Design and development of novel bioadhesive niosomal formulation for the transcorneal delivery of anti-infective agent:In-vitro and ex-vivo investigations
Design and development of novel bioadhesive niosomal formulation for the transcorneal delivery of anti-infective agent:In-vitro and ex-vivo investigations
ARTICLEINFO
Article history∶
Received 23 July 2014
Received in revised form
8 February 2015
Accepted 16 February 2015
Available online 17 March 2015
∶
Ocular
Niosomes
Bioadhesive Chitosan
Fluorescence Gati fl oxacin
Gati fl oxacin eye drops are frequently used in eye infections.However such formulations have a major drawback i.e.short duration of action and usually require 4-6 times installations daily.A chitosan coated niosomal formulation of gati fl oxain was purposed to show a longer retention time on eyes and subsequent reduction in dosing frequency. Vesicles were prepared by solvent injection method using cholesterol and Span-60.An extensive optimization of formulation was done using different ratios of cholesterol,Span-60 and drug,revealed NS60-5(cholesterol:span-60 50:50 and drug content of 20 mg)to be the optimized niosome formulation.NS60-5 had shown a highest entrapment ef fi ciency of 64.9±0.66%with particle size 213.2±1.5 nm and zeta potential-34.7±2.2 mV.Optimized niosomes were also coated with different concentrations of chitosan and evaluated. Permeation studies had revealed that optimized niosomes(86.77±1.31%)had increased the transcorneal permeation of Gati fl oxacin more than two fold than simple drug solution (37.19±1.1%).Longer retention potential of the coated niosomes was further veri fi ed by fl uorescence microscopy.Study revealed that simple dye solution got easily washed out with in 6 h.The uncoated niosomes(NS60-5)showed a longer retention(more than 6 h), which was further enhanced in case of coated niosomes i.e.CNS60-1(more than 12 h). Antimicrobial studies had shown the better ef fi cacy of CNS60-1(zone of inhibition)when compared to marketed formulation.The fi nal chitosan formulation was found to have shown better ocular tolerability as demonstrated by corneal hydration test histopathology investigations.
?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1. Introduction
One of the major problems encountered with most of the eye drops is the rapid and extensive elimination of drugs from the precorneal lachrymal fl uid by solution drainage,lachrymation,and non-productive absorption by the conjunctiva, which may cause undesirable side effects[1].In fact it has been demonstrated through in vivo that 90%of the dose cleared within 2 min for an instilled volume of 50 μL and within 4 min for an instilled volume of 10 μL[2].Consequently, the ocular residence time of conventional solutions is limited to a few minutes,and the overall absorption of a topically applied drug is limited to 1-10%.
Initial attempts to overcome the poor bioavailability of topically instilled drugs typically involved the use of ointments based on mixtures of white petrolatum and mineral oils[3]and suspensions[4].Because these vehicles have the major disadvantage of providing blurred vision,they are nowadays mainly used for either night time administration or for treatment on the outside and edges of the eyelids[5]. Failures of the initial attempts lead to the advent of novel approaches in the fi eld of ocular drug delivery such as ocular inserts,use of polymeric nanoparticles[6],cyclodextrin complexes[7],collagen shields[8],liposomes[9],in-situ gels [10,11],contact lenses[12],niosomes[13]etc.Niosomes however are inexpensive,easy in preparation,stable and reproducible systems.Niosomes and particularly niosomes coated with bioadhesive materials can leads to a steady and sustain release of drug into the ocular cavity without being washed away frequently and could overcome the retention problem of conventional eye drops.Chitosan,which is a well explored and well understood polymer,offers many advantages as a coating material such as:biodegradability,excellent bioadhesive properties at physiological pH,penetration enhancement, mild self antimicrobial action and economical.
Gati fl oxacin is an extensively used antibacterial for wide variety of ocular infections.However a frequent dosing (generally 4-8 times per day)is required to achieve the effective concentration of it in eye.This leads to the need for frequent installation of the drug into eye and hence patient discomfort and patient non-compliance.Therefore,there is a probable need of novel eye formulation for Gati fl oxacin with longer stay in eye and less frequent dosing.
