusion-evaporation method. J Young Pharm 2010;2(1):35-41.
[24]British Pharmacopoeia Commission.British Pharmacopoeia, vol.1.London:2003.p.874-875.
[25]Chiou W,Riegelman S.Pharmaceutical applications of solid dispersion systems.Pharm Acta Helv 1971;60:1281-1301.
[26]Jain SK,Awasthi AM,Jain NK,et al.Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery:preparation and in vitro characterization.J Control Release 2005;107:300-309.
[27]Skoug JW,Mikelsons MV,Vigneron CN,et al.Qualitative evaluation of the mechanism of release of matrix sustained release dosage forms by measurement of polymer release.J Control Release 1993;27:227-245.
[28]Vilhelmsen T,Eliasen H,Schaefer T.Effect of a melt agglomeration process on agglomerates containing solid dispersions.Int J Pharm 2005;303:132-142.
[29]Chiou WL,Riegelman S.Pharmaceutical applications of solid dispersion systems.J Pharm Sci 1971;60:1281-1302.
[30]Simonelli AP,Mehta SC,Higuchi WI.Dissolution rates of high energy polyvinylpyrrolidone(PVP)-sulfathiazole coprecipitates.J Pharm Sci 1969;58:538-549.
[31]Lloyd GR,Craig DQ,Smith A.A calorimetric investigation into the interaction between paracetamol and polyethylene glycol 4000 in physical mixes and solid dispersions.Eur J Pharm Biopharm 1999;48:59-65.
[32]Pokharkar VB,Mandpe LP,Padamwar MN,et al. Development,characterization and stabilization of amorphous form of a low T-g drug.Powder Technol 2006;167:20-25.
[33]Ghaste R,Chougule DD,Shah RR,et al.Solid dispersions:an overview.Pharm Rev 2009;7.
[34]Taylor LS,Zografi G.Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions.Pharm Res 1997;14:1691-1698.
[35]Pouton CW.Formulation of poorly water-soluble drugs for oral administration:physicochemical and physiological issues and the lipid formulation classification system.Eur J Pharm Sci 2006;29:278-287.
*Corresponding author.College of Pharmacy,Mother Theresa Post Graduate and Research Institute of Health Sciences(A Government of Puducherry Institution),Puducherry 605006,India.Tel.:+91 0413 2271200;fax:+91 0413 2277572.
E-mail address:poovinano@gmail.com(P.Ganesan).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.05.001
1818-0876/?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2015年5期
Asian Journal of Pharmacentical Sciences2015年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Montmorillonite/Poly(L-Lactide)microcomposite spheres as reservoirs of antidepressant drugs and their controlled release property
- Microsponge based drug delivery system for augmented gastroparesis therapy:Formulation development and evaluation
- Solid lipid microparticles:An approach for improving oral bioavailability of aspirin
- Transdermal delivery of fluorescein isothiocyanate-dextrans using the combination of microneedles and low-frequency sonophoresis
- Sustained release donepezil loaded PLGA microspheres for injection:Preparation,in vitro and in vivo study
