Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization
Pornsak Sriamornsak,Kanokporn Burapapadh
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization
Pornsak Sriamornsaka,b,*,Kanokporn Burapapadha,b,1
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
ARTICLEINFO
Article history:
Received 29 October 2014
Received in revised form
20 January 2015
Accepted 23 January 2015
Available online 16 February 2015
Itraconazole
The objective of the present study was to alter the crystal habit of itraconazole(ITZ)by cooling and anti-solvent crystallization and characterize its properties.ITZ was recrystallized in different solvents and the effects of each solvent on morphology of crystals, dissolution behavior and solid state of recrystallized drug particles were investigated.The results revealed that ITZ crystals recrystallized by cooling and anti-solvent crystallization showed the different crystal habits from the untreated ITZ.Using cooling crystallization tended to provide needle-shaped crystals while the crystals obtained from anti-solvent crystallization showed more f l aky,plate shape.This indicated the importance of preparation method on nucleation and crystal growth.No change in drug polymorphism was observed,according to determination of thermal property and crystalline state by differential scanning calorimetry and powder X-ray diffractometry,respectively.The recrystallized ITZ showed higher drug dissolution than untreated ITZ and the highest drug dissolution was observed from the samples recrystallized in the presence of PEG 200,which provided the small plate-shaped crystals with tremendously increased in surface area. However,the increasing of drug dissolution is relatively small,therefore,further development may be required.
?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Crystallization is concerned with the evolution from solution or melt of crystalline state[1].The formation of crystals consists of nucleation and crystal growth.Nucleation is the molecular assembly process,in which the molecules of the particular element start combining together.Nucleation occurs via the formation of small embryos of the new phase inside the large volume of the metastable old phase.Nucleation can occur with or without foreign substance,so called homogeneous and heterogeneous,respectively[2].Once the nucleation has been achieved,crystal growth is the dominated process,leading to evaluation of the embryonic crystals into a crystal form of def i ned size and shape.The factors inf l uencing the crystal size and shape are the crystal lattice of the molecular solids and solvent and additive presented in the system.The solventsused in crystallizationstronglyaffect the habit of crystalline material.However,the role played by solvent interactions in enhancing or inhibiting crystal growth is still not completely understood[3].The mechanism of solvent affecting crystal growth and morphology is explained by Bennema and coworkers[4].They proposed that favorable interactions between solute and solvent on specif i c faces leads to reduced interfacial tension,causing a transition from the smooth to a rough interface and a concomitant faster surface growth.In contrast,preferential adsorption at specif i c faces can inhibit their growth as removal of bound solvent acts as additional energy barrier for continued growth.
The difference in crystal faces affects the nature of each crystal habit which inf l uences the dissolution of a drug[5]. AdhiyamanandBasu[6]reportedthatthedissolution enhancement of dipyridamole correlates to alteration of the drug habit by crystallization using different solvents,additives and crystallization conditions.The dissolution rate of rod-shaped particles crystallized from benzene is notably more rapid than that of rectangular needle-shaped crystals produced using methanol.The effect of crystal habit on tolbutamide dissolution was also suggested[7].The smallest particle size,plate-like shape crystals adopted from solventchange method using methanol and ethanol show higher dissolution rate.
Many crystallization methods have been used in pharmaceutical sciences,e.g.,melt crystallization,cooling crystallization,anti-solventcrystallization,gasanti-solvent crystallization and evaporative crystallization.Cooling and anti-solvent crystallization were the most common crystallization method used in pharmaceutical application.In cooling crystallization,the supersaturation is generated by a decrease in temperature.Coolingcrystallization occurswhen a solution containing solute is cooled at a constant concentration of dissolved crystals[8].A similar process occurs for anti-solvent crystallization,instead of cooling the system,a secondary solvent known as anti-solvent is added to the original solvent, resulting in the reduction of the solubility of the solute in the original solvent and consequently generating a supersaturation driving force[9].Many studies reported the effect of various operating conditions.The different concentrations of aqueous and anti-solvent solutions affect crystal shape and distribution[10].The rate of supersaturation generation in anti-solvent crystallization is highly dependent on the antisolvent addition rate.Both supersaturation and solvent composition are also important factors affecting crystal size and habit.