Hence the present study emphasized to search for an effective tool for solving low ocular retention problem of Gati fl oxacin by using the concept of bioadhesive niosomes. The study involved development of chitosan coated niosomes,in-vitro characterization and investigating the safety and ef fi cacy of the developed formulation on in-vitro models.
2. Materials and method
2.1. Materials
Gati fl oxacin was a generous gift from Aristo Pharma,Mumbai. Span 60,and cholesterol were purchased from central drug house.Rhodamine-B and medium molecular weight chitosan with75-85%deacetylationwaspurchasedfromSigma Aldrich.Goat eye cornea was obtained from local slaughter house.Type-I,Millipore water was used for all the practical purposes.
2.2. Method of preparation of niosomes
The solvent injection method was used to prepare gati fl oxacin niosomes.Span-60,cholesterol and drug were mixed in different ratios by weight(Table 1).For each ratio span 60 and cholesterol were weighed accurately and dissolved in 5 ml of chloroform.Drug was then dissolved in the lipid solution.This resulting solution was then taken in a syringe and injected slowly into a beaker containing 20.0 ml of aqueous phase (phosphate buffer pH 7.2)maintained at 60-70°C and agitated slowly.As the lipid solution was injected slowly into aqueous phase,vaporization of chloroform resulted into the formation of niosomes.
2.3. Characterization of niosome
2.3.1. Entrapment ef fi ciency
Thegati fl oxacinentrapmentcapacityofniosomeswas determined by centrifugation method[14].The entrapment ef fi ciency was determined after separating the unentrapped drug by centrifugation at 4°C at 15,000 rpm for 2 h the niosomes were lysed using Triton-X 100(0.1%v/v)and analysed for drug content.Entrapment ef fi ciency was expressed as percentage of total drug entrapped.
Theentrapmentcapacitywascalculated usingthe formula:
where,
T=theoretical amount of drug that was added.
C=amount of drug detected in the supernatant.
2.3.2. Size and size distribution
The niosome size and size distribution were determined by Dynamic Light Scattering(DLS)technique,using a computerized inspection system(Malvern Zetasizer,Nano-ZS,Malvern)with DTS (nano)software?.Forniosomessize measurement,niosomalsuspension wasdiluted with distilled water and the measurements were conducted in triplicate[15].
2.3.3. Zeta potential
Zeta potential of the niosomes was determined using Zeta Sizer(Nano-ZS,Malvern).
2.4. Preparation coated niosomes
Optimized niosomal formulation was coated with bioadhesive polymer chitosan for longer retention on cornea.Optimized niosomal suspension was added with chitosan solution of different concentrations(0.1,0.2,0.3 mg/ml)and stirred for 2 h with magnetic stirrer to get the chitosan coated niosomes coded as CNS60-1,CNS60-2,and CNS60-3 respectively.

2.5. Viscosity
Rheological properties of the niosomal formulations were analysed by using Anton paar MCR 301 rheometer using cone andplate measuring geometry.The samplesweresubjected to a shear rate variation of 0.1-100 sec-1and resulting shear stress was noted.
2.6. Transcorneal permeation studies
The optimized niosomal formulation(uncoated)as well as different coated niosomal formulations were subjected to transcorneal permeation studies.The uncoated niosome (NS60-5)formulation as well as drug suspension was also evaluated for the same.
2.6.1. Treatment of cornea
Fresh whole eye balls of goat were brought from the local butcher's shop to the laboratory in cold normal saline(4°C). The cornea along with 2-4 mm of sclera tissue was excised and was washed with cold normal saline.The washing of cornea was continued till washings tested negative for proteins as estimated by Folin's Phenol reagent and it gives zero UV absorbance at 296.5 nm using 0.9%normal saline as blank. Throughout the preparation great care was taken to avoid physical trauma to the tissue[13].
2.6.2. Preparation of arti fi cial tear fl uid
Arti fi cial tear fl uid(ATF),pH 7.4,was used in all the transcorneal permeation studies which consist of:sodium bicarbonate 0.200 g,sodium chloride 0.670 g,calcium chloride dihydrate 0.008 g,and puri fi ed water q.s.100 g[16].
