Withania somnifera extract reduces the invasiveness of MDA-MB-231 breast cancer and inhibits cytokines associated with metastasis
Department of Biomedical Sciences, School of Veterinary Medicine, Tuskegee University, Tuskegee, Alabama 36088, USA.
2Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, Alabama 35294, USA.
Withania somnifera extract reduces the invasiveness of MDA-MB-231 breast cancer and inhibits cytokines associated with metastasis
Kamel F. Khazal1, Donald L. Hill2
1Department of Biomedical Sciences, School of Veterinary Medicine, Tuskegee University, Tuskegee, Alabama 36088, USA.
2Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, Alabama 35294, USA.
Aim: The aim was to examine the anti-proliferative effect of a Withania somnifera (WS) root extract in cell cultures and nude mouse xenografts of breast cancer cell line MDA-MB-231. Methods: WS root extract was used to treat tumor cells at concentrations up to 100 μg and for nude mouse experiments, the mice received daily WS at 300 mg/kg by oral gavage for 8 weeks. Results: The WS extract reduced viability of MDA-MB-231 cells by 75% and 88% after exposure of the cells to 50 and 100 μg/mL, respectively, compared to vehicle-treated controls. WS extract caused a dose-dependent increase in the percentage of cells in the sub-G1 phase compared to untreated controls by 6% and 10% after exposure to 25 and 50 μg/mL WS extract, respectively. WS extract also inhibited proliferation of xenografted MDA-MB-231 cells. The WS extract caused reductions in xenograft size by 60% compared to the untreated control after 8 weeks of treatment. Six of ten mice in the control group showed tumor metastasis to the lung, whereas there was none in the mice treated with the WS extract. At the gene level, WS caused a 75% reduction in chemokine CCL2 expression (P < 0.05) in the xenografted tumors of the treated mice. Conclusion: WS root extract inhibited proliferation of breast cancer cells in vitro and in vivo and signif cantly reduced expression of the cytokine, CCL2. These results warrant further studies to assess the underlying molecular mechanism of the anti-tumor activity of the WS extract in breast cancer.
Withania somnifera extract, MDA-MB-231, breast cancer, metastasis, animal model
Ⅰntroduction
Invasive breast cancer is considered one of the great challenges for clinicians to control and improve survival of patients. In 2013, an estimated 232,340 new cases of invasive breast cancer were diagnosed in women in the USA, along with other 64,640 cases of non-invasive breast cancer.[1]For women under 45, deadly forms of this type of breast cancer are more common in African-American women than white women, and African-American women are more likely to die of breast cancer.[2]Despite three decades of advances in treatment of breast cancer using hormone receptor modulators, aromatase inhibitors, and surgery,[3-5]mortality remains high due to tumor metastasis to the lymph nodes, liver, and lung.[6]Triple-negative breast cancer (TNBC) accounts for 10-20% of diagnosed breast cancers and is more likely to affect younger African Americans, Hispanics, and/or those with BRCA1 mutations. TNBCs are more aggressive, diff cult to treat, and more likely to spread and recur.[2]TNBCs are different from other kinds of breast cancer in that they are highly metastatic and resistant to conventional therapies, such as anticancer drugs and radiation.[2]
In a search for an agent that inhibits proliferation and invasion of TNBCs, we evaluated an extract derived from an Indian herb, Withania somnifera (WS), which is a nightshade medicinal plant that contains active components for the treatment of a variety of ailments, including cancer.[7-10]The use of WS root extract is practical since it contains the active compounds present in the plant. In TNBC cells, sub-cytotoxic concentrations of withaferin A, derived from WS, reduce various effectors of metastasis.[11]In the present study, we assessed the effect of the WS extract on proliferation and metastasis of MDA-MB-231 cells, derived from a TNBC, in cell cultures, and in mice.
Methods
Preparation of WS extract
Roots of WS were ground to a paste, and then extracted with 5 volumes of 70% ethanol by stirring for 2 days. The alcoholic extract was f ltered, and the solvent was evaporated under a vacuum. The extract was then dried to a powder and kept in a closed container until use.[12]To avoid variations in the activity of different preparations, the suff cient extract was obtained in one batch for use throughout the experiments.
Reagents and antibodies
WS roots were purchased from a local market in the USA and dimethyl sulfoxide (DMSO) from Sigma (St. Louis, MO, USA). Antibodies (anti-chemokine CCL2, CXCL1, CXCL2, CXCL3, PARP, and GAPDH) were from Cell Signaling (Beverly, MA, USA). Human breast cancer MDA-MB-231 cell line and a normal breast cell line, MCF10A, were obtained from ATCC (Manassas, VA, USA). The HCA-II human cytokine primer kit was obtained from Real Time Primers (Elkins Park, PA, USA).
