Lesion localization of global aphasia without hemiparesis by overlapping of the brain magnetic resonance images
Woo Jin Kim, Nam-Jong Paik
1 Department of Rehabilitation Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
2 Department of Physical Medicine and Rehabilitation, Haeundae Paik Hospital, Inje University of Medicine, Busan, South Korea
3 Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, South Korea
Lesion localization of global aphasia without hemiparesis by overlapping of the brain magnetic resonance images
Woo Jin Kim1,2, Nam-Jong Paik1,3
1 Department of Rehabilitation Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
2 Department of Physical Medicine and Rehabilitation, Haeundae Paik Hospital, Inje University of Medicine, Busan, South Korea
3 Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, South Korea
Global aphasia without hemiparesis is a striking stroke syndrome involving language impairment without the typically manifested contralateral hemiparesis, which is usually seen in patients with global aphasia following large left perisylvian lesions. The objective of this study is to elucidate the speci fi c areas for lesion localization of global aphasia without hemiparesis by retrospectively studying the brain magnetic resonance images of six patients with global aphasia without hemiparesis to de fi ne global aphasia without hemiparesis-related stroke lesions before overlapping the images to visualize the most overlapped area. Talairach coordinates for the most overlapped areas were converted to corresponding anatomical regions. Lesions where the images of more than three patients overlapped were considered significant. The overlapped global aphasia without hemiparesis related stroke lesions of six patients revealed that the signi fi cantly involved anatomical lesions were as follows: frontal lobe, sub-gyral, sub-lobar, extra-nuclear, corpus callosum, and inferior frontal gyrus, while caudate, claustrum, middle frontal gyrus, limbic lobe, temporal lobe, superior temporal gyrus, uncus, anterior cingulate, parahippocampal, amygdala, and subcallosal gyrus were seen less signi fi cantly involved. This study is the fi rst to demonstrate the heterogeneous anatomical involvement in global aphasia without hemiparesis by overlapping of the brain magnetic resonance images.
nerve regeneration; global aphasia without hemiparesis; global aphasia; stroke; hemiparesis; brain; magnetic resonance imaging; neural regeneration
Funding: This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, No. A101901.
Kim WJ, Paik NJ. Lesion localization of global aphasia without hemiparesis by overlapping of the brain magnetic resonance images. Neural Regen Res. 2014;9(23):2081-2086.
Introduction
Stroke is a major cause of adult disability (Wolfe, 2000). Aphasia is a loss or impairment of verbal communication occurring as a consequence of brain dysfunction (Sinanovic et al., 2011), and has a heterogeneous phenomenon with severity ranging from complete inability to produce and understand language to mild word fi nding problems (van de Sandt-Koenderman et al., 2012). Aphasia following stroke is one of the most common and devastating manifestations causing long term disability (Kang et al., 2010), and its presence can predict longer hospital stays, increased need of rehabilitation therapy and lower chance of returning home (Dickey et al., 2010; Gialanella et al., 2011), thus leading to lower quality of life and participation in social activities (Hilari, 2011). The incidence of aphasia is reported to be 18 to 38% of stroke population (Wade et al., 1986; Ferro et al., 1999; Pedersen et al., 2004). Patients with aphasia following stroke has a higher mortality risk (Ferro et al., 1999) and has a tendency of rapid spontaneous recovery in the fi rst months (Laska et al., 2001). The percentage of patients with aphasia decreases over time and the incidence is reduced to 20—25% at 3 months post onset of stroke (van de Sandt-Koenderman et al., 2012).
20—40% of post stroke aphasia has been reported to be the global type (Kang et al., 2010), usually occurring after large perisylvian lesions in the left middle cerebral artery territory and is associated with contralateral hemiparesis due to proximity of the language and motor control area in the cortex (Pai et al., 2011).
Global aphasia without hemiparesis (GAWH) is a rare and distinct phenomenon (Legatt et al., 1987). It has been reported to involve receptive and expressive language impairment, without manifestation of the typical hemiparesis in patients with global aphasia following large left perisylvian lesions (Hanlon et al., 1999), due to unusual dissociation of the language from the motor functions.