2.6.3. Permeation experiment
Modi fi ed Franz Diffusion Cell with a diffusion area of 0.785 cm2and a receiver volume of 15 ml were used in passive diffusion studies and all experiments were conducted in triplicate.ATF pH 7.4 was used as the receiver medium. Freshly excised treated cornea was fi xed between donor and receptor compartments of an all-glass modi fi ed Franz diffusion cell in such a way that its epithelial surface faced the donor compartment.Donor compartment was fi xed on the cornea.After fi lling the donor compartment with formulation (niosomal formulations,free drug suspension,or marketed formulation)with equivalent quantities of drug,samples (1 ml)were withdrawn through sampling port of the Franz cell at predetermined time intervals over 24 h and analyzed by UV spectrophotometer at 296.5 nm.The receptor phase was immediately replenished with equal volume of fresh phosphate buffer.Sink condition was maintained throughout the experiment.At the end of each permeation experiment the integrity of the cornea was checked microscopically for the presence of any pore or tearing.
2.7. Shape and surface morphology of the niosomes
Niosomes were visualized using a Philips TEM CM 12 Electron Microscope,with an accelerating voltage of 100 kV.Samples were negatively stained with a 1%aqueous solution of phosphotungstic acid.A niosomal suspension containing drug was dried on a microscopic carbon-coated grid for staining.The excess solution was removed by blotting.After drying,the specimen was viewed under the microscope at a 100 k fold enlargement.
2.8. Bioadhesion testing
The bioadhesive potential of the optimized coated niosomes, uncoated niosomes,marketed formulation(Zymar?)was evaluated by method reported by Bachhav and Patravale,2009 [17].An agar plate(1%w/w)was prepared in pH 7.2 phosphate buffer.Test samples of 2.5 ml were placed at the center of plate.After 5 min,the agar plate was attached to an IP disintegration test apparatus and moved up and down in pH 7.2 phosphate buffer at 37±1°C.The sample(formulation with dye)on the plate was immersed into the solution at the lowest point and was out of the solution at the highest point.Dye loaded formulations were prepared by adding 5 μM Rhodamine B in organic phase(Chloroform)in place of drug during preparation of niosomes and following procedure similar to drug loaded niosomes.The residence time of the test samples on the plate was noted by visual appearance of the formulation over the plate.
2.9. Corneal retention study by fl uorescence microscopyFluorescence microscopic evaluations were done to determine the corneal retention and permeation of the coated and uncoated niosomal formulation.Rhodamine B was used to tag the formulations.Corneal samples were subjected to permeation study with formulation containing fl uorescent dye and fi xed for visualization after 2 h,6 h and 12 h.The blank sampleconsisting of rhodamine solution in water was similarly applied on a corneal sample.All the corneal sample slides were prepared and fi xed in 10%formic acid to washout the applied extra fl uorescent dye from the corneal surface before its microscopic evaluation.Slides were then evaluated using fl uorescent microscope at an excitation wavelength of 540 nm and emission wavelength of 625 nm.
2.10. Evaluation of anti-microbial potency of niosomal formulation
Anti-microbial potency of the niosomal formulation was compared with the marketed formulation using antimicrobial assay.Nutrient agar plates were inoculated with B.subtilis suspension.Standard wells were bored into these plates and poured with equal amount of samplescontaining sterilewater (control),niosomal placebo formulation,optimized niosomal formulation(CNS60-1)and marketed formulations separately at concentration of 0.05 mg/ml,0.1 mg/ml,and 0.2 mg/ml. Plates were incubated for 48 h at 34-37°C.At the end of the experiment the zone of inhibition for different treatments were noted.
2.11. Toxicity studies
Safety prospective of the formulation was investigated by toxicity studies.The following tests were carried out to evaluate any untoward reaction of the formulation on eye cornea:
2.11.1.Corneal hydration test
Goat corneas from permeation studies were used for the determination of corneal hydration.At the end of the experiment,each cornea(freed from adhering sclera)was weighed, soaked in 1 ml methanol,dried overnight at 90°C,reweighed. From the difference in weights,corneal hydration calculated [18].