Cell culture and treatment
Breast cancer MDA-MB-231 cells were maintained in Dulbecco’s Modifed Eagle’s Medium (ATCC) supplemented with 10% fetal bovine serum and penicillin/streptomycin. MCF10A cells were maintained in complete MEGM (Lonza, Houston, TX, USA). All cell cultures were incubated at 37 °C with 5% CO2in a humidif ed incubator.
Assessment of cell viability
To assess the effect of the WS extract on regulation of cell viability, cells were seeded into 96-well, 6-well or 6-cm plates at densities of 103, 104or 105cells per well, respectively. For experiments requiring longer than 48 h, cell numbers were reduced by one half. Viability was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazo lium assay in 96-well plates in triplicate with CellTiter 96?AQueous One Solution cell proliferation kits from Promega (Madison, WI) according to the manufacturer’s instructions. Absorbance was recorded at 490 nm using a Synergy HT multimode plate reader or PowerWave XS2 (BioTek?, Winooski, VT, USA) reader. DMSO was used as a control. To calculate the viability index, absorbance readings from DMSO-treated control wells were set at 100%, and the relative A490 was calculated as a percentage of the control.
Flow cytometry
Cells treated with the WS extract were harvested and prepared for f ow cytometry as described by Samuel et al.,[13]with some modif cations. WS treated and untreated cells were harvested by trypsinization in 0.25% trypsin/ethylenediaminetetraacetic acid. Prior to trypsinization, f oating or loose cells were harvested by gentle rocking of the culture dishes and transferring the culture medium containing the cells into centrifuge tubes. Trypsinized and detached cells were then combined and centrifuged. Cell pellets were suspended in 300 μL of phosphate-buffered saline (PBS), f xed with 700 μL of 100% ethanol with vortexing, and stored at -20 °C overnight. The f xed cells were centrifuged and stained in f uorescence-activated cell sorting staining solution (3 mg/mL RNase A, 0.4 mg/mL propidium iodide) in PBS without calcium or magnesium for 30 min at 37 °C and then f ltered through a 70-μm f lter and analyzed by f ow cytometry (FACScalibur?Becton Dickinson or C6 Accuri?f ow cytometer). Data were analyzed with CellQuest and CFlow software (BD).
Immunocytochemistry
Breast cancer MDA-MB-231 cells were seeded in 4-well plates and grown for 16 h. The cells were then treated with DMSO (vehicle) or with 25 or 50 μg/mL of WS root extract for 18 h. After treatment, the culture medium was removed, and the cells were f xed with 10% neutral buffered formalin. Xenograft tissues were placed in an automatic tissue processor, embedded in paraff n, sectioned at 5-μm thickness, and stained with hematoxylin and eosin (HE). For immunohistochemistry, the f xed cells and tissues from xenografted tumors were stained with CCL2 antibody because this cytokine is considered to be most responsible for metastasis of breast cancer.[14]The sections were de-paraff nized in xylene and rehydrated through a series of graded ethanol (100%, 95%, and 70%) and in water for 5 min each. The sections were then washed three times for 5 min each in PBS containing 0.05% Tween 80 (pH 7.4). Antigen retrieval was achieved by heating the sections in a microwave with 0.01 mol/L sodium citrate (pH 6.0) solution and subsequently cooling down to room temperature. Endogenous peroxidase activity was blocked by incubating the sections for 30 min in 1% hydrogen peroxide in methanol. Non-specif c binding was blocked by incubating the sections for 1 h with a normal horse serum (Vector Laboratories, Inc., Burlingame, CA, USA). The sections were then incubated with mouse anti-CCL2 (MCP-1, eBioscience, San Diego, CA, USA) overnight at 4 °C. On the next day, the sections were rinsed 3 times with PBS at room temperature and then further incubated with goat anti-mouse IgG-FITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. The f uorescence was then read using a wide-f eld f uorescent microscope (Olympus, Center Valley, PA, USA). Stained sections were reviewed and scored according to the intensity of staining (0, +1, +2 or +3) and for the percentage of tumor cells staining positive for CCL2 (0%, 0.1-30%, +1; 31-70%, +2; or > 70%, +3). The score of the intensity of immunostaining was multiplied by the score of percentage of cell staining to obtain the f nal staining index.
