Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients
Zheng-Gui Du, Yong-Gang Wei, Ke-Fei Chen and Bo Li
Chengdu, China
Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients
Zheng-Gui Du, Yong-Gang Wei, Ke-Fei Chen and Bo Li
Chengdu, China
BACKGROUND:Surgical resection is an important curative treatment for hepatocellular carcinoma (HCC); however, some patients experience an unexpected recurrence even after hepatectomy. The present study aimed to investigate risk factors and predictive criteria for early and late recurrence of HCC after resection.
METHODS:A retrospective analysis of 398 Chinese patients who received curative resection for HCC was conducted. Patients were divided into three groups: without recurrence, early recurrence, and late recurrence. Prognostic factors and predictive criteria for early and late recurrence were statistically analyzed.

(Hepatobiliary Pancreat Dis Int 2014;13:153-161)
hepatocellular carcinoma;
intrahepatic recurrence;
hepatectomy;
risk factors;
prognosis
Introduction
Hepatocellular carcinoma (HCC), a common malignancy worldwide, accounts for approximately one million deaths with an increasing trend of new incidences annually[1,2]and a 5-year survival rate of less than 5% without treatment.[3]Surgery, including liver transplantation (LT) and hepatectomy, is the most effective modality for the treatment of patients with HCC.[4,5]However, due to the lack of donor organs, long waiting period, higher perioperative risk, and long-term immunosuppression associated with LT, hepatectomy is widely accepted as the first treatment option for many HCC patients.[6]As surgical techniques and perioperative management of patients with HCC have improved, HCC can be resected safely with very low operative morbidity and mortality rates.[7]Even after surgical resection, however, the longterm prognosis remains poor due to a high incidence of recurrence (68%-96%),[8,9]making effective therapeutic strategies aimed at controlling tumor recurrence critical for prolonging survival after HCC resection.
To date, various factors influencing the recurrence of HCC have been reported, including tumor-relatedfactors,[10-13]host liver-related factors,[14,15]and even the type of surgery (e.g., major or minor resections, anatomical or non-anatomical resections, wide or narrow surgical margins, etc.).[16-18]Nevertheless, the causes of recurrence, intrahepatic metastasis or multicentric occurrences, remain controversial. In the study, we investigated the time of recurrence and a number of potential predictors to clarify the prognostic factors and predictive criteria of early and late recurrence in patients who underwent a curative partial hepatectomy for HCC.

Table 1.Types of hepatectomy
Methods
Patients
Between March 2007 and January 2011, 572 patients underwent resections of HCC at the Department of Liver and Vascular Surgery, West China Hospital of Sichuan University. Of these patients, 174 were excluded from the present study. Two died in the hospital during the operation period; 79 received other treatments before admission to undergo hepatectomy, and another 86 underwent intraoperative ablation because of multinodularity with some nodules left unresected. In addition, the other 7 patients were excluded because of positive macroscopic or microscopic margin (n=5) or disease detected in the liver remnant by ultrasonography or contrast-enhanced CT at 1 month after hepatectomy (n=2), which was considered a residual disease. After these exclusions, 398 patients who had undergone curative resection were enrolled in this study. Curative resection was defined as complete excision of the tumor and macroscopic portal vein tumor thrombi with a clear microscopic margin (R0 resection) and no residual tumors demonstrated by ultrasonography or contrastenhanced CT at 1 month after surgery. All the patients in this study underwent a preoperative indocyanine green (ICG) excretive test which has been described in detail elsewhere.[19,20]Patients who received preoperative and postoperative anti-hepatitis B therapy were recorded and the relationship between anti-hepatitis B therapy and late recurrence was analyzed.
The study protocol for collecting and using human samples was approved by the Institutional Ethics Committee of our hospital. Written informed consent was obtained from all participants involved in this study.
