Serine-threonine protein kinase activation may be an ef ective target for reducing neuronal apoptosis after spinal cord injury
Mu Jin, Yan-wei Yang, Wei-ping Cheng, Jia-kai Lu, Si-yu Hou, Xiu-hua Dong, Shi-yao Liu
Department of Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China
Serine-threonine protein kinase activation may be an ef ective target for reducing neuronal apoptosis after spinal cord injury
Mu Jin#, Yan-wei Yang#, Wei-ping Cheng*, Jia-kai Lu*, Si-yu Hou, Xiu-hua Dong, Shi-yao Liu
Department of Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China
The signaling mechanisms underlying ischemia-induced nerve cell apoptosis are poorly understood. We investigated the ef ects of apoptosis-related signal transduction pathways following ischemic spinal cord injury, including extracellular signal-regulated kinase (ERK), serine-threonine protein kinase (Akt) and c-Jun N-terminal kinase (JNK) signaling pathways. We established a rat model of acute spinal cord injury by inserting a catheter balloon in the left subclavian artery for 25 minutes. Rat models exhibited notable hindlimb dysfunction. Apoptotic cells were abundant in the anterior horn and central canal of the spinal cord. The number of apoptotic neurons was highest 48 hours post injury. The expression of phosphorylated Akt (p-Akt) and phosphorylated ERK (p-ERK) increased immediately after reperfusion, peaked at 4 hours (p-Akt) or 2 hours (p-ERK), decreased at 12 hours, and then increased at 24 hours. Phosphorylated JNK expression reduced after reperfusion, increased at 12 hours to near normal levels, and then showed a downward trend at 24 hours. Pearson linear correlation analysis also demonstrated that the number of apoptotic cells negatively correlated with p-Akt expression. These f ndings suggest that activation of Akt may be a key contributing factor in the delay of neuronal apoptosis after spinal cord ischemia, particularly at the stage of reperfusion, and thus may be a target for neuronal protection and reduction of neuronal apoptosis after spinal cord injury.
nerve regeneration; ischemic spinal cord injury; cell apoptosis; neurological function; serine-threonine protein kinase; extracellular signal-regulated kinase; c-Jun N-terminal kinase; neural regeneration
Funding: This research was supported by the National Natural Science Foundation of China, No. 81271387; the Research Special Fund of Public Welfare and Health Department of China, No. 201402009; the National Key Technology R&D Program in China, No. Z141107002514031.
Jin M, Yang YW, Cheng WP, Lu JK, Hou SY, Dong XH, Liu SY (2015) Serine-threonine protein kinase activation may be an effective target for reducing neuronal apoptosis after spinal cord injury. Neural Regen Res 10(11):1830-1835.
Introduction
Ischemia/reperfusion (I/R) injury can result in nerve cell apoptosis (Coselli et al., 2002; Safi et al., 2003; Yang et al., 2012). However, the precise mechanism of such apoptosis is not fully understood. Several reports have suggested that stress-responsive mitogen-activated protein kinase pathways might play a protective role in ischemic spinal cord injury (Chang and Karin, 2001; Badrian et al., 2006). There are three major forms of kinase: extracellular signal-regulated kinases (ERKs) and c-Jun N-terminal kinases (JNKs), and serine-threonine kinase (Akt). Activation of ERK by I/R injury is believed to confer a survival advantage to neurons (Shackelford and Yeh, 2003; Kilic et al., 2005). Activation of JNK is associated with neuronal death (Willaime-Morawek et al., 2003). JNK is active in the normal spinal cord, and its activity is reduced by ischemia; however, levels of JNK after reperfusion depend on the duration of ischemia (Shackelford and Yeh, 2001). Akt mediates growth factor-induced neuronal survival (Crowder and Freeman, 1998) and its activation in the early stages of reperfusion may be one of the factors responsible for the delay in neuronal death after spinal cord ischemia (Sakurai et al., 2001). Furthermore, the spatiotemporal expression of Akt, JNK, and ERK protein kinases has been described after ischemic brain injury (Kitagawa et al., 1999), but their expression has not been examined in ischemic spinal cord. Therefore, it is possible that Akt, JNK and ERK exert benef cial or harmful ef ects via phosphorylated Akt (p-Akt), JNK and ERK af ecting the induction of apoptosis, with the existence of dif erent expression time windows. This study examined the spatiotemporal expression of phosphorylated Akt, JNK and ERK after permanent aortic occlusion in rats.