Itraconazole(ITZ),a poorly water-soluble drug,is a triazole antifungal agent having a broad spectrum of activity against a variety of pathogens,including Aspergillus species,Candida albicans,Cryptococcus neoformans,Coccidioides immitis,Histoplasma capsulatum,Paracoccidioides brasiliensis,and Sporothrix schenckii[11],which are the major cause of opportunistic infection in human immunodef i ciency virus(HIV)infected patients.The mechanism of action of ITZ is similar to all other azole antifungals.It inhibits cytochrome P450 of the fungi and thus interferes the synthesis of ergosterol,a vital component of the fungal cell membrane,leading to cell death[12].ITZ is a white to slightly yellowish powder.It has a molecular formula C35H38Cl2N8O4and molecular weight of 705.64.It is a weak basic drug(pKa=3.7)which is virtually ionizedat only low pH, possessing extremely low water solubility(about 1 ng/ml at neutral pH and about 6 μg/ml at pH 1).
Theobjectiveofthisstudywasto preparetherecrystallized ITZ by cooling and anti-solvent crystallization methods.The properties of recrystallized ITZ,i.e,morphology,surface area, thermal property,crystalline state and dissolution,were investigated.
2.Materials and methods
2.1.Materials
ITZ raw material used in this study was purchased from Nosch Labs Private(India).Chloroform(lot number K40141945 924,Merck,Germany)and methylene chloride(lot number D3056-1-2501 94556-1112,QReC,New Zealand)were used as crystallizing solvents in halide group while methanol(lot number I520407 004,Merck,Germany),ethanol(lot number R:11S:1-16,Liquor Distillery Organization,Thailand),and isopropanol(lot number K40861440 010,Merck,Germany)were used as crystallizing solvents in alcohol group.Polyethylene glycol(PEG)200(lot number 1294947 51807087)and PEG 400 (lot number 1366280 34407P06)were from Fluka(Germany). Distilled water was used as an anti-solvent in recrystallization of ITZ from alcohols and PEGs while hexane(lot number 09 08 1092,Labscan,Thailand)was used as an anti-solvent for halide solvents.Other chemicals were of reagent or analytical grade and used without further purif i cation.The simulated gastric f l uid USP without pepsin(SGF)was prepared by dissolving 2 g of sodium chloride and 7 ml of hydrochloric acid into distilled water and adjusting volume to 1000 ml,pH to 1.2, and used as dissolution medium.
2.2.Solubility studies
Solubility ofITZwas determined in waterandvarioussolvents by adding ITZ in 1 ml of a pure solvent in Pyrex culture tubes. The drug suspension was equilibrated at 25°C in a thermostatically controlled bath for 48 h.After equilibration,the tubes were centrifuged at 3500 rpm for 15 min and the clear supernatants were analyzed for ITZ with high performanceliquid chromatography,HPLC(Agilent,USA)using Alltima?C18 column(5 μm,4.6×250 mm)(Alltech,Italy).The mobile phase consisted of 63:37:0.05 acetonitrile:water:diethylamine adjusting pH to 2.45 with phosphoric acid and was f i ltered through a membrane f i lter(0.22 μm),and degassed in a sonicator bath before use.The f l ow rate was 1.0 ml/min,and the UV detection wavelength was 263 nm.
2.3.Recrystallization of ITZ
ITZ was recrystallized by two methods in order to alter crystal habit or crystalline state of the drug.Three groups of solvents, that is,alcohols(methanol,ethanol and isopropanol),polyethylene glycols(PEG 200 and PEG 400)and halide solvents (chloroform and methylene chloride)were used.
2.3.1.Cooling crystallization
ITZ was recrystallized in three groups of solvents as described above.Supersaturation was achieved by changing the solution temperature.An appropriate drug amount was dissolved in a particular solvent volume at 40.0±0.5°C.Solution was cooled in water bath to 10.0±0.5°C under continuous stirring, at the cooling rate of about 0.25°C/min.Crystals were recovered by vacuum f i ltration,washed with distilled water for three times,dried at room temperature for 24 h,and then kept in a desiccator.
