FDA’s Critical Path Initiative and Its Enlightenment to Medical Device Market Access System in China
LI Fei, BI Kai-shun
?
FDA’s Critical Path Initiative and Its Enlightenment to Medical Device Market Access System in China
LI Fei1, 2, BI Kai-shun1
Objective To studyFDA’s critical path initiative and summarize the methods and approaches that FDA has adopted to acceleratenew medical device products into the market, thus to provide references for constructing a modernized medical device market access path in China. Methods Literature review was adopted to conduct a study of FDA’s critical path initiative. Results and Conclusion The methods FDA has mainly adopted in the process of implementing the critical path initiative include the following: the critical path initiative should be applied to identify the critical activities in medical device market access regulations; each critical activity should be analyzed and optimized; new and more reliable safety evaluation guidelines, tools and relevant standards should be developed so as to instruct the industry to pass through the critical path with higher probability and efficiency.
FDA; critical path initiative; medical device; market access path
1 Preface
FDA has always been committed to the mission of protecting public health in the following three ways. First and foremost, ensure product safety and effectiveness; secondly, promote product innovations so as to provide safer, more effective and cheaper products to the public; thirdly, help the public to obtain accurate and scientific information as they need. In order to accomplish its mission, FDA proposes the critical path initiative (CPI) to address the growing crisis of moving products from basic research to the market and thus make improvement to the second of the three ways mentioned above, namely, to promote product innovations.
FDA first published the white paper on Critical Path in 2004, entitled “Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products”[1]. The report studied the causes of the crisis and trends of future development, analyzed the trials and development in the past in favor of the critical path initiative, and made plans for future direction. It concluded with a demand for collaboration among the industry, academic and scientific research institutions and the FDA to identify the key issues and work out solutions.
China’s medical device market access path is undergoing a critical reform at the moment. The study of FDA’s critical path initiative will be instructive for building China’s medical device market access path and achieving the long-term goal of ensuring both safety and development for our medical device products. The research scope of this paper includes the annual reports, guides and special projects of FDA from the year 2004 to 2011 and papers published in China’s national medical journals concerning the critical path initiative. Contributions to the research of China’s medical device market access system by these papers as well as their insufficient points are both analyzed in this paper.
2 FDA’s critical path initiative
The Critical Path was originally developed by Dupont Company and applied to project management. A critical path is a sequence of project end points which add up to the longest overall duration and determine the shortest time to complete the project. The duration of the critical path determines the duration of the whole project. Any delay of an end point on the critical path will directly impact the planned project completion date.
The critical path of medical device market access starts with the products selected to be the research object and ends with the products finally being brought to the market. Throughout this process, there exists a series of continuous evaluation activities, which are illustrated in Figure 1 below. The critical path starts from sample design, through clinical, development evaluation and quality system’s evaluation, to market application and premarket review, after which the product will be finally approved for sale on market. But the situation in real practice depends on the regulator’s specific arrangement, which is usually more complex in general terms. For example, in China, the products need to go through more activities such as product registration and examination, product manufacturing permission. The critical path is featured with the following characteristics:
(1) Duration of activities on the critical path determines overall duration of the market access. Duration of all activities on the critical path adds up to market access duration.
(2) Each activity on the critical path is a critical activity. Any delay of an activity will drag the duration of the overall market access progress.
(3) Critical path is the longest path from the start to the end. Any intention to shorten the duration of market access progress must resort to efforts along the critical path.