RNA isolation and quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated from treated and control samples with RNeasy Mini Kits (Qiagen, Valencia, CA, USA) and reversely transcribed into cDNA using Quantitect Reverse Transcriptase Kits (Qiagen) according to the manufacturer’s instructions. All primers were from SABiosciences (Valencia, CA, USA); and quantitative polymerase chain reaction (qPCR) amplif cation was performed using 50 ng of cDNA, 10 μL of Brilliant III Ultra-Fast SYBR Green qPCR Master Mix(Agilent Technologies, Santa Clara, CA, USA), and 500 nM of each primer. β-Actin was used as the internal control, and the f nal reactions were adjusted to a total volume of 20 μL with DNase RNase-free water (Qiagen). All qPCR amplif cation was performed in duplicates with a Stratagene Mx 3005P system (Agilent Technologies), and the conditions were set to initial cycle of denaturation at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min. The f nal segment involved generation of a dissociation curve. This comprised one cycle at 95 °C for 1 min, followed by 55 °C for 30 s and 95 °C for 30 s. Inclusion of a dissociation curve in each qPCR run ensured specif city of the amplicon.
Microarray analysis
To determine the effect of WS extract on expression of cytokines in MDA-MB-231 cells, cells were incubated overnight with either 50 μg/mL WS or DMSO (vehicle) as a control. The analysis was accomplished by use of HCA-II cytokine primer library II according to the manufacturer’s instructions.
Experimental mice and treatments
Athymic Nude-Foxn1numice at 6 weeks of age were obtained from Harlan Sprague-Dawley and housed in animal quarters at 22 °C with a 12 h light/dark cycle. Animals were given free access to water and food. These studies were approved by the Tuskegee University Institutional Animal Care and Use Committee. At 8 weeks of age, mice were injected subcutaneously with 0.2 mL of PBS containing 1.5 × 106human breast cancer MDA-MB-231 cells into the right f anks. Twenty mice that developed tumor sizes of 50-200 mm3were divided into two equal groups. The control group received 0.2 mL of 5% DMSO orally by gavage, and the treated group received 300 mg/kg/day WS root extract dissolved in 5% DMSO orally by gavage daily for 5 days a week for 8 weeks. Tumor sizes were checked weekly in each group. Tumor dimensions in mm (length and width) were measured with vernier calipers and calculated for each tumor by using the following equation : tumor volume = 1/2 (length × width2). At the end of the 8th week, mice were euthanized with CO2. Tumors and lung tissues were collected and f xed with 10% formalin for histopathological and immunochemistry analysis.
Evaluation of lung metastasis
Two pathologists histopathologically evaluated lung metastases in untreated and treated groups after staining of sections with HE, and the results were reported independently. The number of metastatic foci was counted in each stained tissue section.
Statistical analyses
Student’s t-test was used to assess differences between values for the treated and control groups. One-way analysis of variance was used with Dunnett’s test.
Results
WS extract caused a dose-dependent reduction of viability of breast cancer MDA-MB-231 cells by 75% and 88% after treatment with 50 or 100 μg/mL WS extract, respectively, compared to vehicle-treated controls [Figure 1], but WS treatment did not affect the viability of non-cancerous epithelial mammary cells, MCF10A [Figure 2]. Moreover, compared to untreated controls, WS extract caused a concentration-dependent increase in the sub-G1 phase of the cell population, by 6% and 10% after exposure to 25 μg/mL and 50 μg/mL, respectively [Figure 3].
Furthermore, WS extract inhibited proliferation of xenografted MDA-MB-231 cells, reducing the size of xenografted tumors by 60% compared to the untreated control after 8 weeks of treatment (P < 0.05) [Figure 4]. In addition, after euthanasia, six of ten mice in the control group showed tumor metastasis to the lung, whereas none of the mice in WS-treated group developed metastasized tumor lesions in the lung [Figure 5]. This f nding motivated us to explore the underlying molecular mechanism by which the WS extract inhibited tumor metastases to the lung. Microarray analysis of gene expression of cytokines was then performed. WS suppressed expression of CCL2, CXCL1, CXCL2, CXCL3, IL1B, TGFB3, and BMP4 mRNA [Figure 6]. These inhibitory effects were conf rmed by quantitative reverse transcriptionpolymerase chain reaction analysis [Figure 7]. WS caused a 75% reduction in CCL2 expression (P < 0.05) in the xenografted tumors of treated mice [Figure 8].