Surgical modalities
Table 1 shows the types of hepatectomy performed. A resection was defined as "major" if 3 or more segments were removed, according to Couinaud's classification. Major hepatectomies were performed in 207 patients (52.0%), including 20 (5.0%) patients with 3 or more discontiguous segments resected. One hundred and ninety-one patients (48.0%) underwent minor hepatectomies that were predominantly non-anatomical wedge resections (≤two segments) or enucleations (57, 14.3%), and left lateral segmentectomy (51, 12.8%). The average number of hepatic segments resected was 3.2±0.4 (range 0-6). Anatomical resection (AR), defined as any type of systematic resection of the portal regions based on Couinaud's classification, was performed in 299 patients (75.1%), while non-anatomical resection (non-AR) was performed in 99 (24.9%). In this study, incomplete removal of the tumor-bearing portal regions, such as wedge resection or enucleation, was classified as a non-AR, while discontiguous segments resection was included in AR, if every resection in that patient was AR; if not, then it was considered non-AR.[21]
Diagnosis of HCC
HCC was detected by contrast ultrasonography, dynamic CT and MRI. A focal lesion (≤2 cm in diameter) with arterial hypervascularization and venous washout detected by 2 imaging techniques or a single imaging modality associated with a focal lesion >2 cm in diameter was suggestive of HCC.[22]All HCC diagnoses were confirmed histopathologically after resection. Pathological grading was based on the Edmondson-Steiner criteria.[23]
Follow-up
All patients were seen regularly in the outpatient clinic and monitored prospectively for recurrence by a standard protocol. Follow-up consisted of monthly blood tests for monitoring serum alpha-fetoprotein level and ultrasonography or contrast-enhanced CT at least onceevery 3 months after surgery. Changes in tumor markers before and after curative resection and at diagnosis of recurrence were also assessed. The levels obtained before surgery and 2 months after the curative resection were adopted for analysis. Tumor recurrence was defined according to the same criteria applied to the initial HCC, and if the patients underwent hepatic re-resection, the tumor recurrence was diagnosed histopathologically. When tumor recurrence was confirmed, the number, size, and location of intrahepatic recurrences were then verified. Tumor recurrences at other sites were examined by contrast ultrasonography, CT, MRI, or whole body bone scans with single PET-CT.
Statistical analysis
Descriptive statistics included mean, range, standard deviation (SD), and proportion. Pearson's productmoment correlation coefficient test was applied to assess categorical variables significantly associated with recurrence in univariate analysis. Continuous variables were compared using unpaired Student'sttest, and the Cox's proportional hazards model was used for multivariate analysis of prognostic factors for tumor recurrences. All 23 variables were entered into a backward stepwise regression model. Step selections were based on the maximal likelihood ratio tests, and only significant variables were kept in the multiple logistic regression model. Survival rates were evaluated by the Kaplan-Meier method and compared using the log-rank test. All statistical evaluations were performed by SPSS18.0 for Windows (Chicago, IL, USA). For receiver operating characteristic (ROC) curve analysis, MedCalc (version 12.0) was used to calculate the sensitivity, specificity, area under the curve, and to select the optimal cut-off value for predicting tumor recurrence. APvalue <0.05 was considered to be statistically significant.
Results
Patient characteristics
The cohort consisted of 324 men (81.4%) and 74 women (18.6%) and had a mean age of 48.0±12.0 years. None of the patients belonged to Child-Pugh grade C. However, 360 patients were of Child-Pugh grade A and 38 were of Child-Pugh grade B. At the start of hepatectomies, 345 patients had single nodular tumors and 53 multinodular ones. The median nodule diameter was 5.6 cm (range 1.2-16.0). Of all the patients, 310 (77.9%) were HBV-positive and only 3 were HCV-positive. All patients had different degrees of liver fibrosis, and 272 patients (68.3%) had cirrhosis. The demographics of all patients, including preoperative, intraoperative, and tumorrelated parameters of the initial hepatectomy, are shown in Table 2.
Cumulative risk of recurrence
During the follow-up of 3 to 60 months after surgery, 247 patients (62.1%) suffered from tumor recurrence, with a mean time to recurrence of 22.9±16.7 months (range 3-60). Of these recurrent patients, 237 (96.0%) had intrahepatic recurrences, 58 (23.5%) had concurrent or subsequent extrahepatic recurrence, and 10 (4.0%) developed extrahepatic recurrence only. Extrahepatic metastases were confirmed using imaging techniques and pathological analysis of re-resected tissue. The sites of extrahepatic metastasis included the lungs (57 patients), brain (18), bone (4), and extrahepatic bile duct (3). No local recurrence in the vicinity of the cut surface was observed in these patients. Almost all intrahepaticrecurrences were multiple and located in either both liver lobes or the contralateral lobe. One hundred fiftyone patients (37.9%) remained recurrence free after a median follow-up period of 31.5 months. Fig. 1 shows the cumulative Kaplan-Meier curve of recurrencefree survival among the 398 patients. The cumulative recurrence-free survival was 75.5%, 58.2%, 54.1%, 40.5%, and 28.7% at 1, 2, 3, 4, and 5 years, respectively.