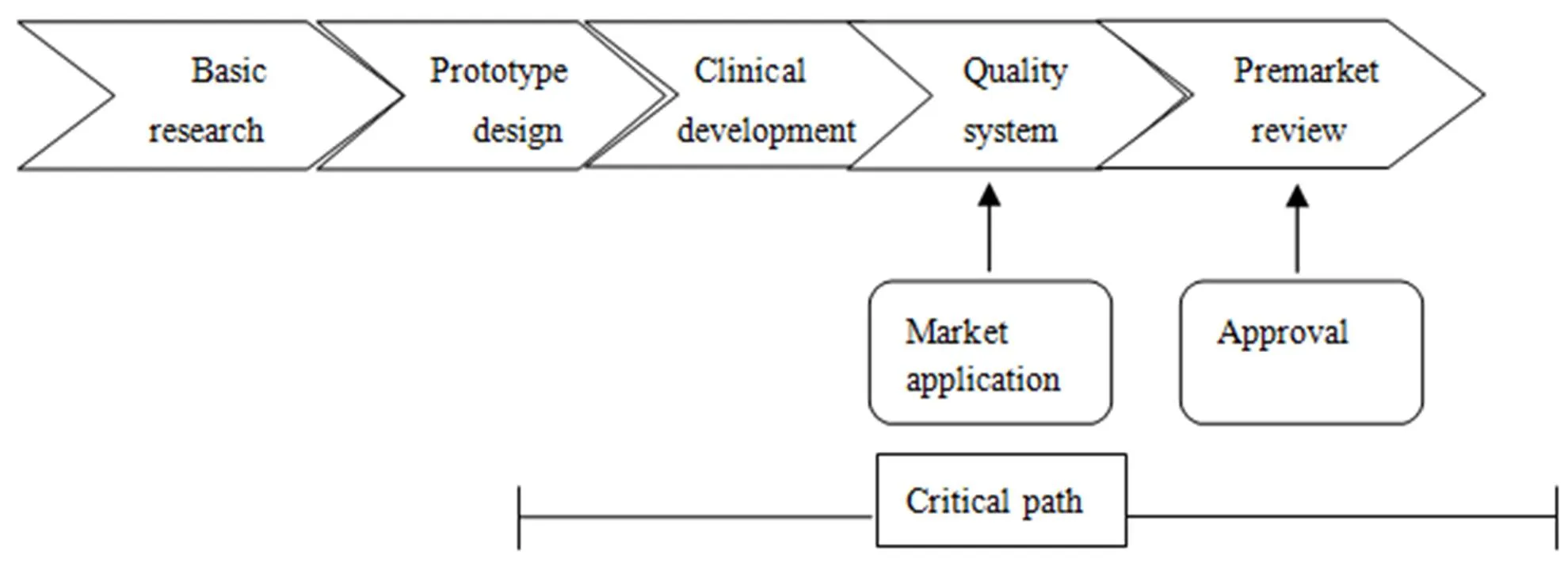
Figure 1 The critical path for medical product development
Due to the widely use of medical device products in medical fields, the safety of medical device products is closely related to public health security now.How to better regulate the medical device products so as to have their safety and effectiveness guaranteed has become a common goal of all countries around the world. Medical product market access, as a must-have before the entry of medical products into market, is also a critical link in the regulation chain. The market access path for medical device products resembles the assembly lines. For example, there are some processes like product examination, clinical trial, review from the quality control system, registration evaluation and so on. FDA found a problem in the research conducted in 2004 that the delivering speed of innovative products onto market was becoming slower rather than being accelerated.
During the ten years before 2004, investment from the industry in medical product research and development has increased 2.5 times and the US government investment in medical product research has increased 2.3 times. In contrast, the number of new compound drug applications has decreased by 10% and applications of new biological products have decreased by 90%[1]. The above-mentioned numbers indicate the fact that the pace of development work has not kept up with the innovations in basic sciences. In FDA’s view, efforts should be concentrated on providing sufficient support to the transformation process from basic scientific innovation to medical product development, which would require urgent breakthroughs in building a modernized critical path infrastructure.
FDA firstly analyses and summarizes four factors that may lead to the low efficiency of the critical path[2]: the growing cost that hinders enterprise’s input into new medical product research and development; the high failure rate in new medical product research and development that has impact upon effective allocation of research and development resources; the shortage of effective predictive tools that gives rise to higher uncertainty in the research and development of new medical products; and the outdated evaluative tools that fail to effectively evaluate current sciences.
In order to solve those problems, FDA planned to identify and set priorities to the following issues:
(1) the most pressing problems concerning research and development path at present;
(2) The greatest opportunities for the industry’s rapid development and public health benefit at present.
All these prioritized issues are done from three dimensions along the critical path: safety assessment, evaluation of medical utility, and product industrialization. FDA believes the most important issue in the list is to construct a new set of supportive tools for the research and development of products so as to enhance the feasibility and efficiency along the critical path.
Since the critical path initiative was officially launched in 2004, FDA has insisted on propelling and implementing it in ten years thereafter and FDA has the implementation of the planned activities and result evaluations published every year. In 2006, FDA published FDA’s Critical Path Opportunity Report, in 2007, FDA issued Key FDA Critical Path Activities Under Way in 2007. In 2008, FDA published Critical Path Activities Receiving Fiscal Support, In 2009, Critical Path Report on Key Achievements was issued. Reports on Projects Receiving Critical Path Support was released in 2010. CPI Supported Project Report to Congress: Improving the Prevention, Diagnosis and Treatment of Rare and Neglected Diseases was issued in 2011. All these efforts highlight the significant achievements that FDA has done in promoting product innovations.