2.11.2.Histological studies
Toxic effects of the optimized niosomal formulation were investigated using histological studies.Goat cornea was kept in niosomal suspension,simple drug suspension,normal saline(Negative control),and saturated KCl solution(Positive control).Cornea was removed from their respective medium at different time interval(1 h,6 h and 12 h)and fi xed in 10% formalin solution.Properly fi xed and stained slides were prepared from the samples and microscopically evaluated for cell disruption and toxic effects.
2.11.3.Effect of niosome formulation corneocytes∶biochemical estimation
A comparative toxicity study was carried out to get an insight of the tissue interference by niosomes on corneocytes.The assembly for transcorneal permeation was set as described using goat cornea.The donor compartments were fi lled with aqueous suspension of optimized niosomes(10 μg/ml of dried niosomes)and equivalent amount of span-60:cholesterol mix,separately for 60 min with Tyrode solution in both the compartments.Normal saline and Triton X 100(1%)were employed as negative and positive controls respectively.Post 60min the samplesweretaken from the receptor compartment and evaluated by using Accurex biomedical kit for LDH assay.
2.12. Statistical analysis
Analysis of variance(Kruskal-Wallis or One-way ANOVA) along with multiple comparison test(Student-Newman-Keuls Method)and t-test(for two samples)were employed by SigmaStat?3.5 software at P<0.05.
3. Results and discussions
3.1. Optimization of uncoated niosomes
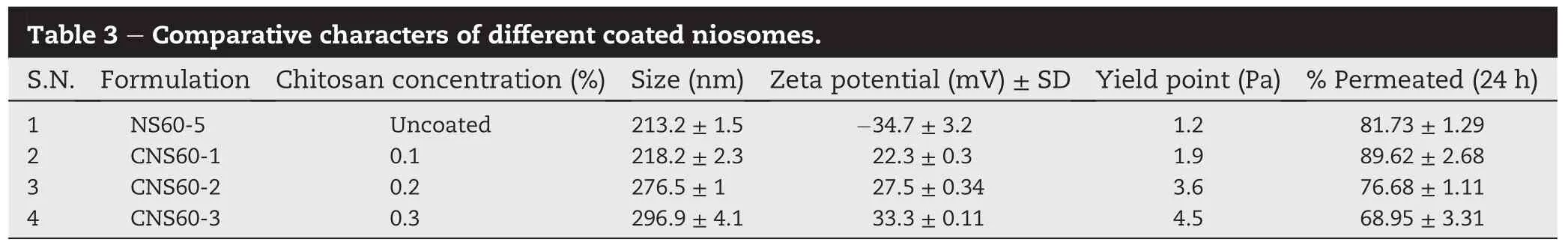
The optimization of the uncoated niosomes that were prepared by solvent evaporation was done on the basis of entrapment ef fi ciency,size and zeta potential.The entrapment ef fi ciency of the niosomes was found to get increased with increase in cholesterol concentration(Table 2).As gatifl oxacin islipophilicin nature,increasesin lipophilic component(cholesterol)lead to more entrapment[19]in the niosomes.Excess of surfactant lead to leakage of drug from the vesicles and hence a lower entrapment was observed with higher concentrations of span-60.A maximum entrapment ef fi ciency of Gati fl oxacin was observed with NS60-5 (64.9±0.66%).
Size of the vesicles was found vary non-signi fi cantly with the surfactant concentration from 5 to 50%.However a sharp increase in particle size was observed with 75%of span-60 (Table 2).The higher concentration may be having destabilizing effect on the vesicles,which lead to a very large particle size.Smallest size was observed for formulation NS60-5 (213.2±1.5 nm).
Zeta-potential of all the formulations were found to be negative.This might be due to the presence of free carboxyl groups in cholesterol and surfactant molecule.There was no statistically signi fi cant difference observed between the zetapotential values of different formulations(Table 2).The zetapotential value suggested suf fi cient kinetic stability of the niosomes.Highest zeta-potential was observed with NS60-5 (-34.7±2.2 mV).