Materials and Methods
Ethics statement
Rats received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutesof Health Publication No. 85-23, revised 1996). All procedures were approved by the Institutional Animal Care and Use Committee of the Capital Medical University, Beijing, China. Precautions were taken to minimize suf ering and the number of animals used in each experiment.
Animals
Ninety clean male Sprague-Dawley rats aged 13 weeks and weighing 280–350 g (310 ± 20 g) were provided by the Experimental Animal Center of Beijing Institute of Heart, Lung and Blood Vessel Diseases in China (license No. SYXK (Jing) 2005-0026). All rats were neurologically intact prior to anesthesia and surgery. Rats were randomly divided into sham group (n = 10) and I/R group (n = 80).
Establishment of ischemic spinal cord injury models
Rats were anesthetized with an intraperitoneal injection of 3% (w/v) sodium pentobarbital (30–50 mg/kg). After endotracheal intubation, an Inspira Advanced Safety Ventilator (Harvard Apparatus, Holliston, MA, USA) was connected. The tidal volume was set at 15 mL/kg, and the respiratory frequency was 80–100 breaths/min with an inspiratory to expiratory ratio of 1:1. The rectal temperature was monitored, and body temperature was maintained at 36.5–37.5°C with an infrared heat lamp and a heating pad. The left carotid artery was cannulated with a 20 gauge catheter (B. Braun Medical Inc., Bethlehem, PA, USA) to measure the mean proximal aortic pressure, which was maintained at 65 ± 3 mmHg throughout the procedure. A 24-gauge catheter was inserted into the tail artery to monitor the mean distal arterial pressure. The carotid artery cannula was connected to a heated (37.5°C) blood collection circuit that was primed with heparinized normal saline at 4 U/mL. The mean proximal aortic pressure, mean distal arterial pressure, and temperature were recorded using a Powerlab/8SP Polygraph (AD Instruments, Sydney, Australia). Spinal cord ischemia was induced by inserting a 2F Fogarty balloon catheter (Edwards Life Sciences, Irvine, CA, USA) via the left femoral artery into the descending thoracic aorta, 10 cm from the femoral arteriotomy, so that the tip of the catheter balloon lay 3–4 mm caudal to the left subclavian artery (Yang et al., 2012). All rats received 200 U of heparin sodium through the carotid artery cannula. The aortic occlusion was conf rmed by an immediate and sustained loss of detectable pulse pressure and a decrease in mean distal arterial pressure. At the end of the 25-minute ischemic period the balloon was def ated, animals received 200 U of protamine sulfate, catheters were removed and spinal cord blood f ow was restored. The surgical incisions were closed and the rats were returned to their cages to recover. Rats in the sham group underwent the same surgical procedure as those in the I/R group but without aortic catheter occlusion and were euthanized with an intraperitoneal injection of sodium pentobarbital 25 minutes after surgery for subsequent histological examination.
The I/R rats were divided into eight groups according to time points after I/R: 0, 1, 2, 4, 12, 24, 48 and 72 hours (n = 10 per group). At 0, 1, 2, 4, 12, 24, 48 and 72 hours after reperfusion, the rats were euthanized for histological examination. Rats in the 4-, 12-, 24-, 48- and 72-hour groups also underwent neurological assessment prior to euthanasia.
Assessment of neurological function
Hindlimb function was scored using the Basso, Beattie, and Bresnahan open-f eld locomotor scale (Basso et al., 1995). Scores ranged from 0 (no detectable hindlimb movement) to 21 (normal hindlimb locomotion).
Sample preparation
Rats were euthanized with an intraperitoneal injection of sodium pentobarbital (30–50 mg/kg). Lumbar (L3–5) spinal cord segments were dissected and post-f xed in 4% (w/v) paraformaldehyde for 2–4 days. The cord was embedded in paraf n, and serial sections (5 μm thick) were cut for Nissl staining and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) to detect cellular apoptosis.