Major progresses of FDA’s critical path initiative include:
(1) FDA’s critical path opportunities list[3];
(2) Guidelines and principles and critical path opportunities[4];
(3) Clinical trial transformation plan[5];
(4) Biomarkers and other tools;
(5) Product safety securing plan;
(6) FDA’s full electronic product and data management.
In the toolkit that constructed and practiced by FDA to support product research and development, guideline and principle documents are the most effective market access tools that FDA has always used. The purpose of FDA’s release of such guidelines and principles is to explain government policies and address regulative issues representing FDA’s current understanding of the government policies and regulative issues. These guidelines and principles are mainly targeted at the medical device product industry, including stake holders and FDA staff. Contents of guidelines and principles include design, manufacturing, sale and trial; application processing and content evaluation; examination and enforcement; and science-based issues. The Center for Devices and Radiological Health of FDA has published more than 700 guidelines and principles till now, which have effectively increased the predictability and efficiency of the critical path for medical device product research and development.
3 Implications for China’s medical device market access system
Taking China’s specific situation into consideration, the core values of FDA’s critical path initiative are reflected in two aspects: prioritizing scientific evaluation and optimizing the market access path.
Prioritizing scientific evaluation means to set the government service to more prior place in the market access process, which will facilitate the products’ integration with regulations and market access requirements earlier from the research and development stage so that the products will be designed to meet the standards of the market access system at the first place. This is critically important to improve product quality and shorten market access duration.
Optimizing market access path means to reduce the risks and uncertainties along the market access process, which is not only conducive to the development of new products and new enterprises but also is helpful for the existing products to be further enhanced and innovated, thus having a decisive role in promoting product innovation and development.
FDA’s critical path initiative was implemented for fulfilling its mission of ensuring the safety and effectiveness of the medical products, and promoting innovation, and the public is therefore offered with more effective and more affordable products, which is just in line with China’s regulating mission at the current stage. Therefore, China should use the critical path initiative for reference so as to reform the regulatory way, links and measures so as to improve efficiency, simplify procedures and promote medical device product’s safety and development.
In order to ensure the safety and development of medical device products on market, every country attaches great importance to the market access system and has it well established. Compared with FDA, the market access system for medical device products in China is faced with different situations:
In 1976, FDA published amendments to the Federal Food, Drug, and Cosmetic Act regarding medical devices and a mature market access system for medical device products was gradually formed from then on, which now involves classification, 510(k) procedures, PMA approval, GMP, clinical trial approval and monitoring, and so on. However, medical device products were brought into regulatory scope just in the year of 2000 in China and the first administrative rule of medical device product registration was published in 2004. Problems continued to appear in management during years thereafter and there has been no amendments to this rule yet till now. Therefore, the market access system in China is still at an initial stage and is unable to meet requirements of regulation and industry development.
For quite a long time, medical care, education and housing are the three major topics concerning people’s livelihood in China. The medical device industry, which plays an important supporting role in the construction of a social medical system, is attracting more and more attention from the public. The development of the medical device industry also brings new challenges to government’s regulation. Firstly, the medical device industry is experiencing rapid development due to its dual nature combining both that of the IT industry and that of the manufacturing industry. Public and private investment in medical device research and development continuously grows, however, the delivering speed of more effective and more affordable medical device products to the public becomes increasingly slower. This is because the industry development is hindered by the inefficient and costly medical device market access path which needs to be elevated at any cost. Secondly, the medical device product development needs to absorb novel achievements from other professional fields so as to improve clinical therapy and diagnosing standards. The current market access path includes complicated procedures for alteration registration, thus discouraging the industry to constantly improve and innovate the products. This is clearly not the goal that the industry’s regulation is expected to achieve. Thirdly, some key activities in the medical device market access system, such as classification, registration and technical review are in lack of a scientific mode based on objective evidences and risk control. As the system has not been formed yet, there are too many subjective and uncertain factors in the market access system that may affect the regulation and sustainable development of the industry.