Table 2.Main characteristics of 398 patients with HCC
Fig. 2 shows the distribution of the time from tumor resection to recurrence among the 247 patients. From the distribution curve, two distinct peaks of tumor recurrence were detected. The first peak with a steep slope observed 1 year after hepatectomy showed the maximum probability of HCC recurrence, whereas another peak was observed 4 years after hepatectomy. The distribution also suggested that the recurrence can be divided into early phase (before 2 years) and late phase (after 2 years).
Univariate analysis of early and late recurrence
One hundred sixty-four patients had early phase recurrence, but 83 patients had late phase recurrence. Univariate analysis of preoperative and intraoperative conditions and pathological findings after hepatectomy associated with early and late tumor recurrences are shown in Table 3. Univariate analysis identified 1 host-related factor and 4 tumor-related factors that significantly correlated with early tumor recurrence, namely Child-Pugh grade (P=0.045), multiplicity of tumors (P=0.008), tumor size (P=0.001), pTNM grade (P=0.023), and venous infiltration (P=0.029). Univariate analysis also identified 6 parameters that significantly correlated with late tumor recurrence, specifically serum albumin level (P=0.010), indocyanine green retention rate at 15 minutes (ICG-R15) (P=0.0002), Child-Pugh grade (P=0.041), HBeAg status (P=0.0003), liver histology (P=0.029), and tumor size (P=0.006).
Multivariate analysis of early and late recurrence
Results from the Cox's multivariate proportional hazard model analysis identifying the significant independent adverse prognostic factors for early and late recurrence are shown in Table 4. In contrast to the univariate analysis, only two independent risk factors were identified for early recurrence: multiplicity of tumors [odds ratio (OR)=1.767,P=0.004, 95% confidence interval (CI): 1.193-2.616] and venous infiltration (OR=1.448,P=0.002, 95% CI: 1.142-1.837). Conversely, 3 adverse independent factors were identified to contribute to late phase recurrence: ICG-R15 (OR=1.098,P=0.007, 95% CI: 1.025-1.176), serum albumin level (OR=0.971,P=0.045, 95% CI: 0.943-0.999), and HBeAg status (OR=1.657,P=0.028, 95% CI: 1.055-2.603).
Predictors of early recurrence
On the basis of the Cox's multivariate proportional hazard model analysis of early phase recurrence, patients were stratified into high-risk (presence of at least 1 adverse predictive variable,n=180) and low-risk (absence of any adverse predictive variable,n=218) groups. Cumulative recurrence rates for the high- and low-risk groups for early phase recurrence are shown in Fig. 3. A significant difference was observed in the cumulative recurrence rate at 24 months between the 2 groups (P=1.36e-4, log-rank test). The recurrence rates at 1 and 2 years were 32.5% and 52.2% in the high-risk group and 17.9% and 33.3% in the low-risk group, respectively.
Predictors of late recurrence

Fig. 1.Cumulative recurrence-free survival curve of the patient cohort.

Fig. 2.The distribution of the time from resection to recurrence among the 247 patients.