The following studies addressed the topographic correlates and the specificity of GAWH in the literature. Neuroana-tomical correlates of post-stroke aphasias were analyzed with cerebral blood flow single photon emission tomography (SPECT) scanning, and it was found that most extensive damage was throughout the perisylvian region of the left hemisphere in global aphasia, highlighting the integrative role of some subcortical structures in language and speech functions (Jodzio et al., 2003). Lesion analysis for the GAWH using diffusion-weighted imaging (DWI) and SPECT images identi fi ed the lesion sites in three groups of classical, single and extra-sylvian lesions, suggesting the complex functional anatomy of aphasia and that different lesion localization would depend on the pathogenic mechanism (Bang et al., 2004).
However, anatomical regions accounting for GAWH have never been demonstrated by overlapping brain magnetic resonance (MR) images in the literature. The purpose of this study was to determine the neuroanatomical correlates of GAWH by mapping of overlapped brain MR images.
Subjects and Methods
Subjects
Medical records of stroke patients with left hemisphere lesions from 2005 to 2011 without symptoms of hemiplegia or hemiparesis were reviewed retrospectively. Patients who undertook brain MR imaging (MRI) exam and speech evaluation within 14 days from stroke onset, which revealed left hemisphere lesion and global aphasia according to the Korean version of Western Aphasia Battery (KWAB) test (Kim and Na, 2004) were enrolled. Those with previous history of stroke, bilateral hemisphere lesion, left handedness and premorbid lingual or language problems were excluded. Severe cognitive impairment affecting conversation in one patient and lack of complete speech evaluation due to poor cooperation in two patients rendered exclusion of three of the nine patients. Speech evaluation data and brain MR images of the remaining six patients with GAWH were obtained for analysis. The demographic data included the duration from stroke onset to initial speech evaluation. The Western Aphasia Battery (WAB), a routinely used evaluation tool for language function with high internal consistency, test-retest reliability and validity (Shewan and Kertesz, 1980), and discern the presence, type, and severity of aphasia (Shewan and Kertesz, 1980). In the current study, the Korean version of the WAB (KWAB; Kim and Na, 2004) was used to evaluate fl uency, comprehension, repetition, and naming. Classi fi cation of aphasia type and overall severity of language impairment expressed in aphasia quotients were recorded (Kang et al., 2010). The T1 and FLAIR views of brain MR images were reviewed thoroughly with reference to the official readings by an expert neuroradiologist.
MRI and FLAIR imaging acquisition
MRI was conducted using a 3T Philips Achieva TX with parameters as follows: acquisition matrix = 352 × 264, reconstructed to matrix = 512 × 512, field of view = 182 × 230 mm2, repetition time = 11,000 ms, echo time = 125 ms, parallel imaging reduction factor (SENSE factor) = 1, number of excitations = 1, slice gap = 1, slice thickness = 5 mm, number of slices = 25—27, and RC SENSE-NV-16 channel coil. We obtained the fluid attenuated inversion recovery (FLAIR) images (acquired voxel size = 0.5 × 0.5 × 1 mm3, transverse orientation) for visualizing the lesion. Sixty-eight axial images were collected for each subject, encompassing the whole brain. DICOM files were acquired and spatially normalized into reconstructed images of isotropic voxel size of 2 × 2 × 2 mm3using SPM8 implemented in Matlab (Version7.8.0, The Mathworks Inc, Natick, MA, USA) as described elsewhere (Marchina et al., 2011).
Lesion mapping
MRIcro software program (www.mricro.com, Columbia, SC, USA) was adopted to manually define the outline of the hyperintense lesions in the spatially normalized FLAIR images with reference to the co-registered T1-weighted images for additional guidance using BambooTM(Wacom, Kazo-shi, Saitama, Japan) to increase precision (Kim et al., 2013). Slice 34 of the 68 axial slices was set as the median level and three additional levels both below and above the median slice were selected for analysis, with a total of seven slices separated by fi ve slices. The regions of interest (ROI) were drawn on the slices of 19, 24, 29, 34, 39, 44 and 49. Six slices at the same level of each patient were overlaid on each other to obtain the overlapped lesion onto the template. Color coding was adopted to display the overlapped areas with different colors according to the number of overlapped ROIs (red = 6, yellow = 5, green = 4, blue = 3, navy = 2, purple = 1) at each level for all of the selected slices. Talairach space coordinates at the center of each most overlapped region were gained using the MRIcro software for identi fi cation of the exact anatomical region of the most overlapped area. The coordinates gained were then inputted to the Talairach-Client program (www.talairach. org, San Antonio, TX, USA) for conversion of the coordinates to the anatomical names at the marked coordinate as well as anatomical structures within 5 mm or nearest gray matter of the center of the ROI. Anatomical lesions were considered significant when images overlapped in more than three patients. The names of the anatomical regions are listed inTable 1in order of frequency of appearance.