2.3.2.Crystallization by anti-solvent addition
A solution of ITZ was supersaturated by adding an antisolvent to reduce the solubility of the drug in the solution. The anti-solvent should be miscible with the solvent at any proportion,and the solute should be relatively insoluble in it. Thus,for this method,two anti-solvents were selected.Hexane was used as anti-solvent for water-immiscible systems to generate crystals,whereas distilled water went with water miscible systems.An appropriate drug amount was dissolved in a particular solvent volume at 40.0±0.5°C.Crystallization was started in the warm solution,maintained under continuous stirring,by adding an anti-solvent in a ratio of 2:1(antisolvent:ITZ)solution.After beginning of crystallization,the liquid was cooled down to 10.0±0.5°C under continuous stirring.Then,thecrystals werevacuum-f i ltered,washedwith distilled water for three times,dried at room temperature for 24 h,and kept in the same conditions as mentioned above.
2.4.Morphology examination
ITZ crystals were investigated by a scanning electron microscope(SEM;Maxim-2000,CamScan Analytical,England), under an accelerating voltage of 15 keV.Crystal samples were fi xed on SEM stubs with double-sided adhesive tape and then coated in a vacuum with thin gold layer before investigation.
2.5.Surface area measurement
Crystal samples were degassed for at least 3 h at 200°C under a light vacuum(0.013 Torr)to remove physisorbed material such as water from the particle surface.Nitrogen gas adsorption and desorption isotherms were collected using a surface area analyzer(Nova 2000e,Quantachrome,USA)at 77 K.The specif i c surface area was calculated using Brunnaur, Emmett and Teller(BET)theory,for three to f i ve adsorption points in the relative pressure range of 0.05-0.30 using ultrahigh-purity nitrogen(cross-sectional area 16.2 A?2)as the adsorbate.
2.6.Differential scanning calorimetry(DSC)
The thermal properties of ITZ after treatment in various conditions were observed by a Sapphire DSC(Perkin Elmer, Germany).An accurately weighed amount of sample was placed inside standard crimped aluminum pan and heated from 25 to 250°C at a heating rate of 10°C/min under nitrogen fl ow(30 ml/min).
2.7.Powder X-ray diffractometry(PXRD)
PXRD analysis was used to investigate the effect of recrystallization condition on the crystalline state of ITZ.PXRD patternsofITZcrystalswereobtainedusingtheX-ray diffractometer(JDX-3530,JEOL,Japan)at 30 kV,40 mA over the range of 5-40°2θ by the scanning speed of 2°/min using Cu Kα radiation wavelength of 1.5406 A?.
2.8.In vitro dissolution study
DissolutionstudiesofITZcrystalswereperformedintriplicate at 37±0.5°C employing USP apparatus I(basket,100 mesh) with a speed of 100 rpm(DT70,Erweka,Germany).The dissolution vessels were loaded with 1000 ml of dissolution medium(i.e.,SGF,pH 1.2).An accurately weighed amount of ITZ crystals(0.3 mg)was used to ensure the sink condition in dissolution medium.Samples were withdrawn from the dissolution vessels at 5,10,20,30,60,90,and 120 min and passed through 0.45-μm cellulose membrane.Then,the analysis of ITZ content was done by HPLC assay.
After dissolution test,the sampling medium was immediately analyzed by HPLC(JASCO PU-2089plus quaternary gradient inert pump,and a JASCO UV-2070plus multiwavelengthUV-visdetector,Jasco,Japan)atawavelength of 263 nmusinga Cosmosil 5C18-MS-II(4.6×250 mm)column. The system was operated under isocratic f l ow at 1 ml/min using a mobile phase consisting of acetonitrile:water:diethylamine,63:37:0.05(by volume),adjusting pH to 2.45 with phosphoric acid and being f i ltered through a 0.22-μm membrane f i lter and degassed in a sonicator bath before use. Samples collected from dissolution test were injected in the volume of 100 μl.Data were collected and analyzed by ChromNav program(Jasco,Japan).The retention time of ITZ was approximately 5 min.