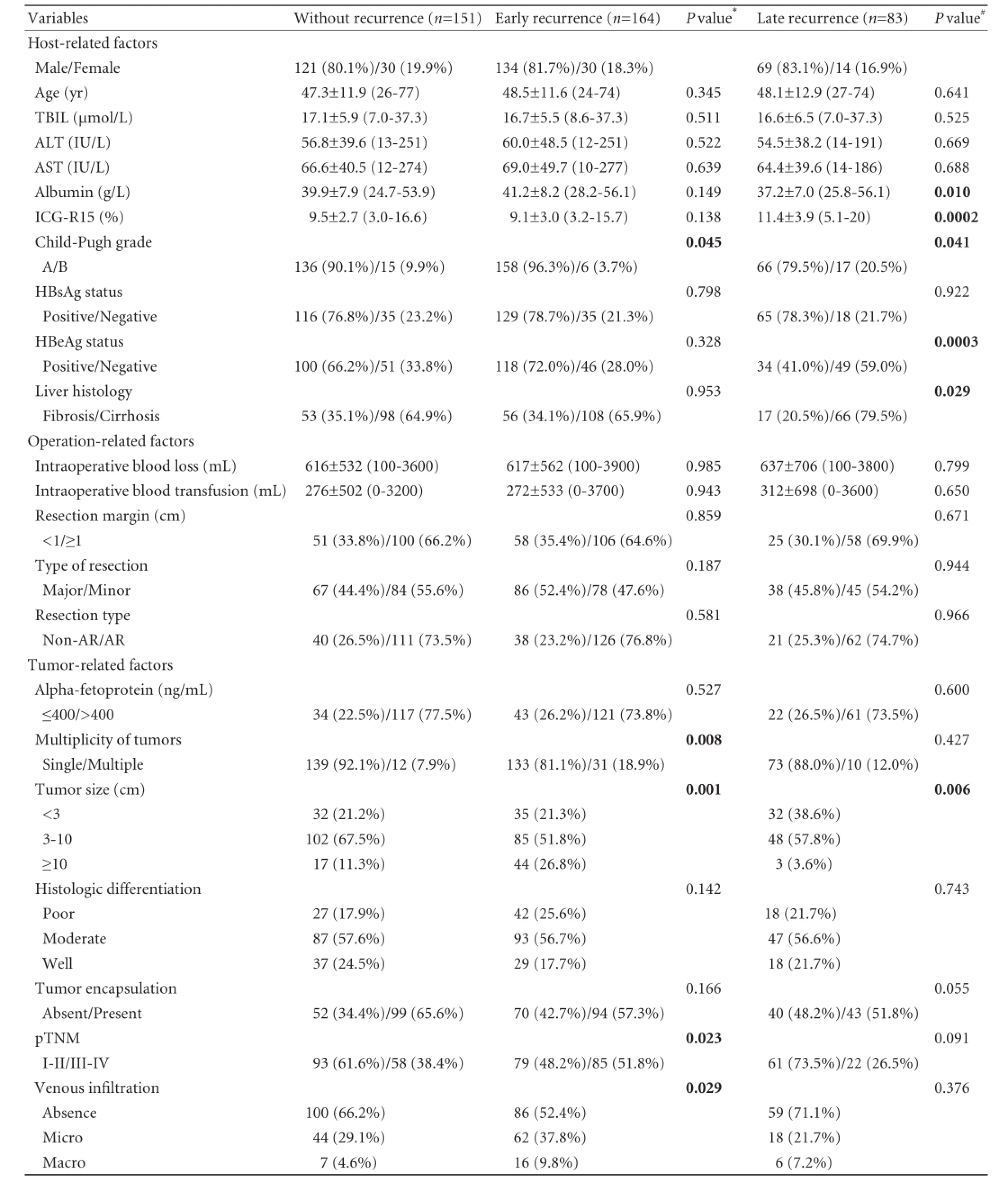
Table 3.Univariate analysis of early and late recurrence
ROC curve analysis of serum albumin level and ICG-R15 was performed to detect the cut-off value for predicting late phase recurrence. A cut-off value was determined by seeking the largest sum of the sensitivity and specificity values. The cut-off values for serumalbumin level and ICG-R15 were 45 g/L and 13%, respectively (areas under the curve was 0.595 and 0.604, withPvalues of 0.020 and 0.010, respectively). The area under the curve of HBeAg status was 0.627 (P=0.002). Though the predictive power of each of these 3 adverse independent parameters was not strong separately, the combination of the 3 independent risk factors showed a strong predictive power for late recurrence. The area under the curve was 0.747 (95% CI: 0.678-0.816,P<0.001), with a sensitivity of 67.5% and a specificity of 76.3%.