Results
Clinical data of patients
The age of the six patients ( fi ve males, one female) at the time of stroke ranged from 40 to 74 years, with mean of 57 years. The duration from onset of the stroke to the initial speech evaluation ranged from 8 to 14 days, with mean of 11.2 days. The KWAB results of fluency, comprehension, repetition, and naming are summarized inTable 2. Assessment results showed that fl uency ranged from 31% to 37%, comprehension from 25% to 59%, repetition from 25% to 75%, naming from 29% to 49%, and aphasia quotient for overall severity of aphasia from 29% to 49%. At follow up of speech evaluation, global aphasia evolved to transcortical motor aphasia in three patients, Wernicke aphasia in one patient, and completerecovery in one patient, while one patient was lost during follow up. Brain MRI showed that the lesion sites in the left hemisphere were frontal, temporal lobes, deep white matter, thalamus, basal ganglia and insula. The size and extent of the lesions varied; some patients had focal lesions, others had large lesions, and some had cortical infarcts while deep structures were affected in some patients. They were all acute infarctions except for patient 6, whose MR fi nding suggested subacute infarction.
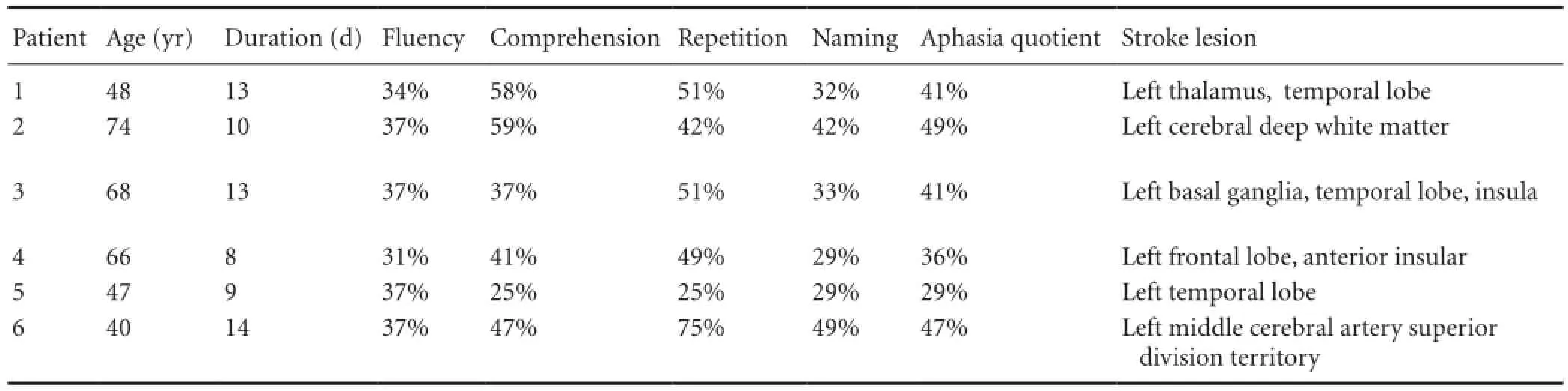
Table 2 Demographic data and KWAB results
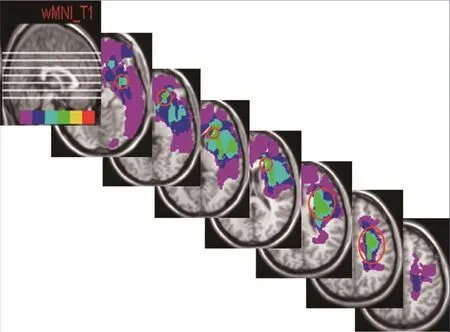
Figure 1 FLAIR MRI of axial brain slices of six patients, showing distribution of all patient’s lesion area on a brain template.