Discussion
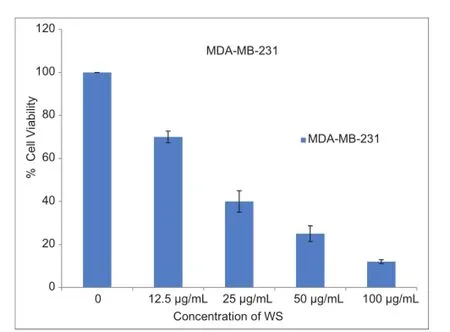
Figure 1: Effect of WS on viability of breast cancer MDA-MB-231 cells. The bars represent the mean ± standard deviation of six 24-h treatments for the vehicle and different concentrations of WS. The results are statistically signif cant (P < 0.05) compared to the DMSO-treated (control) cells as determined by one-way ANOVA with Dunnett’s test. WS: Withania somnifera; ANOVA: Analysis of variance; DMSO: Dimethyl sulfoxide
The current study assessed the effect of an alcoholic extract of WS roots on proliferation and metastasis ofbreast cancer MDA-MB-231 cells in vitro and in nude mice, respectively. WS roots have been used in ayurvedic medicine for their anti-inf ammatory, analgesic, anticancer, and anti-stress properties.[7,8]These diverse effects are attributed to the presence of active steroidal compounds that are called withanolides.[15]Our current data showed that the WS extract inhibited proliferation and metastasis of MDA-MB-231 cells in vitro and in nude mice. This inhibition was greater than that caused by withaferin A.[16]The difference in inhibition may be attributed to the fact that the whole extract contains active ingredients that have a synergistic effect against breast cancer cells.[7,17]Since MDA-MB-231 cells are“triple-negative” form estrogen-independent tumors in vivo, the anti-proliferative effect of WS is apparently estrogen-independent. The WS extract caused increases in the percentage of MDA-MB-231 cells in the sub-G1 phase, indicating that WS causes apoptosis. Withaferin A, one of the active compounds of WS, causes G (2)/M cell cycle arrest, associated with modulation of cyclin B1, p34(cdc2), and PCNA levels, decreases the levels of STAT3 and its phosphorylation at Tyr(705) and Ser(727), and alters expression levels of p53-mediated apoptotic markers-Bcl2, Bax, caspase-3, and cleaved PARP.[18]
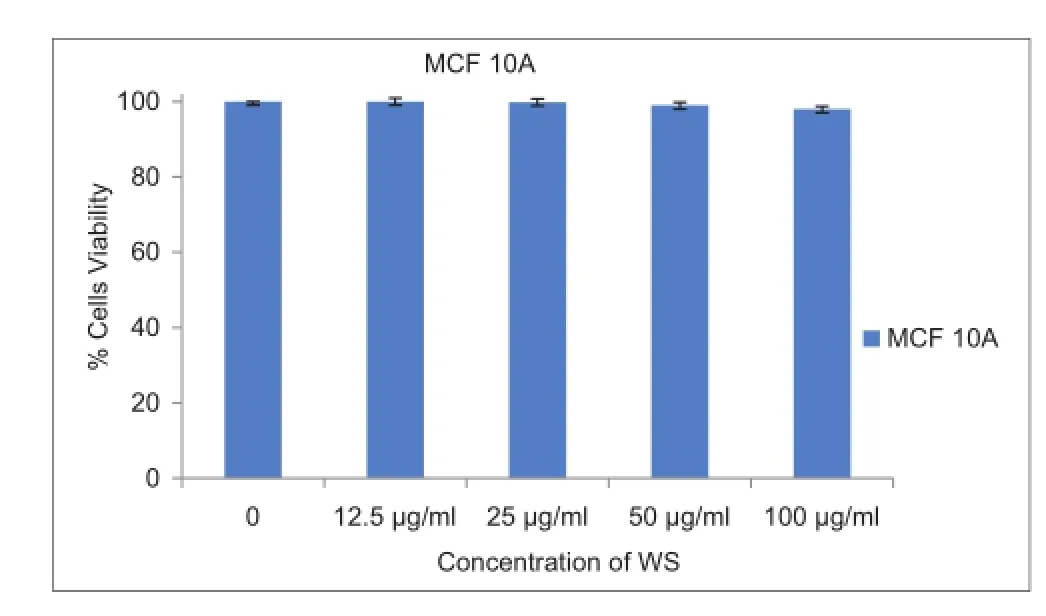
Figure 2: Effect of WS on the viability of non-cancerous epithelial mammary cells, MCF10A.The bars represent the mean ± standard deviation of six 72-h treatments for the vehicle and different concentrations of WS. As determined by one-way ANOVA, results of treated cells are not statistically signif cant compared to the DMSO-treated (control) cells. WS: Withania somnifera; ANOVA: Analysis of variance; DMSO: Dimethyl sulfoxide