3.Results and discussion
3.1.Solubility of ITZ in various solvents
ITZ has very low solubility in water.This is because of the predominant nonpolarity of the drug molecules.ITZ cannot effectively break into the lattice structure of water,hence,the solubility of the drug in deionized water was only 2.8 μg/ml,asshown in Table 1.Solubility of ITZ was found to be fairly high in methanol and PEGs.Among alcohols,solubility decreased with increased chain length while increasing of chain length of the PEGs increased solubility of the drug.Very high solubility of ITZ has been observed in organic solvents,i.e.,30,720 and 28,400 μg/ml for chloroform(CHCl3)and methylene chloride(CH2Cl2),respectively.
Polarity of the solvent is an important factor governing the solubility of the drug.However,it is not the only factor involved.Among alcohols,solubility did not increase with a decrease in polarity.At 25°C,dielectric constant of methanol, ethanol and isopropanol is 33.77,24.35 and 19.45,respectively [13,14].The solubility was maximum in methanol and decreased with an increase in the chain length of alcohol.This effect indicated that the ability of the solvent to form hydrogen bonds with hetero-atom in the drug molecule is another important factor that inf l uenced solubility of the drug in alcohols[15].As the alkyl chain length in alcohols increased,their ability to form hydrogen bonds with the drug molecules decreased,especially for alcohol with branched methyl group,hence,the solubility decreased[16].For the same reason,in case of PEGs,even though the polarity of PEG is less than ethanol[17],the solubility of the drug could be higher because of extensive hydrophobic interactions with the solvents of long nonpolar part in PEG molecules.
3.2.Crystal morphology
ITZ crystals were columnar in shape with a wide range of size, between 5 and 20 μm(Fig.1a).After recrystallization,the crystal morphology was changed depending on both types of solvent and recrystallization methods.Fig.1b-f show the morphology of ITZ crystals obtained from cooling crystallization.Supersaturation in cooling crystallization process is a resultfrom decreasingof thesolutiontemperature.During the cooling process,nucleation occurred and solute molecule could rest on the crystal surface.After the cooling crystallization process,there was no crystal observed from the PEG 200 and PEG 400 systems.This may be due to lack of nucleation occurred in PEG in the crystallizing temperature[18]. The inf l uence of crystallization solvent on habit modif i cation of ITZ crystals was clearly shown.Cooling crystallization process in methanol(Fig.1b)and ethanol(Fig.1c)provided needle-shapedcrystals.Theuseofisopropanol(Fig.1d),which has lower polarity,as crystallizing solvent resulted in various sizes of blade-shaped crystals.Using methylene chloride and chloroform as crystallizing solvent provided different crystalhabits.Small plate-shaped crystals were obtained from cooling crystallization in methylene chloride(Fig.1e)but the small blade-shaped crystals were obtained after crystallization in chloroform(Fig.1f).Comparing SEM images of crystals obtained from chloroform with those obtained from other solvents,thecrystalshapeofITZwassigni fi cantlychangedtothe plate shape,which is quite different from the previous needle shape.The variation in face dimension or the appearance or disappearance of some faces could be the cause of change in morphologyofITZcrystalsusingdifferentsolventsin recrystallization[3].The results also demonstrated the difference in size of recrystallized crystals compared to the untreated ITZ.
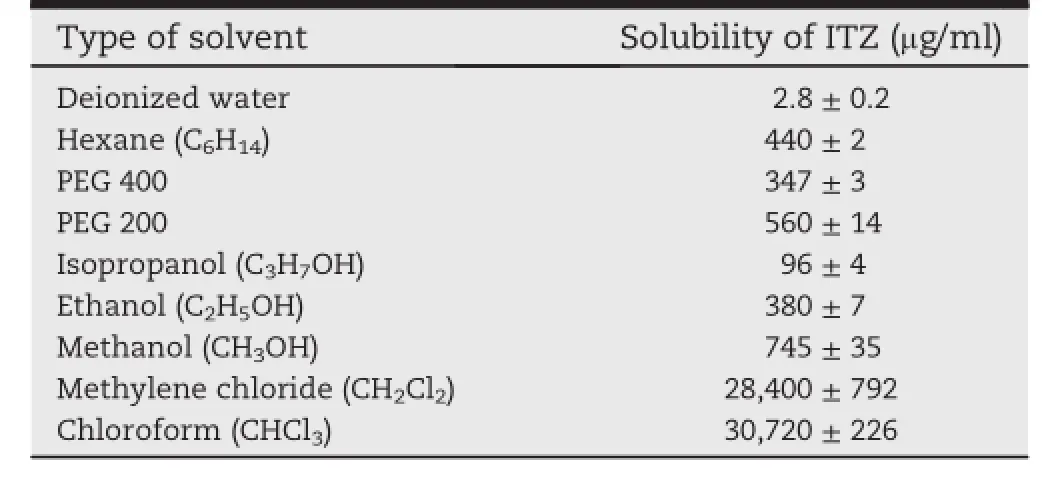
Table 1-Solubility of ITZ in various solvents(n=3).