Nissl staining
After dewaxing and rehydration, spinal cord sections were immersed in 0.5% (w/v) cresyl violet. Neurons containing Nissl substance in the cytoplasm, loose chromatin, and prominent nucleoli were considered normal neurons. Damaged neurons were identif ed as cells with reduced cytoplasmic Nissl substance, cavitations around the nucleus, and pyknotic homogenous nuclei. A pathologist who was unaware of the study groups and the neurological outcomes examined each spinal cord section and counted the total number of normal motor neurons in half the gray matter of each section. The number of normal motor neurons in each animal was obtained by averaging counts from three dif erent Nisslstained slides. A pathologist unaware of the neurological outcomes examined the tissue and counted the total number of normal motor neurons in the gray matter of each section. TUNEL staining
To detect DNA fragmentation in cell nuclei, TUNEL assay was performed using an apoptosis kit (Roche Diagnostics, Mannheim, Germany) in accordance with the manufacturer’s instructions. To determine the number of motor neurons undergoing apoptosis, two independent and blinded pathologists counted the numbers of positive or negative motor neurons in TUNEL-stained sections using a f uorescence microscope (ECLIPSE90i, Nikon, Tokyo, Japan). For each slide, color images of 10 separate f elds were captured randomly and digitized in half the gray matter of each section. Cells with clear nuclear labeling were def ned as TUNEL-positive cells and the number of TUNEL-positive cells in the gray matter of each section was counted.
Western blot assay
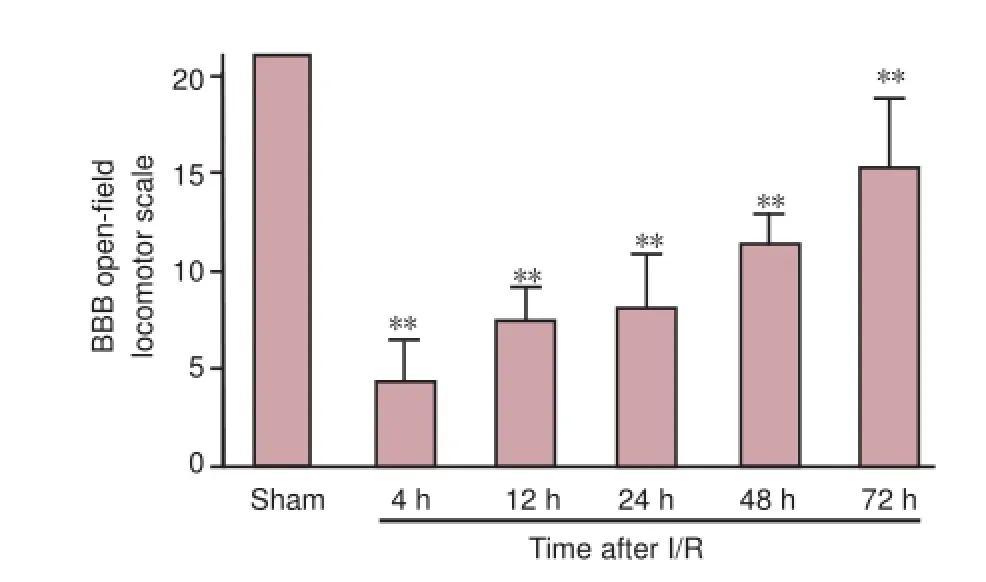
Figure 1 BBB open-f eld locomotor scale in rats with ischemic spinal cord injury.