The comparative analysis of particle size,zeta-potential and entrapment ef fi ciency of different niosomal formulations had suggested that Formulation NS60-5 was the best optimized formulation.NS60-5 contained surfactant:cholesterol(50:50)and drug 20 mg.NS60-5 was considered for further formulation development into coated niosomes.
3.2. Optimization of coated niosomes
The optimized uncoated niosomal formulation(NS60-5)was coated with solutions of different chitosan concentrations and evaluated for their size,zeta-potential,viscosity and transcorneal permeation.
An increase in particle size was observed with the chitosan concentration for the coating of niosomes.The original size of uncoated niosomes was 213.2±1.5 nm which got subsequently increased to 218.2±2.3,276.5±1,and 296.9±4.1 nmfor 0.1,0.2 and 0.3 mg/ml concentration of chitosan,respectively(Table 3).
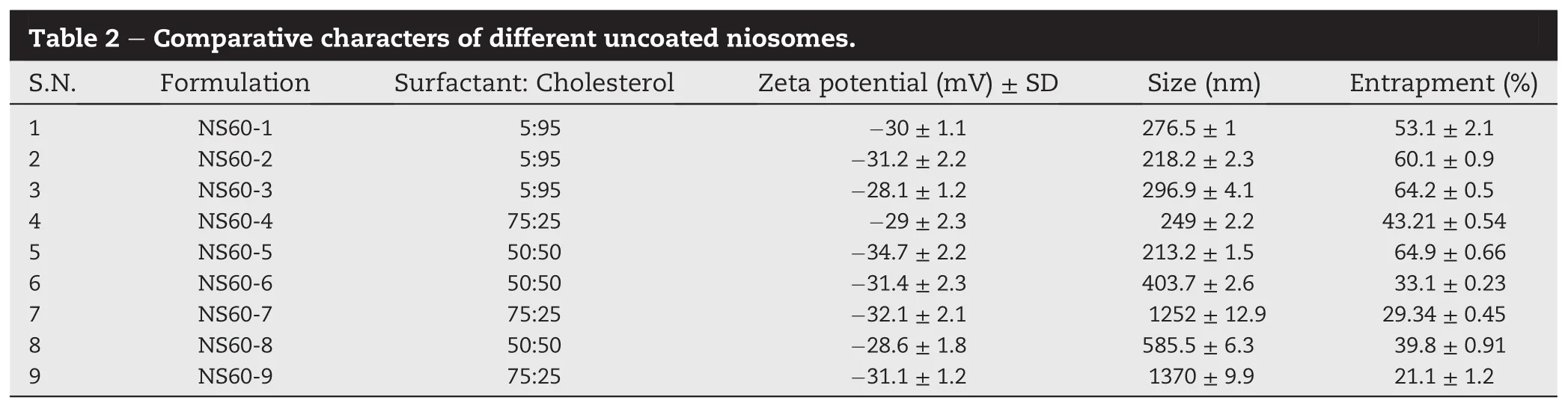
A very good correlation was observed with the percentage of chitosan coating and zeta potential of the niosomes.Originally the zeta-potential of the uncoated niosomes(NS60-5) was-34.7 mV.However,niosome particles were found to have carried a positive charge after coating with chitosan. Hence,this can be concluded that chitosan get adsorbed on the surface of the negatively charged niosomes and imparted an overall positive surface charge to them.Further,an increase in zeta-potential was observed with the increase in concentration of chitosan(Table 3).
All the niosomal formulations were found to have shown non-Newtonian behaviour(shear thinning).There relative yield point values gave an indication that chitosan coated niosomes with higher concentration of coating had higher viscosity(Table 3).Furthermore,it was observed that on coating the niosomes with chitosan initially an increase in total drug permeation(89.62%)was found with 0.1%coating (CNS60-1).This might be attributed to bioadhesive as well as permeation enhancing action of chitosan.Chitosan was reported to have a property of loosening the tight junctions of the cell layers[20].However,on further increase in chitosan concentration a sharp decrease was observed in permeation. The increase in overall viscosity of the formulation at higher chitosan concentration might be a possible reason for that.
Over all formulation CNS60-1 was found to be a potential candidate as it had shown a better permeation pro fi le and suf fi ciently low viscosity than other formulations.Higher viscosity of an eye formulation may lead to dif fi culty in administration as well as smudging and blurring of vision.