A mature market access system should not be confined to the control of products before they are brought to market. It should further gradually form a set of management concepts based on theories and principles so as to provide guidance to the industry for its sound development. Such guidance include encouraging continuous product innovation, improving effectiveness with lowered risk in the meantime, propelling enterprises to improve quality management, facilitating industry’s integration and enhancement and so on. For example, the risk management is not only a requirement of the medical device manufacturer’s self control but also a compulsory requirement of China’s regulations. As the liable entities, the manufacturing enterprises should implement risk management at all stages through the product’s complete life cycle[6]. However, the risk management activities of many Chinese enterprises are usually ended with a risk management report prepared for market access when it is needed. The market access fails to play a role that propels the enterprises to carry out risk management concepts.
Therefore, FDA has formed a mature market access system, with its critical path initiative focusing on developing new and more reliable safety evaluation tools and related standards. The market access system in China is still at an initial stage and a more scientific and mature market access system is needed for the industry development. The emphasis of the reform should be laid on constructing a modernized market access system for the medical device products.
In conclusion, the regulatory authorities of the government should make effective and reasonable use of the regulatory resources and approaches, reform and construct a science-based, rational and adaptable market access path so as to realize the goal of regulating the medical device products, namely, to provide safer, more effective products to patients with affordable prices and at a faster speed.
At present, few systematic study of China’s medical device market access has been carried out. Most previous studies are mainly focused on the introduction of regulating modes and experiences of foreign governments. They neither identify the problems exiting in China’s market access path nor objectively evaluate the standards of the market access path. FDA’s CPI gives some valuable implications on the following regards:
(1) Apply Critical Path Method to identify the critical activities in medical device market access regulations;
(2) Analyze the problems at every step on the critical path, coordinate all relevant parties to resolve the hindering factors thoroughly;
(3) Proactively reform the mechanisms of medical device product classification, clinic trial and review, so that the market access path for medical device products becomes safer and more efficient;
(4) Develop new and more reliable safety review guides, tools and corresponding standards and instruct the industry to pass through critical path with efficiency.
Therefore, the critical efforts to build a medical device market access system in China are: to discuss and analyze the problems existing in current medical device market access path, meanwhile, explore their causes and propose corresponding solutions, by applying management theories and critical path method, to objectively analyze and elaborate on the critical path of the market access and its regulations; to construct critical path models by applying modeling methodology; to evaluate the models’ impact upon the safety and effectiveness of medical device products by applying risk analysis methodology; employing economic methodology to evaluate the models’ impact upon efficiency and cost of the medical device market access. It will be ready then to have an objective evaluation of the critical path, seek optimization to it and further propose feasible modes and approaches on how to improve the path. From perspectives of model constructing, critical path method and model evaluating, comparative studies are conducted to find how foreign countries reform their classification modes, clinical trials and 510(k) registration mode in order to adapt to the medical device industry and technological development so that a market access system for medical device products that fits China can be constructed.
[1] FDA. Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products [EB/OL]. http://www.fda.gov, 2004-03-16.
[2] FDA. Innovation/Stagnation: Critical Path Opportunity Report [EB/OL]. http://www.fda.gov, 2007-06-15.
[3] FDA. Innovation/Stagnation: Critical Path Opportunity list [EB/OL]. http://www.fda.gov, 2006-03-21.
[4] FDA. Key FDA Critical Path Activities under Way in 2007 [EB/OL]. http://www.fda.gov, 2008-06-15.
[5] FDA. The Critical Path Initiative: Report on Key Achievements in 2009 [EB/OL]. http://www.fda.gov, 2009-06-30.
[6] LI Fei, LV Da-lei. An Impact Factors Study on the Risk Management Level of Medical Device Manufactures [J]. China Medical Device Information, 2010, 16 (4): 74-77.
Author’s information: BI Kai-shun, Professor. Major research area: National drug system. Tel:024-23986012, E-mail: bikaishun@126.com
- 亞洲社會(huì)藥學(xué)雜志的其它文章
- E-commerce Marketing Models of Fisher Scientific Company in Canada: Analysis and Its Enlightenment
- The Development of Chinese Medicinal Materials and Decoction Pieces Industry: Problems and Countermeasures
- Online Pharmacy ModelChoice of Pharmaceutical Retail Chain Enterprises
- An Research on Establishing the System of Drug Safety Mandatory Liability Insurance
- On Digital Drug Management in One Hospital Pharmacy
- Current Status of Pharmaceutical Retail Market: Problems and Countermeasures