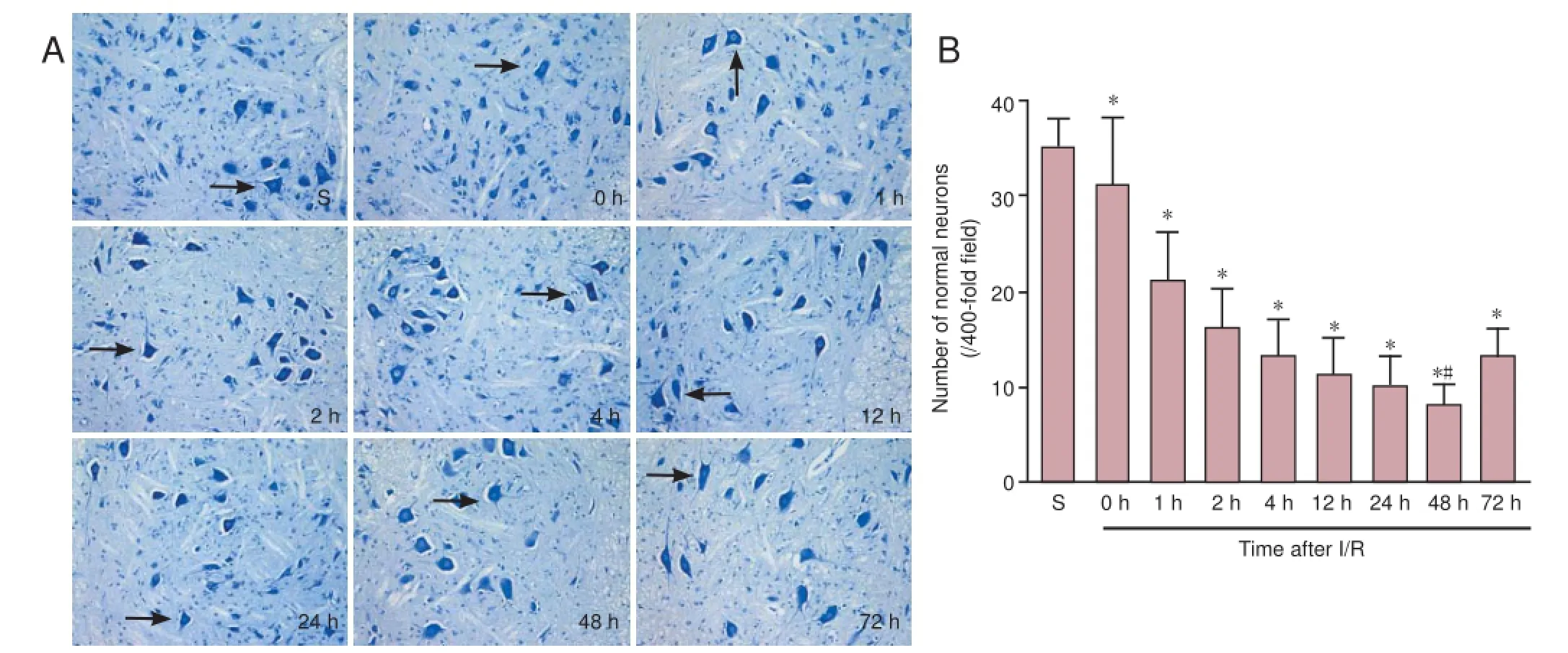
Figure 2 Histological changes in the spinal cord of rats with ischemic spinal cord injury.

Figure 3 Apoptosis in the spinal cord of rats with ischemic spinal cord injury.
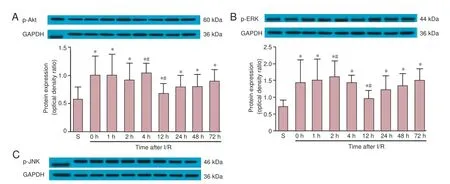
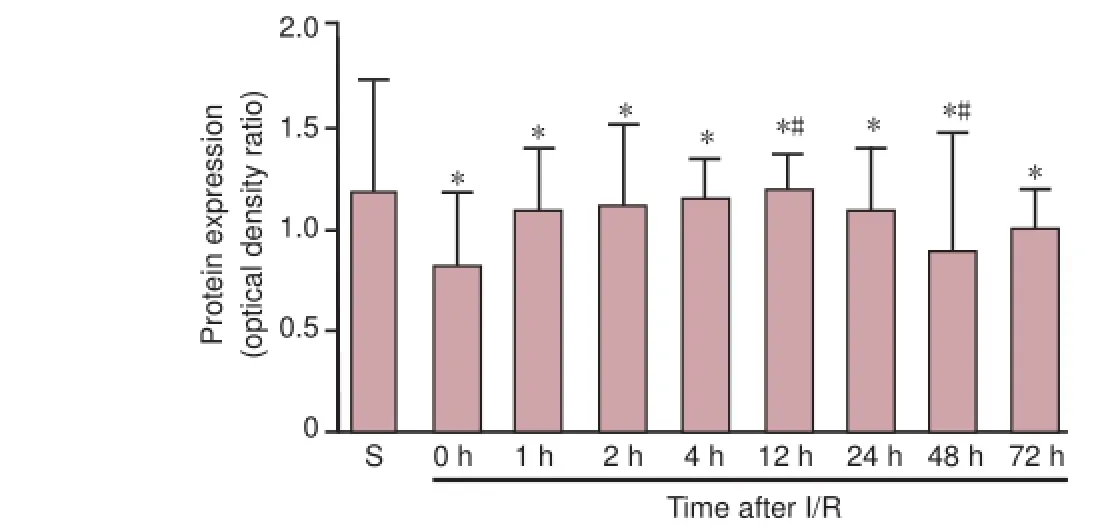
Figure 4 p-Akt (A), p-ERK (B) and p-JNK (C) protein expression in S and I/R groups at dif erent time points after reperfusion (0–72 hours).