3.3. Shape and surface morphology of niosomes
Pictures from transmission electron microscope showed clear structural differences between coated and uncoated niosomal formulations(Fig.1a and b).Uncoated niosomes(NS60-5)were found to be circular in size with well de fi ned boundaries.On the other hand a clear coating layer with uneven boundaries can be seen over the chitosan coated niosomes(CNS60-1).
3.4. Transcorneal permeation
Transcorneal permeation pro fi le of the optimized formulation (CNS60-1)was compared with free drug suspension and marketed gati fl oxacin eye drop(Zymar?).Due to the low aqueous solubility,transcorneal permeation of gati fl oxacin was found to be very slow and incomplete from drug suspension.Only 35.03±1.1%of the drug was able to permeate in 24 h from the drugsuspension.Ontheotherhandtherewasamorecomplete permeationofgati fl oxacinfromniosomalformulation(NS60-5) 81.73±2.1%of drug was permeated in 24 h(Fig.2).The coated niosomal formulation(CNS60-1)has also shown an enhanced permeation(86.77±1.31%)in 24 h.This observation could be attributed to the permeation enhancement effect of the niosomes.Furthermore,it was observed that permeation from the free drug suspension was concentration dependent,and a decreaseinpercentage permeationwasobserved withincrease in time.However,permeation of drug from the niosomes was found to be concentration independent.This could be due the dominance of hydrotaxis forces for the transport of the niosomes through the cornea over the concentration gradient due to the presence of lecithin[21].Hence,if retained for a longer time over the cornea,niosomes can provide a suf fi cient concentration(minimum inhibitory concentration)of drug over the period of time.
3.5. Bioadehesion testing
Thebioadhesivepotentialofchitosancoatedniosomal formulation(CNS60-1)was compared to marketed formulation as well as uncoated niosomal formulation(NS60-5)by using the in house bioadhesion assembly(Fig.3).The results clearly indicate that the chitosan coated niosomes had the longer retention time(176±5.5 min)over the agar plate than marketed formulation(2±0.5 min)and uncoated niosomal formulation(28±2.25 min).
3.6. Corneal retention study by fl uorescence microscopy
The fl uorescent study with rhodamine-b dye solution showed initially fl uorescence(Fig.4A1),which subsequently disappeared with time(Fig.4A2 and A3).Dye solution had poor retention over the goat cornea which represents normal drug solution.In contrast to this use of niosomal formulation NS60-5,had increased the overall retention time of the dye in the corneal tissues.There was signi fi cant amount of fl uorescence left in the tissues even at 6th hour of treatment(Fig.4B2). However,at the 12 h there was very less amount of fl uorescence left in the tissues.On the other hand chitosan coated niosomal formulation CNS60-1,had further extended the dye retention in the corneal tissues.A signi fi cant amount of fl uorescence was left in the tissues even at 12th hour(Fig.4C3)as compared to uncoated niosomal formulation NS60-5(Fig.4B3). Therefore it can be safely concluded that chitosan coated niosome formulation(CNS60-1)had good retention capacity in corneal tissues,and can be ef fi ciently prolong the retention of gati fl oxacin in eye for the reduction of dosing frequency.
3.7. Evaluation of anti-microbial potency of niosomal formulation
An antimicrobial study was performed to evaluate the relative potency of the niosomal formulation.The study was performed using three different concentrations of gati fl oxacin (0.05,0.1,and 0.2 mg/ml)in niosomal formulations and marketed eye drop(Table 4).Concentration higher than 0.2 mg/ml had shown overlapping of zone of inhibitions.The placebo niosomal formulations had shown a weak antimicrobial activity itself.This might be due to presence of chitosan in the formulation which was itself reported to carry antimicrobial effects.There was an increase in zone of inhibition with increased concentration of gati fl oxacin in CNS60-1 niosome formulation.It was also found to be more potent than marketed eye drop at every concentration.It was observed with the marketed formulation that initially there was an increase in zone of inhibition with the increase in concentration.However on further increase in concentration,no signi fi cant change was observed in the zone of inhibition.This could be attributedtothe lowdiffusionofthedrugthroughthenutrient agar.A lower solubility of gati fl oxacin found to have limited its diffusion after an optimum concentration.On contrary the niosomes had resulted in better permeation of the drug,and there was constant increase in zone of inhibition with increase in drug concentration.