The morphology of ITZ crystals obtained from anti-solvent addition method is shown in Fig.2.For crystallization by antisolventadditionin alcohols,higher polarityalcohols tendedto provide more fl aky crystals than lower polarity ones.In methanol,the crystals became plate-like(Fig.2a)while in other alcohols,ITZ crystals turned to needle shape with more fl akes(Fig.2b and c).Anti-solvent crystallization of ITZ in methylene chloride resulted in long and thin needle-shaped crystals(Fig.2d).Using chloroform as crystallizing solvent, irregular shape and fl aky crystals were observed(Fig.2e), similar to those in the case of using PEGs(Fig.2f and g).
The growth of one set of crystal faces can be inhibited or the other set of faces can be induced to grow faster when particular solvents are used[3].In this study,using higher polarity alcohols tended to provide the plate-shaped crystals while needle-shaped crystals occurred when using the lower polarity one.This can be explained by the interaction between the solvent molecules and different crystal faces,which is believed to change the crystal morphology[19].It is suggested that polar solvents were adsorbed by polar faces and nonpolar solvents by the non-polar faces.Since the interaction of methanol is stronger than isopropanol,the growth of crystalfromthe polarfaceswasmore inhibitedand thecrystal growth was continued from other sides[20].
3.3.Surface area
Reduction of crystal size or increase in surface area of the drug isa widelyusedmethodto increase dissolutionrate,under the Noyes-Whitney equation.The surface area of ITZ crystals is shown in Table 2.The untreated ITZ provided the lowest surface area of 4.17 m2/g while all processed crystals showed an increase in the surface area.The crystals prepared by antisolvent crystallization in PEG 200 demonstrated the highest surface area of 524.90 m2/g,which is 125-fold higher than the untreated ITZ.Therefore,the improvement of ITZ dissolution by anti-solvent recrystallization in PEG 200 is expected.The crystalsobtainedfromcoolingcrystallizationwerenot included in this study due to the very low amount of crystal samples.
3.4.Thermal properties of ITZ crystals
The polymorphs always have different levels of thermodynamics stability and an unstable,so called metastable form, can melt at a temperature less than the melting point of stable form[4].Therefore,the DSC was used as primary screening ofITZ polymorph that may be occurred during crystallization. Fig.3 shows the DSC thermograms of ITZ crystals recrystallized by cooling crystallization and anti-solvent addition methods.
The onset melting temperature and enthalpy(ΔH)of recrystallized ITZ crystals are given in Table 3.It is observed that there was a noticeable reduction in the enthalpy of the obtained crystals in comparison with untreated ITZ.Crystals obtained from crystallization by anti-solvent addition in methanol showed the lowest enthalpy of 92.63 J/g along with a lower onset temperature.A decrease in onset melting temperature of the obtained crystals may be due to the decreased drug crystallinity or residual solvent presented as impurities in the drug crystals.It is well known that the melting temperature and enthalpy rely on crystal size[21].In particular, the decreasein crystal size ref l ected in the decreasein melting temperature/enthalpy.The explanation of these phenomena is based on the different structure of surface and bulk phases. Atoms at the surface are in a less limited arrangement than in the bulk;therefore,their energy is higher than that of bulk atoms.Hence,lattice break-down on crystal surface would require less energy and would be favored with respect to bulk lattice break-down[21].
From the results,it is clear that there are alterations of thermal properties of ITZ crystals recrystallized by various solvents and conditions.To check the drug polymorphism, PXRD was used(discussed later).Due to very low yield obtained and very high amount of solvent required in preparation of drug crystals by cooling crystallization,it may be not practical to use in pharmaceutical industry.Therefore,the experiments on this technique were excluded.The further experiment focused on anti-solvent addition technique which could alter crystal habit and also yield high amount of crystals.