Lesion location in the brain determined by imaging
The numbers of patients overlapping in slices 1, 2, 3, 4, 5,6 and 7 were four, four, five, five, four, four and two, respectively. In the seventh slice, only two patients’ images overlapped and therefore were discarded from analysis. The rendered Talairach space coordinates at the center of the overlapped ROI were —32 × —2 × —14 in the fi rst slice, —20 × 32 × —4, —20 × 24 × 6, —22 × 22 × 16, —28 × 4 × 26 and —28 × —6 × 36 in the 2nd, 3rd, 4th, 5thand 6thslices, respectively.Figure 1shows the axial view of each level with open circle indicating the region with the highest number of ROIs (more than four overlaps). There is no open circle in the last slice since only two ROIs overlapped. Sagittal view of the seven levels at which the ROI analysis was performed is shown in the fi rst slice. The overlapped ROIs of six patients revealed the involved areas as follows in order of frequency of appearance with number of patients for each overlapped ROI in the brackets: frontal lobe (6), sub-gyral (6), sub-lobar (5), extra-nuclear (5), corpus callosum (3), inferior frontal gyrus (3), caudate (2), claustrum (2), middle frontal gyrus (2), limbic lobe (2), temporal lobe (1), superior temporal gyrus (1), uncus (1), anterior cingulated (1), parahippocampal (1), amygdala (1) and subcallosalgyrus (1) (Table 1).
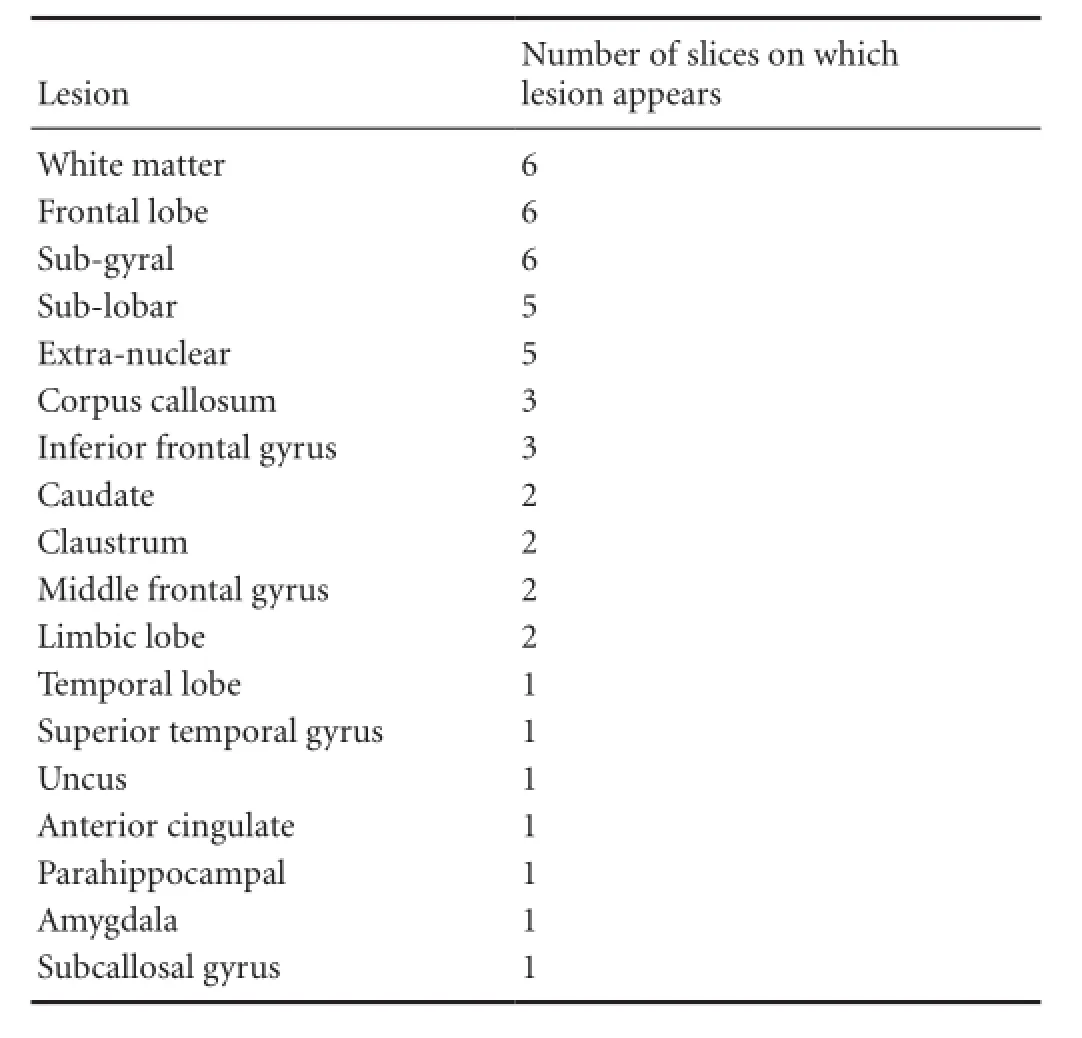
Table 1 Number of slices of each lesion
Discussion
Stroke is the cause of aphasia in 80—90% of all aphasic patients (van de Sandt-Koenderman et al., 2012) and aphasia is a common consequence of left hemispheric lesions (Sinanovic et al., 2011). Global aphasia typically results from large presylvian lesions, also affecting adjacent motor area, responsible for accompaniment of right hemiplegia and facial weakness in right handed patients (Damasio, 1992). The absence of hemiparesis in GAWH syndrome suggests that the cortical representation of motor and language function may not always follow classic description.