ROC curve analysis also identified that the cutoff value for the number of risk factors was less than 2 (Fig. 4). Based on this result, patients were stratified into high-risk (presence of at least 2 adverse predictive variables,n=87) and low-risk (presence of less than 2 adverse predictive variables,n=127) groups. Cumulative recurrence rates for the high- and low-risk groups for late phase recurrence are shown in Fig. 5. The recurrence rates at 3, 4, and 5 years were 9.5%, 42.0%, and 72.6% in the high-risk group respectively and 5.1%, 20.0%, and 30.2% in the low-risk group respectively (P=1.0e-6, log-rank test).

Table 4.Independent risk factors for recurrences identified by multivariate analysis using Cox's regression models

Fig. 3.Cumulative recurrence-free survival curve for early recurrence. Patients with solitary HCC were stratified into high-risk (dotted line,n=180) and low-risk (solid line,n=218) groups.
The relationship between anti-hepatitis B therapy and late recurrence
Two hundred and fourteen patients were followed up for more than 2 years after hepatectomy. Of these patients, 63 (29.4%) received preoperative anti-hepatitis B therapy and 95 (44.4%) received postoperative anti-hepatitis B therapy. Anti-hepatitis B therapy comprised lamivudine. For patients with lamivudine resistance, potential choices include the addition of adefovir or switching to entecavir. Patients who did not receive preoperative anti-hepatitis B therapy were not significantly different from those who received preoperative anti-hepatitis B therapy in terms of the late recurrence rate (Fig. 6A,P=0.077, log-rank test). However, the cumulative recurrence rate at 60 months in patients who received no postoperative anti-hepatitis B therapy was significantly higher than postoperative in those who received postoperative anti-hepatitis B therapy (Fig. 6B,P=0.011, log-rank test).
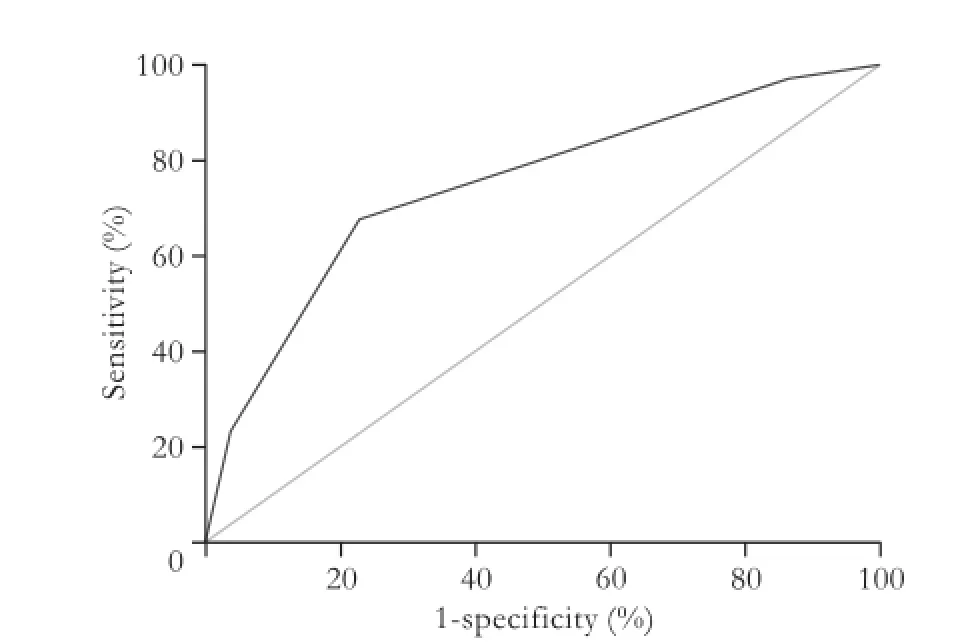
Fig. 4.ROC curve for the combination of the three adverse independent factors for late recurrence. The corresponding area was 0.747, with a sensitivity of 67.5% and a specificity of 76.3%, respectively.