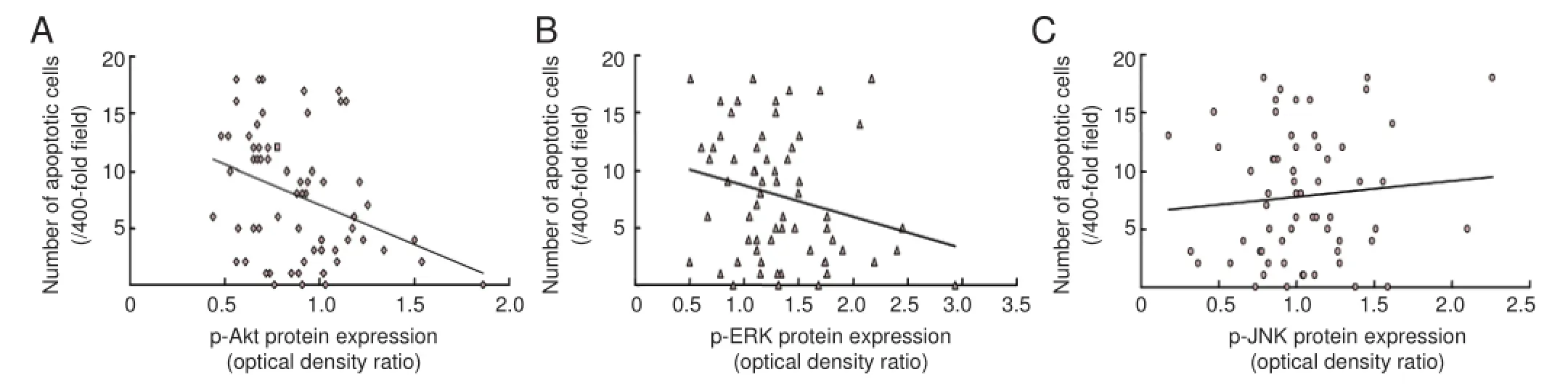
Figure 5 Correlation between apoptosis and p-Akt, p-ERK or p-JNK expression.
The homogenates of spinal cord were used for western blot assay. Forty micrograms of protein per animal was resolved on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred onto polyvinylidene dif uoride membranes. The samples were electrophoresed in a 10% polyacrylamide gel. Protein samples were boiled in 2.5% sodium dodecyl sulfate and 5% β-mercaptoethanol. A total of 20 μg protein samples and markers (MagicMark XP Western Standard; Invitrogen, Carlsbad, CA, USA) of each group were electrophoresed at 20 mA for 90 minutes. Electrophoresis buf er was prepared by 25 mM tris(hydroxymethyl)aminomethane, 250 mM glycine and 0.1% sodium dodecyl sulfate. Proteins were transferred onto polyvinylidene f uoride membrane (LC2002; Invitrogen) using transmembrane buf er and 10% methanol. The membrane was incubated with rabbit anti-p-ERK, p-Akt, p-JNK and GAPDH polyclonal antibodies (1:1,000; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at room temperature for 1 hour, washed with PBS, and then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:1,000; Beijing Biosynthesis Biotechnology Co., Ltd.) at room temperature for 90 minutes. The samples were visualized with ECL Plus Kit (Amersham Bio-sciences, Piscataway, NJ, USA). Optical density values were analyzed using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was conducted using SPSS for Windows 17.0 (SPSS Inc., Chicago, IL, USA). All results are presented as the mean ± SD. All data were compared using one-way analysis of variance, and correlations were explored using the Pearson linear correlation analysis. A value of P < 0.05 was considered statistically signif cant.
Results
Change in hindlimb function in rat models of ischemic spinal cord injury
In all rats, heart rate, blood pressure, and body temperaturewere stable and did not dif er between groups before, during, or after surgery. Sham-operated rats did not show any neurological impairment. However, in the I/R groups all rats exhibited notable hindlimb dysfunction, which persisted for 72 hours after reperfusion (Figure 1).