The underlying pathogenic mechanisms of GAWH are not completely understood, but multiple etiologies including embolic cerebral infarction, subarachnoid hemorrhage, arterial atherosclerosis, brain tumors and dural arteriovenous fi stula have been previously reported as causes of GAWH in the literature (Shindo et al., 2013; Togawa et al., 2014). Underlying mechanism for GAWH has been recently studied by transcranial magnetic stimulation (Shindo et al., 2013), in which authors suggested sparing of the decussated pyramidal tract in cases of GAWH caused by cardioembolism. In their description of stroke mimics, the so-called neuroimaging-negative strokes (Artto et al., 2012; Zinkstok et al., 2013) and its related language impairment, compared with the true ischemic stroke, the authors reported a high proportion of stroke mimic patients presenting with GAWH, as in several others studies (Winkler et al., 2009; Chen et al., 2011; Guillan et al., 2012). However, all of the six patients in our study were neuroimaging-positive, suggesting that GAWH occurs both in neuroimaging-negative and -positive stroke patients.
Diversity of anatomical regions associated with global aphasia with hemiparesis has been documented, showing heterogenous and variable lesion constellation responsible for global aphasia with hemiparesis (Hanlon et al., 1999). In a series of 46 patients with global aphasia, Scarpa et al. (1987) reported only 53% had anterior-posterior lesion profi le while 32% of patients had deep lesion. Similarly, Vignolo et al. (1986) found only 59% of their 37 patients with global aphasia had large left perisylvian lesion, involving both Broca’s and Wernicke’s areas. Four (11%) involved deep lesions of insula and lenticular nucleus, while 22% and 8% had anterior and posterior lesions, respectively. Global language impairment was also reported after left thalamic hemorrhage (Kumar et al., 1996).
Van Horn and Hawes (1982) fi rst described involvement of two discrete ischemic lesions in the domnant hemisphere in patients with GAWH, which was con fi rmed by Tranelet et al. (1987) by demonstrating lesions in the anterior language cortices or language-related subcortical area, and one in the posterior language cortices. Hanlon et al. (1999) explored whether a single or multiple lesions could cause GAWH, demonstrating heterogenous lesion pro fi le.
Ferro (1983) reported two cases with left middle cerebral artery involving both Broca’s and Wernicke’s areas with spared posterior limb of the internal capsule whereas other studies reported various etiologies and lesions relating to GAWH, stating that GAWH does not imply a single topographic correlate and etiology. Deleval et al. (1989) reported only a single lesion of the posterior part of F2 and F3, suggesting that functional disconnection of posterior language area is responsible for GAWH. Location and size of brain lesions between the global aphasia with hemiparesis group and GAWH group were compared in an earlier report (von Keyserlingk et al., 1997). The authors observed that the infarcted areas of patients with hemiparesis always extended to the wall of the lateral ventricle, including the whole corona radiata with the pyramidal tract, while parts of the deep white matter were spared in the GAWH group. However, subcortical lesions causing GAWH have been reported byBang et al. (2004) who suggested functional reorganization following earlier lesion of the motor pathway, such as cortical plasticity in right hemispheric control of right limb motor functions, may have played a role in sparing of the motor skills despite the likely involvement of the motor pathways.
In the current study, the frontal lobe, sub-gyral, white matter, sub-lobar, extra-nuclear, corpus callosum and inferior frontal gyrus were overlapped in more than three slices, while caudate, claustrum, medial frontal gyrus, limbic lobe, temporal lobe, superior temporal gyrus anterior cingulate, parhippocampal gyrus, amygdale and subcallosal gyrus were less signi fi cantly involved, demonstrating signi fi cant involvement of deeper structures (Table 2). Naeser et al. (1989) reported subcortical lesions involving the medial subcallosal fasciculus and the middle third of the paraventricular white matter, which contains the body of the caudate nucleus and motor-sensory projections of the mouth involved in speech production, affected spontaneous speech due to loss of pathways for speech initiation, motor execution or sensory feedback, and emphasized the essential role of white matter pathways for speech and comprehension by reporting on patients with putamen and internal capsule infarction with subcortical aphasia with anterior, posterior and superior white matter extension (Naeser and Palumbo, 1994). Okuda et al. (1994) measured hypoperfusion in perisylvian language areas with a SPECT scan, and suggested white matter lesions in those areas were critical in development of global subcortical aphasia (Okuda et al., 1994). By applying diffusion tensor tractography in recent years, identi fi cation of several white matter structures interconnecting cortical language areas has been demonstrated (Smits et al., 2012). The arcuate fasciculus connects the Broca’s area (a frontal expressive language area) with the Wernicke’s area (a posterior temporoparietal deceptive language area), and is the best known language related white matter pathway (Smits et al., 2012). Although we did not analyze the speci fi c white matter involvement, our observation of white matter involvement is in agreement with those of previous studies (Naeser and Palumbo, 1994, Okuda et al., 1994, Smits et al., 2012). On the contrary, Bates et al. (2003) revealed lesions in the insula and arcuate/superior longitudinal fasciculus most affect speech production, and lesions in the middle temporal gyrus most affect speech comprehension, neither of which appeared in our results (Table 1).
Hanlon et al. (1999) demonstrated composite lesion analysis according to the three subtypes of GAWH based on acute language profiles and evolvement of aphasia in the first 3 months after stroke (persistent, transcortical motor aphasia, and Wernicke). In our study, the subtypes of speech at follow up speech evaluations are transcortical motor aphasia in three patients, Wernicke aphasia in one, complete recovery in one and one was lost in follow up. Such results may raise issue of misdiagnosed GAWH initially due to cognitive de ficit, apraxia, but complete exclusion of cognitive de fi cit when not definitive is difficult in aphasic patients, and there is always the possibility of actual recovery of the global aphasia over the period in those with initial clinical manifestations of GAWH.
However, because its relationship with lesion extent and aphasia severity was not studied, further studies on this topic are needed. Also, more extensive studies correlating the lesion mapping with the subtypes of aphasia based on aphasic evolvement in few months, and as well as overlapping the lesions according to the different types of GAWH with larger number of cases would further specify the responsible or most commonly involved anatomical region.
This study is the first to use the brain MR image overlapping technique to demonstrate the lesions involved in global aphasia without hemiparesis. Our results identify the involvement of heterogeneous structures in six acute GAWH patients. Further studies with larger number of cases, together with exploration of the functional correlation speci fi c structures contributing to GAWH would enhance our understanding of GAWH.