3.8. Toxicity studies
Normal cornea has a hydration level of 75-80%[22].Corneal hydration observed in the present experiments was between 76and 79%,indicating no damage to cornea.Lack of toxicity of the optimized chitosan coated formulation was further demonstrated by histological studies(Fig.5).KCl solution(positive control)had shown marked damage to the corneal tissues (Fig.5A).An initial swelling was observed with KCl solution in fi rsthourwhichleadtosubsequentdamageofcornealcelllayers till 6th and 12th hour.Normal saline was taken as a negative controlinthestudyandcornealtissuesshowednodamagewith it(Fig.5B).There were no toxic responses were seen with drug suspension and CNS60-1 formulation(Fig.5C and D).
The toxicity studies were further extended to biochemical estimation of LDH.High concentrations of LDH are often associated with the tissue injury.Thus,the estimation of LDH is often employed as biochemical estimation of toxicity[23,24]. Triton X-100 treatment showed a marked release of LDH due to tissue destruction,while LDH release was minimal in case of normal saline(Fig.6).Furthermore,high LDH release was also observed in case of free span-60-cholestrol mix.On contrary, niosome formulation had signi fi cantly lower LDH release (P<0.05).We have demonstrated in our previous work that surfactants bound to vesicular system had lower potential to cause cellular damage than free surfactant molecules[24]. Thus,comprehensive toxicological investigation revealed that thedevelopedniosomalformulationshadlowertoxicpotential.
4. Conclusion
The novel niosomal formulation of gati fl oxacin was found to be capable of increasing the corneal retentionof the drug.Also there was a signi fi cant enhancement of transcorneal permeation by the formulation.Hence this novel formulation was found to be a good replacement for conventional eye drops with decreased dosing frequency and an effective drug levels in eyes.The future perspectives include study of effects of different bioadhesives,pharmacokinetic studies and clinical trials for developing a clinically viable formulation.
REFERENCES
[1]Lee VHL,Robinson JR.Mechanistic and quantitative evaluation of precorneal pilocarpine disposition in albino rabbits.J Pharm Sci 1979;68:673-684.
[2]Chrai SS,Patton TF,Mehta A,et al.Lacrimal and instilled fl uid dynamics in rabbit eyes.J Pharm Sci 1973;62:1112-1121.
[3]Greaves J,Wilson C,Birmingham A.Assessment of the precorneal residence of an ophthalmic ointment in healthy subjects.Br J Clin Pharmacol 1993;35:188.
[4]Mazor Z,Ticho U,Rehany U,et al.Piloplex,a new long-acting pilocarpine polymer salt.B:comparative study of the visual effects of pilocarpine and piloplex eye drops.Br J Ophthalmol 1979;63:48-51.
[5]Loftsson T,Stefˊansson E.Cyclodextrins in eye drop formulations:enhanced topical delivery of corticosteroids to the eye.Acta Ophthalmol Scand 2002;80:144-150.
[6]Verma P,Gupta RN,Jha AK,et al.Development,in vitro and in vivo characterization of Eudragit RL 100 nanoparticles for improved ocular bioavailability of acetazolamide.Drug Deliv 2013;20:269-276.
[7]Loftsson T,Frithriksdottir H,Stefansson E,et al.Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits.J Pharm Pharmacol 1994;46:503-504.
[8]Hwang DG,Stern WH,Hwang PH,et al.Collagen shield enhancement of topical dexamethasone penetration. Archives Ophthalmol 1989;107:1375-1380.
[9]Nagarsenker M,Londhe VY,Nadkarni G.Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery.Int J Pharm 1999;190:63-71.
[10]Lou J,Hu W,Tian R,et al.Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles.Int J Nanomedicine 2014;9:2517-2525.
[11]Shen Y,Ling X,Jiang W,et al.Formulation and evaluation of cyclosporinA emulgelfor ocular delivery.DrugDeliv 2014:1-7.