Neuronal damage in the spinal cord of rats with ischemic spinal cord injury
Histological examination by Nissl staining of the spinal cord from the sham group at 25 minutes after surgery revealed that spinal cord tissue was well maintained and that neurons were morphologically normal with clear karyosomes and a uniformly stained cytoplasm. Compared with the sham group, there were fewer morphologically normal neurons in the I/R groups, and cells appeared swollen with a darkly stained and shrunken cytoplasm. Pyknosis and vacuolation were also observed in the I/R groups. The lowest number of morphologically normal neurons was observed at 48 hours after reperfusion (Figure 2).
Apoptosis in the spinal cord of rats with ischemic spinal cord injury
TUNEL-positive cells appeared brown and were mainly distributed in the anterior horn and central canal of the spinal cord. Very few TUNEL-positive cells were observed in sham-operated rats. In the I/R groups, signif cantly more apoptotic neurons were noted at all time points after reperfusion. The highest number of apoptotic neurons was observed at 48 hours after reperfusion (P < 0.05; Figure 3).
Akt, ERK and JNK phosphorylation in the spinal cord of rats with ischemic spinal cord injury
Western blot assay revealed that in sham-operated rats, p-Akt (serine-473) and p-ERK were constitutively expressed in motor neurons. In the I/R group, p-Akt and p-ERK expression levels peaked at 4 hours and 2 hours after reperfusion, respectively. p-Akt and p-ERK expression then decreased at 12 hours after reperfusion, f nally returning to an elevated level at 24 hours after reperfusion, which was sustained until the end of the observation period. Signif cant dif erences in p-Akt and p-ERK expression were observed between the sham and I/R groups at all time points after reperfusion (P < 0.05). Furthermore, Akt expression at 4 hours and 12 hours was signif -cantly dif erent from the other time points in the I/R groups (P < 0.05). p-JNK expression after I/R injury was lower than that in sham-operated animals immediately after reperfusion (P < 0.05), but approached sham levels at 12 hours (P < 0.05, vs. all other time points). However, from 24 hours, expression returned to below sham levels until the end of the observation period (P < 0.05, vs. all other time points) (Figure 4).
Relationship between cell apoptosis and Akt, ERK and JNK phosphorylation in the spinal cord of rats with ischemic spinal cord injury
The result of Pearson linear correlation analysis showed that apoptosis negatively correlated with p-Akt expression (r =?0.352, P < 0.05); p-ERK and p-JNK expression did not show any correlation with apoptosis (r = ?0.24, P = 0.056; r = 0.09, P = 0.47; Figure 5).
Discussion
ERKs, JNKs and Akt are all crucial to the survival and apoptosis of neuronal cells in the spinal cord (Maulik et al., 2008). Recently, it has been demonstrated that Akt mediates growth factor-induced neuronal survival (Yamauchi et al., 2006). JNK, a subfamily of mitogen-activated protein kinases, was considered a degenerative signal in the nervous system. In this study, our principal f ndings using this acute spinal cord injury rat model are: (1) levels of p-Akt and p-ERK increase at the beginning of reperfusion and then start to decrease after 12 hours with the respective peak of p-Akt and p-ERK expression being 4 hours and 2 hours after reperfusion; (2) p-JNK expression is lowest immediately after reperfusion but then increases and peaks at 12 hours; and (3) p-Akt expression correlates negatively with the number of apoptotic neurons in the f rst 72 hours of reperfusion. This conf rms that the spinal cord injury was responsible for the high expression of ERK, Akt and JNK.
Akt mediates growth factor-induced neuronal survival (Crowder and Freeman, 1998), and a transient increase in p-Akt is observed in some models of cerebral ischemia (Noshita et al., 2001; Yano et al., 2001), similar to the increased neuronal expression of p-Akt observed in the present study after spinal cord ischemia. Yu et al. (2005) showed that p-Akt expression influences the number of motor neurons that survive at 1 day after spinal cord injury, and that inhibition of phosphatidylinositol 3-kinase reduces the expression of p-Akt. Here we have demonstrated that p-Akt expression in motor neurons increases in the f rst 12 hours after reperfusion, but decreases thereafter. Furthermore, neuronal apoptosis correlates negatively with Akt activation. The optimal time window for this approach was within 12 hours of reperfusion. As a result, the optimal time window for inhibiting apoptosis was within 12 hours of reperfusion.