Author contributions:Paik NJ was responsible for conception and design of the study, fundraising, provided assistance in technical performance or material use, supervised the study, and provided critical revision of the manuscript for intellectual contents. Kim WJ collected and analyzed data and wrote the manuscript. Both of these two authors interpreted the data and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Artto V, Putaala J, Strbian D, Meretoja A, Piironen K, Liebkind R, Silvennoinen H, Atula S, Happola O (2012) Stroke mimics and intravenous thrombolysis. Ann Emerg Med 59:27-32.
Bang OY, Heo KG, Kwak Y, Lee PH, Joo IS, Huh K (2004) Global aphasia without hemiparesis: lesion analysis and its mechanism in 11 Korean patients. J Neurol Sci 217:101-106.
Chen Y, Bogosavljevic V, Leys D, Jovanovic D, Beslac-Bumbasirevic L, Lucas C (2011) Intravenous thrombolytic therapy in patients with stroke mimics: baseline characteristics and safety pro fi le. Eur J Neurol 18:1246-1250.
Damasio AR (1992) Aphasia. N Engl J Med 326:531-539.
Deleval J, Leonard A, Mavroudakis N, Rodesch G (1989) Global aphasia without hemiparesis following prerolandic infarction. Neurology 39:1532-1535.
Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S (2010) Incidence and pro fi le of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil 91:196-202.
Ferro JM (1983) Global aphasia without hemiparesis. Neurology 33:1106.
Ferro JM, Mariano G, Madureira S (1999) Recovery from aphasia and neglect. Cerebrovasc Dis 9 Suppl 5:6-22.
Gialanella B, Bertolinelli M, Lissi M, Prometti P (2011) Predicting outcome after stroke: the role of aphasia. Disabil Rehabil 33:122-129.
Guillan M, Alonso-Canovas A, Gonzalez-Valcarcel J, Garcia Barragan N, Garcia Caldentey J, Hernandez-Medrano I, Defelipe-Mimbrera A, Sanchez-Gonzalez V, Terecoasa E, Alonso de Lecinana M, Masjuan J (2012) Stroke mimics treated with thrombolysis: further evidence on safety and distinctive clinical features. Cerebrovasc Dis 34:115-120.
Hanlon RE, Lux WE, Dromerick AW (1999) Global aphasia without hemiparesis: language pro fi les and lesion distribution. J Neurol Neurosurg Psychiatry 66:365-369.
Hilari K (2011) The impact of stroke: are people with aphasia different to those without? Disabil Rehabil 33:211-218.
Jodzio K, Gasecki D, Drumm DA, Lass P, Nyka W (2003) Neuroanatomical correlates of the post-stroke aphasias studied with cerebral blood fl ow SPECT scanning. Med Sci Monit 9:MT32-41.
Kang EK, Sohn HM, Han MK, Kim W, Han TR, Paik NJ (2010) Severity of post-stroke aphasia according to aphasia type and lesion location in Koreans. J Korean Med Sci 25:123-127.
Kim H, Na DL (2004) Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol 26:1011-1020.
Kim WJ, Yang EJ, Paik NJ (2013) Neural substrate responsible for crossed aphasia. J Korean Med Sci 28:1529-1533.
Kumar R, Masih AK, Pardo J (1996) Global aphasia due to thalamic hemorrhage: a case report and review of the literature. Arch Phys Med Rehabil 77:1312-1315.
Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M (2001) Aphasia in acute stroke and relation to outcome. J Intern Med 249:413-422.
Legatt AD, Rubin MJ, Kaplan LR, Healton EB, Brust JC (1987) Global aphasia without hemiparesis: multiple etiologies. Neurology 37:201-205.
Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G (2011) Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 42:2251-2256.
Naeser MA, Palumbo CL (1994) Neuroimaging and language recovery in stroke. J Clin Neurophysiol 11:150-174.
Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML (1989) Severe non fl uency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain 112:1-38.