Table 2-Surface area of ITZ crystals.
3.5.Crystalline state of ITZ crystals
The PXRD pattern of untreated ITZ crystals is shown in Fig.4. There are many peaks associated with crystallinity.However, the most intense peak was located at the same position of ITZ reported in the previous study[22]in which the PXRD peaks at values of 2θ were at 17.45 and 17.95(doublet),20.30,and 23.45. A polymorphic form of ITZ was pointed out in 2007[23],whichwas characterized by peaks at values of 2θ of approximately 7.3,19.9,21.9,26.1,and 32.2°.
Even though DSC thermograms of recrystallized ITZ showed the shift of the onset melting temperature,indicating the possible polymorphic formation,the PXRD patterns of these crystals were not different from that of untreated ITZ. The PXRD patterns still showed the sharp peaks with straight base lines referred to crystallinity.However,a decrease in intensity was observed in case of anti-solvent addition crystallization using alcohols and PEGs as primary solvent,as shown in Fig.4.This may be due to the decrease in crystallinity of ITZ or the presence of residual solvents.However,theresidual PEGs were not found according to the PXRD patterns in the Fig.4b.The PXRD patterns of ITZ crystals recrystallized by anti-solvent addition using methylene chloride and chloroform(Fig.5)as the crystallizing solvent showed high intensity and sharp peakssimilar to untreated ITZ,indicating no change in the polymorphism.It is suggested that,in these cases,only the change in crystal habit occurred.
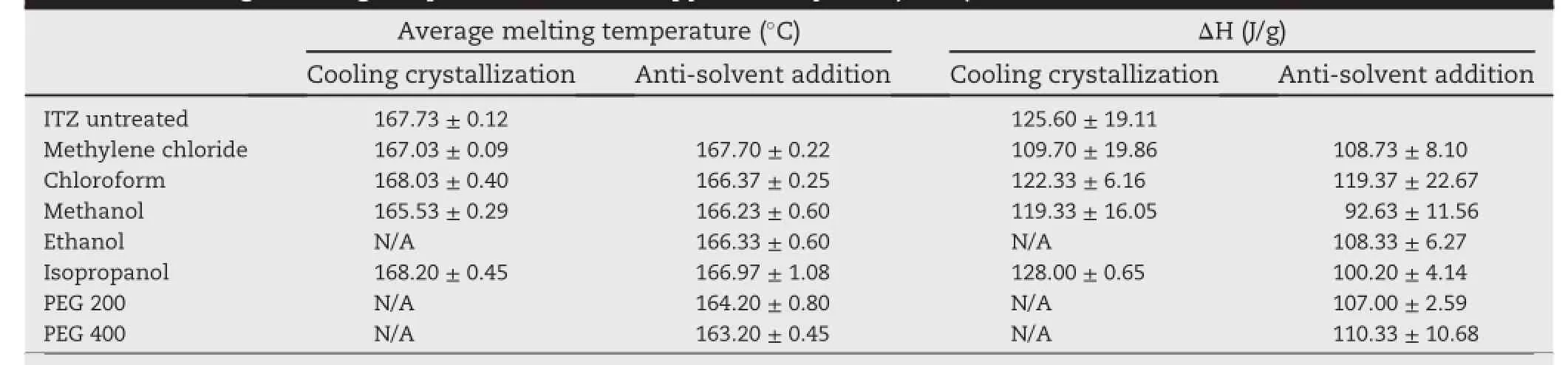
Table 3-Average melting temperature and enthalpy of ITZ crystals(n=3).