While dif erent models of cerebral or spinal cord ischemia have consistently implicated p-Akt as a mediator of neuroprotection, conf icting results have been obtained with p-ERK. In animal models of brain injury caused by ischemia or trauma, elevated p-ERK expression is involved in neuronal death; for example, ERK1/2 is activated upon deprivation of growth factors in neurons and renal epithelial cells, but inhibition of the ERK pathway blocks apoptosis (Zhuang and Schnellmann, 2006). In other studies, ERK was also shown to be involved in neuroprotective ef ects of pre- and post-conditioning in rat hippocampus and spinal cord (Choi et al., 2006; Jiang et al., 2009). Choi et al. (2006) demonstrated that ERK1/2 activation shows characteristic time- and cell-dependent patterns in a rat model of ischemic tolerance induction; basal levels of ERK1/2 phosphorylation were observed in CA1 neurons after 30 minutes of reperfusion, then in the CA3 and granule cells by 1 hour, and f nally in dentate hilar neurons at 12 hours. By contrast, phosphorylation of ERK1/2 in mossy f bers and the CA1 dendritic f eld was sustained for at least 3 days. In the present study, ERK activation was suggestive of a negative correlation with apoptosis. Therefore, upregulation of p-ERK expression in the spinal cord may be required for sustained spinal cord protection.
Activation of JNK has been associated with induction of apoptosis, protection from cell death, proliferation, or dif erentiation in response to extracellular signals in various cell types (Minden and Karin, 1997; Ip and Davis, 1998; Lepp? and Bohmann, 1999; Mielke and Herdegen, 2000). Lepp? and Bohmann (1999) suggested that p-JNK might trigger the induction of the apoptotic ef ector p53. Activation of JNK was also implicated in neuronal cell death (Xia et al., 1995; Maroney et al., 1999). Xia et al. (1995) demonstrated that activation of JNK and concurrent inhibition of ERK are critical for the induction of apoptosis in neuronal cells, and considered that the dynamic balance between ERK and JNK-p38 pathways may be important in determining whether a cell survives or undergoes apoptosis. The f ndings from the sham group in our study show that JNK has a high basal activity in the spinal cord, supporting a previous report (Cof ey et al., 2000); however, after 25 minutes of ischemia, activity of JNK was signif -cantly lower than that in sham-operated animals at the start of reperfusion. However, JNK activity increased to sham levels by 12 hours, indicating that ischemia caused a decrease in JNK activity in this model. No evidence of a correlation between JNK activity and apoptosis was observed in this part of the spinal cord. Therefore, it is unlikely that inhibition of JNK will be neuroprotective in rat models of ischemic spinal cord injury.
In summary, Akt activation in spinal cord neurons correlates with local apoptosis in the f rst 72 hours of reperfusion after ischemia. The activation of Akt during the f rst 12 hours may be one of the factors responsible for the delay in neuronal apoptosis after spinal cord ischemia. Activated Akt is a strong candidate for use as a therapeutic agent in the treatment of ischemic spinal cord injury in the near future.
Author contributions: WPC, YWY, MJ and JKL participated in study concept, design, data analysis and paper writing. SYH, XHD and SYL reviewed this paper. All authors approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Badrian B, Casey TM, Lai MC, Rakoczy PE, Arthur PG, Bogoyevitch MA (2006) Contrasting actions of prolonged mitogen-activated protein kinase activation on cell survival. Biochem Biophys Res Commun 345:843-850.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open f eld testing in rats. J Neurotrauma 12:1-21.
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37-40.
Choi JS, Kim HY, Cha JH, Lee MY (2006) Ischemic preconditioning-induced activation of ERK1/2 in the rat hippocampus. Neurosci Lett 409:187-191.
Cof ey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ (2000) Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci 20:7602-7613.
Coselli JS, LeMaire SA, Conklin LD, K?ksoy C, Schmittling ZC (2002) Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 73:1107-1115; discussion 1115-1116.
Crowder RJ, Freeman RS (1998) Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and suf cient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 18:2933-2943.
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)--from inf ammation to development. Curr Opin Cell Biol 10:205-219.