Results of our current mouse experiments are consistent with in vitro data. The WS extract, administered orally, inhibited formation and growth of MDA-MB-231 cell xenografts in nude mice, indicating that the active ingredients of the WS extract are bioavailable after oral administration.[19]Six mice of the untreated group developed tumor metastasis to the lung, whereas none of the treated mice showed such tumor metastases. This effect may be attributed to inhibition of CCL2 in xenografted tumors after treatment with WS root extract. These results are consistent with a previous study[20]concerning the inhibition of CCL2 in animals. Inhibition of CCL2/CCR2 signaling by anti-CCL2 antibodies blocks recruitment of inf ammatory monocytes, inhibits metastasis, and prolongs the survival of tumor-bearing mice. Depletion of tumor cell-derived CCL2 also inhibits metastatic seeding. Moreover, CCL2 mediates development of cancer stem cell (CSC) phenotypes. Promotion of CSC is relevant since these cells, through self-renewal, maintain heterogeneity and give rise to metastasis of breast cancer.[21]
a
b
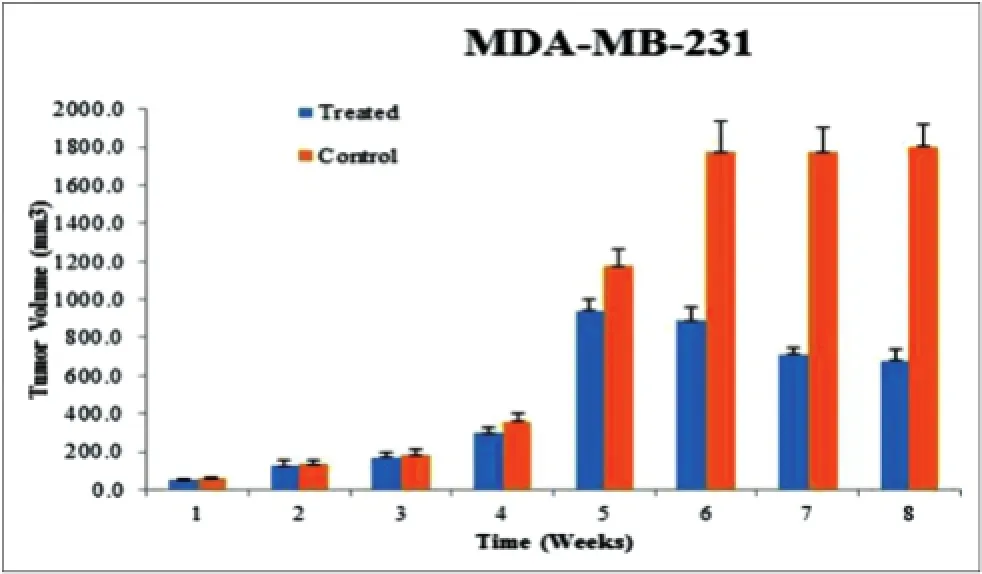
Figure 4: Effect of WS on suppression of growth of xenografted MDA-MB-231 cells in nude mice. The bars represent the means ± standard deviations of tumor size (mm3) (n = 10). The highest reduction (60%) relative to the untreated control was shown after 8 weeks of WS treatment (P < 0.05). Student’s t-test was used to assess significant differences between treated groups and the untreated control group. WS: Withania somnifera
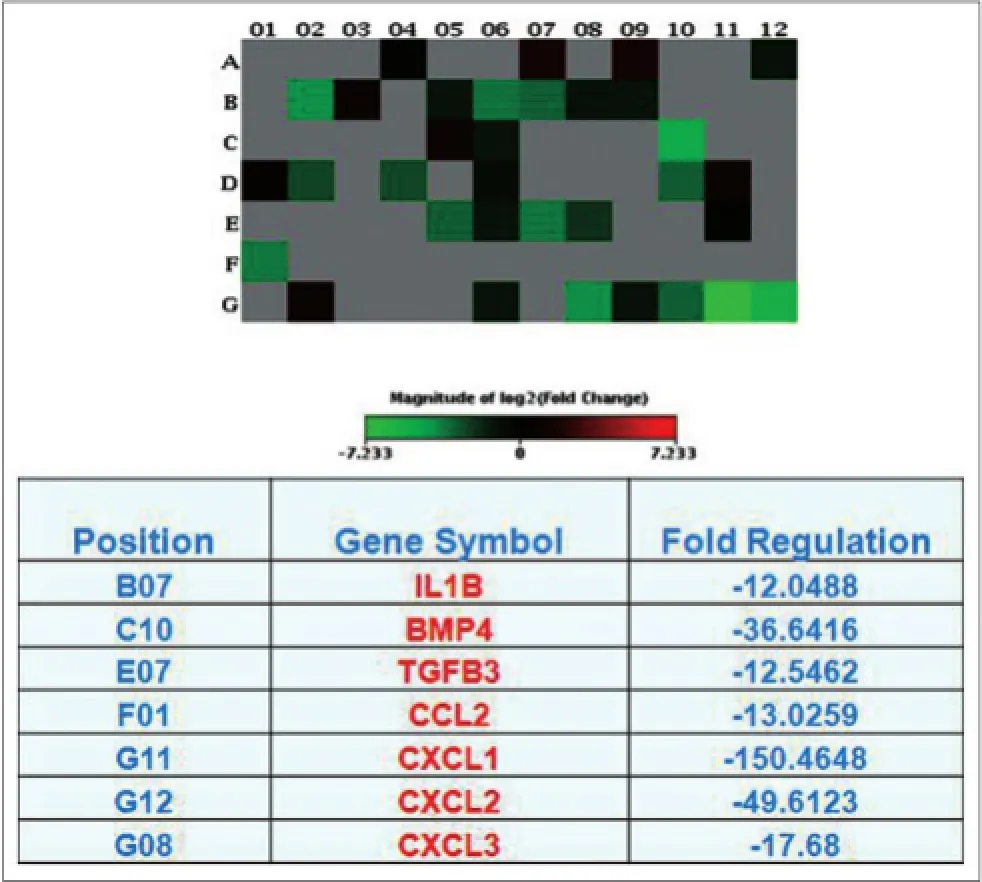
Figure 6: Effect of WS on inhibition of cytokine/chemokine expression. WS: Withania somnifera
Our current data are consistent with those reported by others.[17]A root extract of WS showed dose-dependent inhibition of tumor growth and metastatic lung nodule formation with the minimal toxicity to mice.[17]The extract apparently inhibited cancer metastasis through inhibition of the epithelial-mesenchymal transition (EMT). Furthermore, withaferin A treatment of MCF-10A cells inhibited EMT and in mice, reduced mammary cancer growth, effects of which were associated with reduced vimentin expression.[22]In the present study, the oral dose of WS extract used to inhibit tumor metastasis to the lungs was 300 mg/kg/day body weight. This dose was extrapolated from the cell culture experiments regarding the effect of WS extract on MDA-MB-230 cells. This dose was selected based on a pilot study involving a range of doses to estimate the optimal dose. In addition, the in vitro cytotoxic concentration, ranging between 50 and 100 μg/mL, gave us an idea about the dose. In a previous study, WS root extract inhibited lung metastasis of xenografted MDA-MB-231 cells at a dose of 8 mg/kg body weight, administered 3 times a week for 4 weeks.[19]This dose is 37.5 times less than the dose used in our current study. There is no obvious explanation for the difference in the two doses. Differences in the source of roots, age of roots, and extraction yield may contribute to different dose-responses when using crude plant extracts. However, the WS extract, at a dose of 150 mg/kg/day for 155 days, caused a 23% reduction in development of mammary tumors in rats administered the carcinogen, methylnitrosourea.[23]
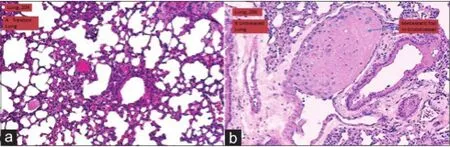
Figure 5: Effect of WS treatment on inhibition of lung metastasis in nude mice. HE staining of lung tissue sections after treatment with or without WS for 8 weeks (×20). (a) WS treated mouse lungs showed no tumor metastasis (n = 10); (b) six of ten mice showed tumor metastasis to the lungs, with a total of 12 metastatic foci in the blood vessels and the parenchyma of the lungs in control mice. WS: Withania somnifera

Figure 7: Effect of WS treatment on the regulation of cytokine expression. Quantitative reverse transcription-polymerase chain reaction was used to measure cytokine expression in cells treated or not treated with 50 μg/mL WS. WS: Withania somnifera
In transgenic (MMTV/Neu) mice that received a diet containing the extract (750 mg/kg of diet) for 10 months, mice in the treated group (n = 35) had an average of 1.66 mammary tumors, and mice in the control group (n = 33) had 2.48, a reduction of 33%. Moreover, in treated mice, WS caused a 50% reduction in the expression of CCL2.[24]
WS caused in vitro and in vivo inhibition of breast cancer MDA-MB-231 cells and caused a signif cant reduction in expression of the cytokine, CCL2, a marker of the metastasis of breast cancer to other organs. These results warrant further studies to assess the underlyingmolecular mechanism of WS extract antitumor activity in the breast cancer metastasis.