3.6.In vitro dissolution study
The dissolution of untreated ITZ was about 6%in 2 h while most of the prepared crystals showed higher drug dissolution. Crystals obtained from anti-solvent crystallization using PEG 200,which provided the highest surface area,showed the highest drug dissolution of about 10%(Fig.6).The amount of drugdissolution fromanti-solvent additioncould berankedas PEG200>methanol>chloroform>PEG 400>isopropanol>ethanol>methylene chloride.This may be due to the reduction of melting temperature and enthalpy (Table 3)and also the change in surface area(Table 2)and morphology of the crystals(Figs.1-2).The amount of drug dissolution from cooling crystallization using chloroform and methylene chloride was slightly lower than that of untreated ITZ and ITZ prepared from anti-solvent addition using the same solvent.However,the amount of drug dissolution from anti-solvent addition using methylene chloride was not signif i cantly different(P>0.05)from that of untreated drug.It is likely due to the insignif i cant difference between the melting temperature of both untreated ITZ and ITZ prepared from anti-solvent addition using methylene chloride(Table 3).
In order to examine the relationship between enthalpy of the crystals obtained from various conditions and dissolution rate,we have plotted the dissolution rate against the enthalpy and calculated the regression line of this system(Fig.7).The experimental data gave a straight line(r2=0.8035)and its slope was-0.0024,indicating a good correlation between enthalpy of the crystals and dissolution rate.The decrease in enthalpy tended to increase the drug dissolution.These data suggested that the drug dissolution could be estimated from the enthalpy data obtained by thermal analysis[24].However, deviation is found for crystals with high enthalpy which showed a high amount of drug dissolution.The non-related dissolution and enthalpy is demonstrated as(*)and(**)in Fig.7,which represented the crystals obtained from antisolvent addition using isopropanol and PEG 200 as the crystallizing solvent,respectively.The amount of drug dissolution from crystals obtained from anti-solvent addition using PEG 200,which is plate-shaped and high enthalpy,was more than that from anti-solvent addition using isopropanol,which is needle-shaped and lower enthalpy.Small f l aky,plate-like crystals providing more surface area leaded to higher drug dissolution,than larger needle-or blade-like crystals.Keraliya and coworkers[7]also reported the inf l uence of crystalmorphology on dissolution of tolbutamide crystals.The crystals with small plate-like shape showed higher dissolution rate than the large crystals with needle,cubic,and prismatic crystal habits.
4.Conclusion
Different crystal habits of ITZ were prepared by cooling crystallization and anti-solvent addition techniques using various crystallizing solvents.There was no polymorphic form of ITZ observed from PXRD pattern;therefore,the change of crystals may result from the alteration of crystal habit and enthalpy. Dissolution rate of the crystals was also inf l uenced by crystal habit and enthalpy.The dissolution rate of recrystallized ITZ showed almost linear relationship with the enthalpy,however,some exceptions were found.Although the enthalpy of the crystals preparedfrom anti-solvent additionusingPEG 200 was high,they could enhance the drug dissolution by 2 folds, compared to the untreated ITZ,which was the highest amount in this study.This may be due to their small plate-like morphology which could provide high surface area.Thus,the dissolution of the drug could be enhanced more than larger crystal habit.Recrystallization is a promising technique to alter physicochemical properties of the poorly water-soluble drugs.The change of recrystallizing methods and conditions also alters the properties of drug crystals.
Acknowledgements
Financial support from The Thailand Research Fund(grant number BRG5480013)is greatly acknowledged.
REFERENCES
[1]Blagden N,Matas D,Gaven PT,et al.Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates.Adv Drug Del Rev 2007;59:617-630.
[2]Fievet F,Lagier JP,Blin B,et al.Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ionics 1989;32-33:198-205.
[3]Lahav M,Leiserowitz L.The effect of solvent on crystal growth and morphology.Chem Eng Sci 2001;56:2245-2253.
[4]Bennema P,Eupen J,Wolf BMA,et al.Solubility of molecular crystals:polymorphism in the light of solubility theory.Int J Pharm 2008;351:74-91.
[5]Heng JYY,Thielmann F,Williams DR.The effects of milling on the surface properties of form I paracetamol crystals. Pharm Res 2006;23(8):1918-1927.
[6]Adhiyaman R,Basu SK.Crystal modif i cation of dipyridamole using different solvents and crystallization conditions.Int J Pharm 2006;321:27-34.
[7]Keraliya RA,Soni TG,Thakkar VT,et al.Effect of solvent on crystal habit and dissolution behavior of tolbutamide by initial solvent screening.Dissolution Technol 2010;17(1):16-21.