Jiang X, Ai C, Shi E, Nakajima Y, Ma H (2009) Neuroprotection against spinal cord ischemia-reperfusion injury induced by dif erent ischemic postconditioning methods: roles of phosphatidylinositol 3-kinase-Akt and extracellular signal-regulated kinase. Anesthesiology 111:1197-1205.
Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM (2005) Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19:2026-2028.
Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K (1999) Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett 274:45-48.
Lepp? S, Bohmann D (1999) Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18:6158-6162.
Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM, Park DS, Yan CY, Troy CM, Greene LA (1999) CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally dif erentiated PC12 cells from death evoked by three distinct insults. J Neurochem 73:1901-1912.
Maulik D, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M (2008) Activation of p38 mitogen-activated protein kinase (p38 MAPK), extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) during hypoxia in cerebral cortical nuclei of guinea pig fetus at term: Role of nitric oxide. Neurosci Lett 439:94-99.
Mielke K, Herdegen T (2000) JNK and p38 stresskinases--degenerative ef ectors of signal-transduction-cascades in the nervous system. Prog Neurobiol 61:45-60.
Minden A, Karin M (1997) Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta 1333:F85-104.
Noshita N, Lewen A, Sugawara T, Chan PH (2001) Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 21:1442-1450.
Safi HJ, Miller CC, Huynh TT, Estrera AL, Porat EE, Winnerkvist AN, Allen BS, Hassoun HT, Moore FA (2003) Distal aortic perfusion and cerebrospinal f uid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg 238: 372-381.
Sakurai M, Hayashi T, Abe K, Itoyuama Y, Tabayashi K (2001) Induction of phosphatidylinositol 3-kinase and serine-threonine kinase-like immunoreactivity in rabbit spinal cord after transient ischemia. Neurosci Lett 302:17-20.
Shackelford DA, Yeh RY (2001) Differential effects of ischemia and reperfusion on c-Jun N-terminal kinase isoform protein and activity. Brain Res Mol Brain Res 97:178-192.
Shackelford DA, Yeh RY (2003) Activation of extracellular signal-regulated kinases (ERK) during reperfusion of ischemic spinal cord. Brain Res Mol Brain Res 115:173-186.
Willaime-Morawek S, Brami-Cherrier K, Mariani J, Caboche J, Brugg B (2003) C-Jun N-terminal kinases/c-Jun and p38 pathways cooperate in ceramide-induced neuronal apoptosis. Neuroscience 119:387-397.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing ef ects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331.
Yamauchi T, Sakurai M, Abe K, Takano H, Sawa Y (2006) Neuroprotective ef ects of activated protein C through induction of insulin-like growth factor-1 (IGF-1), IGF-1 receptor, and its downstream signal phosphorylated serine-threonine kinase after spinal cord ischemia in rabbits. Stroke 37:1081-1086.
Yang YW, Lu JK, Qing EM, Dong XH, Wang CB, Zhang J, Zhao LY, Gao ZF, Cheng WP (2012) Post-conditioning by xenon reduces ischaemia-reperfusion injury of the spinal cord in rats. Acta Anaesth Scand 56:1325-1331.
Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y, Hamada J, Miyamoto E, Ushio Y (2001) Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subf eld of gerbil hippocampus. J Cereb Blood Flow Metab 21:351-360.
Yu F, Sugawara T, Maier CM, Hsieh LB, Chan PH (2005) Akt/Bad signaling and motor neuron survival after spinal cord injury. Neurobiol Dis 20:491-499.
Zhuang S, Schnellmann RG (2006) A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther 319:991-997.
Copyedited by Paul P, de Souza M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Wei-ping Cheng, M.D. or Jia-kai Lu, M.D., ch_eng9735@sina.com.cn or lujiakai620@163.com.
# These authors contributed equally to this work.
orcid: 0000-0002-8948-5432 (Wei-ping Cheng) 0000-0001-7737-8114 (Jia-kai Lu)
10.4103/1673-5374.170313 http://www.nrronline.org/
Accepted: 2015-08-31
- 中國神經(jīng)再生研究(英文版)的其它文章
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke
- Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury
- Injury of corticoreticular pathway and corticospinal tract caused by ventriculoperitoneal shunting
- Susceptibility weighted imaging in the evaluation of hemorrhagic dif use axonal injury
- Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords