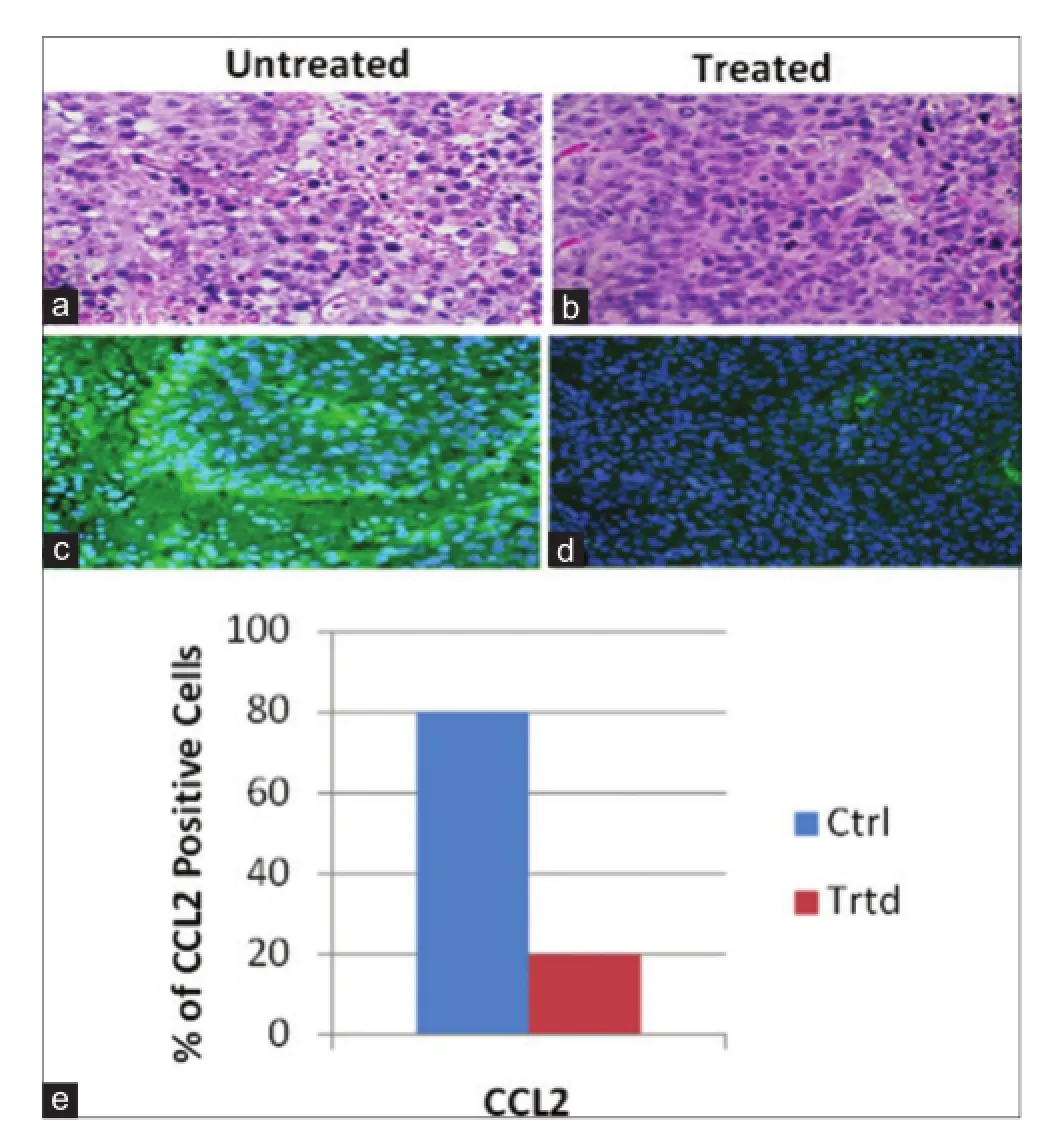
Figure 8: Effect of WS on expression of CCL2 in MDA-MB-231 xenografted tumors. (a and b, ×20) Hematoxylin and eosin sections of untreated and WS treated tumors, respectively; (c and d, ×20) immunohistochemical staining of CCL2 in untreated and WS treated tumors; (e) summarized data. There was a signif cant reduction (P < 0.05) in CCL2 expression in WS-treated tumors compared to untreated tumors as determined by Student’s t-test. WS: Withania somnifera; CCL2: Chemokine (C-C motif) ligand 2
Acknowledgments
This project was supported by NIH Grant U54 CA 118948.
1. Cancer Facts and Figures. American Cancer Society; 2014. Available from : http://www.cancer.org/research/cancerfactsstatistics/ cancerfactsf gures 2014/. [Last accessed on 2015 Mar 14].