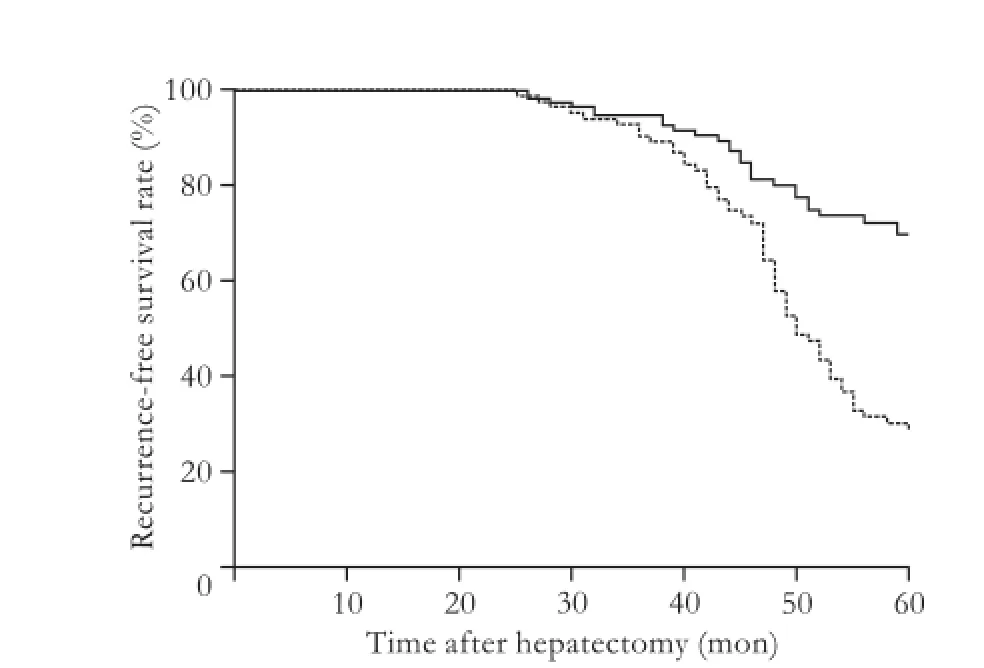
Fig. 5.Cumulative recurrence-free survival curve for late recurrence. Patients with solitary HCC were stratified into high-risk (dotted line,n=87) and low-risk (solid line,n=127) groups.

Fig. 6. Cumulative recurrence-free survival curve for late recurrence according to anti-hepatitis B therapy administration. A: Patients were stratified according to the administration of preoperative: the solid line denotes those who did not receive preoperative antihepatitis B therapy (n=151) and the dotted line denotes those who received preoperative anti-hepatitis B therapy (n=63). B: Patients were stratified according to the administration of postoperative: the solid line denotes patients who did not receive postoperative anti-hepatitis B therapy (n=119) and the dotted line denotes those who received postoperative anti-hepatitis B therapy (n=95).
Discussion
Hepatectomy of the main tumor along with the surrounding tissue may contain nondetectable micrometastases and can be performed without a waiting time, making hepatectomy the first treatment choice for HCC patients. Although hepatectomy greatly improves the hospital mortality rate, tumor recurrence is the most common cause of treatment failure after hepatectomy for HCC. Several studies[10-13]have reported that intrahepatic recurrence was mainly associated with aggressive pathological tumor factors such as vascular infiltration, tumor size, tumor capsule, and satellite nodule or dissemination of tumor cells during the hepatectomy, causing intrahepatic metastasis. Others[14,15]have found that the underlying liver status had a significant influence on tumor recurrence and suggested that tumor recurrence was related to multicentric occurrence. Thus, the mechanisms of recurrence after surgical resection for HCC are still controversial.
In the present study, the distribution of the time to recurrence among the 247 patients suggested that recurrence can be divided into early phase recurrence (before 2 years) and late phase recurrence (after 2 years). Moreover, different risk factors correlate with the different phases of recurrence. These findings may at least in part explain the conflicting results from many previous tumor recurrence studies, since these studies did not distinguish between early and late phase recurrence. Poon et al[24]first divided tumor recurrence as early and late phase recurrence and found different risk factors associated with the different phases. They suggested that early recurrences arise mainly from intrahepatic metastases, whereas late recurrences are more likely to be multicentric in origin. However, the demarcation point of time between early and late recurrence was 1 year after hepatectomy, which is different from the value of 2 years after hepatectomy detected from the distribution of the time to recurrence in our study, as well as different adverse factors with the present study. Imamura et al[25]also reported the same demarcation point of time between early and late recurrence as that found in the present study, although different risk factors were detected because of different diseases of the patients (HCV predominated in the previous study vs HBV in the present study).
In our patients, only 2 tumor-related parameters were significantly associated with early intrahepatic recurrence. The identification of venous invasion as an adverse factor emphasizes that the main route of early intrahepatic recurrence after resection of HCC is spreading via the portal vein. Previous studies[10-14]have shown that the presence of vascular invasion often leads to fulminant recurrence. Tumor multiplicity was another adverse prognostic indicator of recurrence-free survival that may reflect microscopic vascular invasion in the early days. Some studies[10-13,26-29]reported that serum alpha-fetoprotein level, pTNM grade, histological differentiation, tumor encapsulation and tumor size, alone or in combination, were useful prognostic factors of tumor recurrence for hepatectomy. However, none of those factors appear to play an important role in the early tumor recurrence in our patients. Of note, though Child-Pugh grade was different between the early and no recurrence groups by univariate analysis, none of the liver-related parameters in patients with solitary HCC or in patients with or without cirrhosis were identified as factors related to early phase recurrence by our Cox's proportional hazards model analysis. Furthermore, surgical procedures (AR vs non-AR, major resectionvs minor resection, or resection margin <1 vs ≥1 cm) were not identified as independent factors contributing to this type of recurrence, contrary to the results from other studies.[16-18]This finding indicates that the venous invasion behavior of the tumor is the key factor to early recurrence.
In contrast to factors influencing early recurrence, factors associated with late phase recurrence, including albumin, ICG-R15, and HBeAg status, are thought to reflect the degree of damage to the remnant liver. A widely accepted hypothesis for the mechanism of late recurrence is that damage to the remnant liver can stimulate cell proliferation, presumably by an increased rate of random mutations and promotion.[30,31]In support of this hypothesis, several studies[32-35]have reported that the risk factors significantly associated with tumor recurrence included Child-Pugh grade, albumin level, transaminase level, and chronic active hepatitis.
Our data showed that a lower albumin level, a higher ICG-R15 value, and a positivity of HBeAg were independent risk factors of late tumor recurrence. The cut-off values identified by ROC analysis were ≤45 g/L and >13% for albumin and ICG-R15, respectively. A low albumin level and a high ICG-R15 level may reflect serious liver cirrhosis, which is significantly associated with multicentric occurrence of a new tumor.[36]In particular, many studies[14,15,24]have reported that liver cirrhosis was a key adverse factor to tumor recurrence, especially to late recurrence. Though liver cirrhosis was identified as different between the late and no recurrence groups in our study by univariate analysis, it was not an independent risk factor of late phase recurrence identified by our Cox's proportional hazards model analysis. These findings suggest that continuous variables such as albumin level and ICG-R15 value can more accurately reflect the condition of the remnant liver than categorical variables like cirrhosis state.
Although whether patients with chronic hepatitis are susceptible to late tumor recurrence or needed antiviral therapy has been disputed,[35,37-39]our findings did show that HBeAg positivity is an adverse factor for late tumor recurrence and that late recurrence was significantly decreased in patients who received postoperative antihepatitis B therapy. Chronic hepatitis continuously destroyed hepatocytes and increased hepatocyte proliferation. This higher level of degeneration may be a key factor in generating hepatocyte gene mutations that result in a new secondary lesion after hepatectomy.
In conclusion, our findings indicate that both intrahepatic metastasis from the primary tumor and multicentric occurrence of a new tumor are involved in the underlying mechanisms of recurrence after surgical resection of HCC. It is noteworthy that early recurrence is mainly due to intrahepatic metastasis, which is tightly correlated with tumor-related parameters, whereas late recurrence is mainly due to multicentric occurrence, which is tightly correlated with the condition of the remnant liver. Patients with either venous invasion or multiplicity of tumors may exhibit a higher early HCC recurrence rate after hepatectomy, whereas those with at least 2 adverse factors among albumin ≤45 g/L, ICG-R15>13%, and HBeAg positivity exhibit a higher late HCC recurrence rate. Thus, patients exceeding these criteria should be closely monitored and receive liver-protecting and anti-HBV treatment.