[12]Tieppo A,Boggs AC,Pourjavad P,et al.Analysis of release kinetics of ocular therapeutics from drug releasing contact lenses:bestmethodsandpracticestoadvancethe fi eld.Contact lens&anterior eye.J Br Contact Lens Assoc 2014;37:305-313.
[13]Ahuja M,Singh G,Majumdar DK.Effect of formulation parameters on corneal permeability of o fl oxacin.Sci Pharm 2008;76:505.
[14]Bendas ER,Tadros MI.Enhanced transdermal delivery of salbutamol sulfate via ethosomes.AAPS PharmSciTech 2007;8:213-220.
[15]El Maghraby GM,Williams AC,Barry BW.Skin delivery of 5- fl uorouracil from ultradeformable and standard liposomes in-vitro.J Pharm Pharmacol 2001;53:1069-1077.
[16]MotwaniSK,ChopraS,TalegaonkarS,etal.Chitosan-sodium alginatenanoparticlesassubmicroscopicreservoirsforocular delivery:formulation,optimisation and in vitro characterisation.Eur J Pharm Biopharm 2008;68:513-525.
[17]Bachhav YG,Patravale VB.Microemulsion-based vaginal gel of clotrimazole:formulation,in vitro evaluation,and stability studies.AAPS PharmSciTech 2009;10:476-481.
[18]Monti D,Saccomani L,Chetoni P,et al.Effect of iontophoresis on transcorneal permeation'in vitro'of two beta-blocking agents,and on corneal hydration.Int J Pharm 2003;250:423-429.
[19]Tamizharasi S,Dubey A,Rathi V,et al.Development and characterization of niosomal drug delivery of gliclazide. J Young Pharm 2009;1:205.
[20]Kaur IP,Aggarwal D,Singh H,et al.Improved ocular absorption kinetics of timolol maleate loaded into a bioadhesive niosomal delivery system.Graefe's Archive Clin Exp Ophthalmol 2010;248:1467-1472.
[21]Khan MA,Pandit J,Sultana Y,et al.Novel carbopol-based transfersomal gel of 5- fl uorouracil for skin cancer treatment: invitrocharacterizationandinvivostudy.DrugDeliv2014:1-8.
[22]Maurice D.The tonicity of an eye drop and its dilution by tears.Exp Eye Res 1971;11:30-33.
[23]Negi LM,Tariq M,Talegaonkar S.Nano scale self-emulsifying oil based carrier system for improved oral bioavailability of camptothecin derivative by P-Glycoprotein modulation. Colloids and surfaces B.Biointerfaces 2013;111C:346-353.
[24]Mohan Negi L,Fatma S,Talegaonkar S,et al.Development of ethanolic nano vesicles of tenoxicam,investigation of transdermal penetration ef fi ciency and histological safety comparison with common penetration enhancers.Nanosci Nanotechnol Lett 2013;5:600-605.
Yahaya Zubairu,Lalit Mohan Negi,Zeenat Iqbal,Sushama Talegaonkar*
Department of Pharmaceutics,Faculty of Pharmacy,Jamia Hamdard,New Delhi 110062,India
*Corresponding author.Department of Pharmaceutics,Faculty of Pharmacy,Jamia Hamdard,New Delhi 110062,India.
E-mail address:stalegaonkar@gmail.com(S.Talegaonkar).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.02.001
1818-0876/?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2015年4期
Asian Journal of Pharmacentical Sciences2015年4期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Effect of addition of inulin and fenugreek on the survival of microencapsulated Enterococcus durans 39C in alginate-psyllium polymeric blends in simulated digestive system and yogurt
- Zingiber cassumunar blended patches for skin application:Formulation,physicochemical properties,and in vitro studies
- Taste masking of cipro fl oxacin by ion-exchange resin and sustain release at gastric-intestinal through interpenetrating polymer network
- Effect of formulation variables on in vitro release of a water-soluble drug from chitosan-sodium alginate matrix tablets
- Dendritic macromolecules as nano-scale drug carriers:Phase solubility,in vitro drug release, hemolysis and cytotoxicity study