[8]Karashima M,Kimoto K,Kojima T,et al.Rational polymorph screening based on slow cooling crystallization of poorly soluble mebendazole.J Cryst Growth 2014;390:30-37.
[9]Mostafa Nowee S,Abbas A,Romagnoli JA.Antisolvent crystallization:model identif i cation,experimental validation and dynamic simulation.Chem Eng Sci 2008;63:5457-5467.
[10]Paulino AS,Rauber GS,Campos CEM,et al.Hollow crystal anti-solvent preparation process as a promising technique to improve dissolution of poorly soluble drugs.J Cryst Growth 2013;366:76-81.
[11]Glasmacher A,Prentice A.Current experience with itraconazole in neutropenic patients:a concise overview of pharmacological properties and use in prophylactic and empirical antifungal therapy.Clin Microbiol Infect 2006;12(Suppl.7):84-90.
[12]Gestel JV,Beule K.Pharmacology of itraconazole.Drugs 2001;61(1):27-37.
[13]Wohlfarth C.Dielectric constant of methanol.In: Lechner MD,editor.Springer materials-The Landolt-Bornstein database.Berlin:Springer-Verlag;2008.
[14]Wohlfarth C.Dielectric constant of ethanol.In:Lechner MD, editor.Springer materials-the Landolt-Bornstein database. Berlin:Springer-Verlag;2008.
[15]Seedher N,Bhatia S.Solubility of cox-2 inhibitors using various solvent systems.AAPS Pharm Sci Tech 2003;4(3). Article 33.
[16]Aida T,Aizawa T,Kanakubo M,et al.Relationship between volume expansion and hydrogen bond networks for CO2-alcohol mixtures at 40°C.J Phys Chem B 2010;114(43):13628-13636.
[17]Sengwa RJ,Kaur K,Chaudhary R.Dielectric properties of low molecular weight poly(ethylene glycol)s.Polym Int 2000;49:599-608.
[18]Swanson SE.Relation of nucleation and crystal-growth rate to the development of granitic textures.Am Mineral 1977;62:966-978.
[19]Berkovitch-Yellin Z.Toward an ab initio derivation of crystal morphology.J Am Chem Soc 1985;107:8239-8253.
[20]Nokhodchi A,Bolourtchain N,Dinarvand R.Crystal modif i cation of phenytoin using different solvents and crystallization conditions.Int J Pharm 2003;250(1):85-97.
[21]Hasa D,Voinovich D,Perissutti B,et al.Reduction of melting temperature and enthalpy of drug crystals:theoretical aspects.Eur J Pharm Sci 2013;50(1):17-28.
[22]Overhoff KA,Moreno A,Miller DA,et al.Solid dispersions of itraconazole and enteric polymers made by ultra-rapid freezing.Int J Pharm 2007;336:122-132.
[23]Werling J,Doty MJ,Rebbeck CL,et al.Polymorphic form of itraconazole.United States Patent.2007.US7193084B2.
[24]Yonemochi E,Yoshihashi Y,Terada K.Quantitative relationship between solubility,initial dissolution rate and heat of solution of chiral drugs.Pharm Res 2000;17(1):90-93.
*Corresponding author.Department of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand.Tel.:+66 34 255800;fax:+66 34 255801.
E-mail address:sriamornsak_p@su.ac.th(P.Sriamornsak).
Peer review under responsibility of Shenyang Pharmaceutical University.
1Current address:Faculty of Pharmacy,Rangsit University,Pathum Thani 12000,Thailand.
http://dx.doi.org/10.1016/j.ajps.2015.01.003
1818-0876/?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Poorly water-soluble drug Cooling crystallization
Anti-solvent crystallization
 Asian Journal of Pharmacentical Sciences2015年3期
Asian Journal of Pharmacentical Sciences2015年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- UPLC-MS/MS for the determination of azilsartan in beagle dog plasma and its applicationin a pharmacokinetics study
- Design and comparative in-vitro and in-vivo evaluation of starch-acrylate graft copolymer based salbutamol sulphate sustained release tablets
- Enhanced bioavailability of rebamipide nanocrystal tablets:Formulation and in vitro/in vivo evaluation
- Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation
- Chlorogenic acid loaded chitosan nanoparticles with sustained release property,retained antioxidant activity and enhanced bioavailability