2. Triple Negative Breast Cancer. Available from: http:// www.nationalbreastcancer.org/triple-negative-breast-cancer. [Last accessed on 2015 Mar 14].
3. Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88.
4. Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, Farrerons J, Karasik A, Mellstrom D, Ng KW, Stepan JJ, Powles TJ, Morrow M, Costa A, Silfen SL, Walls EL, Schmitt H, Muchmore DB, Jordan VC, Ste-Marie LG. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat 2001;65:125-34.
5. Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H; NCIC CTG MAP. 3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381-91.
6. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7.
7. Mishra LC, Singh BB, Dagenais S. Scientif c basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 2000;5:334-46.
8. Winters M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern Med Rev 2006;11:269-77.
9. Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod 2007;70:461-77.
10. Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 2003;74:125-32.
11. Szarc vel Szic K, Op de Beeck K, Ratman D, Wouters A, Beck IM, Declerck K, Heyninck K, Fransen E, Bracke M, De Bosscher K, Lardon F, Van Camp G, Vanden Berghe W. Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specif c to triple negative breast cancer cells. PLoS One 2014;9:e87850.
12. Senthilnathan P, Padmavathi R, Magesh V, Sakthisekaran D. Modulation of TCA cycle enzymes and electron transport chain systems in experimental lung cancer. Life Sci 2006;78:1010-4.
13. Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res 2005;65:210-8.
14. Li M, Knight DA, Snyder LA, Smyth MJ, Stewart TJ. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology 2013;2:e25474.
15. Namdeo AG, Sharma A, Yadav KN, Gawande R, Mahadik KR, Lopez-Gresa MP, Kim HK, Choi YH, Verpoorte R. Metabolic characterization of Withania somnifera from different regions of India using NMR spectroscopy. Planta Med 2011;77:1958-64.
16. Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a-and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 2008;68:7661-9.
17. Subbaraju GV, Vanisree M, Rao CV, Sivaramakrishna C, Sridhar P, Jayaprakasam B, Nair MG. Ashwagandhanolide, a bioactive dimeric thiowithanolide isolated from the roots of Withania somnifera. J Nat Prod 2006;69:1790-2.
18. Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011;32:1697-705.
19. Yang Z, Garcia A, Xu S, Powell DR, Vertino PM, Singh S, Marcus AI. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS One 2013;8:e75069.
20. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inf ammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222-5.
21. Palacios-Arreola MI, Nava-Castro KE, Castro JI, Garcia-Zepeda E, Carrero JC, Morales-Montor J. The role of chemokines in breast cancer pathology and its possible use as therapeutic targets. J Immunol Res 2014;2014:849720.
22. Lee J, Hahm ER, Marcus AI, Singh SV. Withaferin A inhibits experimental epithelial-mesenchymal transition in MCF-10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog 2013;54:417-29.
23. Khazal KF, Samuel T, Hill DL, Grubbs CJ. Effect of an extract of Withania somnifera root on estrogen receptor-positive mammary carcinomas. Anticancer Res 2013;33:1519-23.
24. Khazal KF, Hill DL, Grubbs CJ. Effect of Withania somnifera root extract on spontaneous estrogen receptor-negative mammary cancer in MMTV/Neu mice. Anticancer Res 2014;34:6327-32.
How to cite this article:Khazal KF, Hill DL. Withania somnifera extract reduces the invasiveness of MDA-MB-231 breast cancer and inhibits cytokines associated with metastasis. J Cancer Metastasis Treat 2015;1:94-100.
Received:23-01-2015;Accepted:15-04-2015.
Source of Support:Nil,Conf ict of Interest:None declared.
Dr. Kamel F. Khazal, Department of Biomedical Sciences, School of Veterinary Medicine, Tuskegee University, Tuskegee, Alabama 36088, USA. E-mail: kamel@mytu.tuskegee.edu
Website:
www.jcmtjournal.com
10.4103/2394-4722.157601
 Journal of Cancer Metastasis and Treatment2015年2期
Journal of Cancer Metastasis and Treatment2015年2期
- Journal of Cancer Metastasis and Treatment的其它文章
- Introduction to Volume 1 Issue 2 of Journal of Cancer Metastasis and Treatment
- Spinal intradural mature teratoma in an elderly patient
- Orbital metastasis from anorectal carcinoma
- Metastatic inguinal lymph nodes with two different histological types in a case of carcinoma of unknown primary site
- Squamous cell carcinoma of the lung: clinical criteria for treatment strategy
- Prognostic signif cance of transcription factors FOXA1 and GATA-3 in ductal carcinoma in situ in terms of recurrence and estrogen receptor status