Okuda B, Tanaka H, Tachibana H, Kawabata K, Sugita M (1994) Cerebral blood fl ow in subcortical global aphasia. Perisylvian cortical hypoperfusion as a crucial role. Stroke 25:1495-1499.
Pai AR, Krishnan G, Prashanth S, Rao S (2011) Global aphasia without hemiparesis: A case series. Ann Indian Acad Neurol 14:185-188.
Pedersen PM, Vinter K, Olsen TS (2004) Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis 17:35-43.
Scarpa M, Colombo A, Sorgato P, De Renzi E (1987) The incidence of aphasia and global aphasia in left brain-damaged patients. Cortex 23:331-336.
Shewan CM, Kertesz A (1980) Reliability and validity characteristics of the Western Aphasia Battery (WAB). J Speech Hear Disord 45:308-324.
Shindo A, Satoh M, Naito Y, Asahi M, Takashima S, Sasaki R, Furukawa K, Narita Y, Kuzuhara S, Tomimoto H (2013) Global aphasia without hemiparesis: the underlying mechanism examined by transcranial magnetic stimulation. Neurologist 19:11-14.
Sinanovic O, Mrkonjic Z, Zukic S, Vidovic M, Imamovic K (2011) Poststroke language disorders. Acta Clin Croat 50:79-94.
Smits M, Visch-Brink EG, van de Sandt-Koenderman ME, van der Lugt A (2012) Advanced magnetic resonance neuroimaging of language function recovery after aphasic stroke: a technical review. Arch Phys Med Rehabil 93:S4-14.
Togawa J, Ohi T, Kawarazaki S (2014) Global aphasia without hemiparesis caused by a dural arteriovenous fi stula. Intern Med 53:135-138.
Tranel D, Biller J, Damasio H, Adams HP Jr, Cornell SH (1987) Global aphasia without hemiparesis. Arch Neurol 44:304-308.
van de Sandt-Koenderman ME, van der Meulen I, Ribbers GM (2012) Aphasia rehabilitation: more than treating the language disorder. Arch Phys Med Rehabil 93:S1-3.
Van Horn G, Hawes A (1982) Global aphasia without hemiparesis: a sign of embolic encephalopathy. Neurology 32:403-406.
Vignolo LA, Boccardi E, Caverni L (1986) Unexpected CT-scan fi ndings in global aphasia. Cortex 22:55-69.
von Keyserlingk AG, Naujokat C, Niemann K, Huber W, Thron A (1997) Global aphasia-with and without hemiparesis. A linguistic and CT scan study. Eur Neurol 38:259-267.
Wade DT, Hewer RL, David RM, Enderby PM (1986) Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry 49:11-16.
Winkler DT, Fluri F, Fuhr P, Wetzel SG, Lyrer PA, Ruegg S, Engelter ST (2009) Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke 40:1522-1525.
Wolfe CD (2000) The impact of stroke. Br Med Bull 56:275-286.
Zinkstok SM, Engelter ST, Gensicke H, Lyrer PA, Ringleb PA, Artto V, Putaala J, Haapaniemi E, Tatlisumak T, Chen Y, Leys D, Sarikaya H, Michel P, Odier C, Berrouschot J, Arnold M, Heldner MR, Zini A, Fioravanti V, Padjen V, Beslac-Bumbasirevic L, Pezzini A, Roos YB, Nederkoorn PJ (2013) Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke 44:1080-1084.
Copyedited by Kremer C, Micu I, Li CH, Song LP, Zhao M
10.4103/1673-5374.147935
Nam-Jong Paik, M.D., Ph.D., Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 166 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, South Korea, njpaik@snu.ac.kr.
http://www.nrronline.org/
Accepted: 2014-10-28
- 中國神經(jīng)再生研究(英文版)的其它文章
- Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
- A more consistent intraluminal rhesus monkey model of ischemic stroke
- Human bone marrow mesenchymal stem cell transplantation attenuates axonal injury in stroke rats
- Pathogenesis of glaucoma: how to prevent ganglion cell from axonal destruction?
- Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the in fl ammatory response
- Pretreatment with scutellaria baicalensis stem-leaf total fl avonoid protects against cerebral ischemia/ reperfusion injury in hippocampal neurons