Contributors:DZG proposed the study. DZG and WYG performed research and wrote the first draft. DZG and CKF collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. LB is the guarantor.Funding:None.
Ethical approval:This study was approved by the Institutional Ethics Committee of West China Hospital, Sichuan University and written informed consent was obtained from all participants.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kim JH, Choi MS, Lee H, Kim do Y, Lee JH, Koh KC, et al. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol 2006;21:588-594.
2 Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg 2008;207:20-29.
3 Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics 2005;25:S3-23.
4 Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 2007;4:424-432.
5 Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 2005;25:181-200.
6 Yi NJ, Suh KS, Kim T, Kim J, Shin WY, Lee KU. Current role of surgery in treatment of early stage hepatocellular carcinoma: resection versus liver transplantation. Oncology 2008;75:124-128.
7 Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-330.
8 Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7:237-257.
9 Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-termsurvival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-382.
10 Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000;127:603-608.
11 Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003;197:753-758.
12 Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, et al. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg 2008;32:2218-2222.
13 Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375-1382.
14 Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996;83:1219-1222.
15 Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993;105:488-494.
16 Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 2008;143:469-475.
17 Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 2005;242:252-259.
18 Hasegawa K, Kokudo N. Surgical treatment of hepatocellular carcinoma. Surg Today 2009;39:833-843.
19 Du ZG, Li B, Wei YG, Yin J, Feng X, Chen X. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2011;10:265-269.
20 Sakka SG, Meier-Hellmann A. Non-invasive liver function monitoring by indocyanine green plasma disappearance rate in critically ill patients. Int J Intensive Care 2002;9:66-72.
21 Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford) 2013;15:31-39.
22 Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 2006;25:3848-3856.
23 Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7: 462-503.
24 Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500-507.
25 Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-207.
26 Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;101:796-802.
27 Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P'eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg 2005;92:348-355.
28 Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol 2008;34:900-905.
29 Kim H, Park MS, Park YN, Kim H, Kim KS, Choi JS, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J 2009;50:789-795.
30 Tarao K, Ohkawa S, Shimizu A, Harada M, Nakamura Y, Ito Y, et al. Significance of hepatocellular proliferation in the development of hepatocellular carcinoma from anti-hepatitis C virus-positive cirrhotic patients. Cancer 1994;73:1149-1154.
31 Ruà S, Comino A, Fruttero A, Torchio P, Bouzari H, Taraglio S, et al. Flow cytometric DNA analysis of cirrhotic liver cells in patients with hepatocellular carcinoma can provide a new prognostic factor. Cancer 1996;78:1195-1202.
32 Morris-Stiff G, Gomez D, de Liguori Carino N, Prasad KR. Surgical management of hepatocellular carcinoma: is the jury still out? Surg Oncol 2009;18:298-321.
33 Bigourdan JM, Jaeck D, Meyer N, Meyer C, Oussoultzoglou E, Bachellier P, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl 2003;9:513-520.
34 Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology 1997;25:87-92.
35 Wakai T, Shirai Y, Yokoyama N, Nagakura S, Hatakeyama K. Hepatitis viral status affects the pattern of intrahepatic recurrence after resection for hepatocellular carcinoma. Eur J Surg Oncol 2003;29:266-271.
36 Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-235.
37 Du Y, Su T, Ding Y, Cao G. Effects of antiviral therapy on the recurrence of hepatocellular carcinoma after curative resection or liver transplantation. Hepat Mon 2012;12:e6031.
38 Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol 2012;38:683-691.
39 Lok AS. Does antiviral therapy prevent recurrence of hepatitis B virus-related hepatocellular carcinoma after curative liver resection? JAMA 2012;308:1922-1924.
Received March 15, 2013
Accepted after revision June 17, 2013
Author Affiliations: Department of Liver and Vascular Surgery, Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, China (Du ZG, Wei YG, Chen KF and Li B)
Bo Li, MD, Department of Liver and Vascular Surgery, Liver Transplantation Center, West China Hospital, Sichuan University, 37 Guoxue Street, Chengdu 610041, China (Tel: 86-28-85422476; Fax: 86-28-85423724; Email: doclibo@gmail.com)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60025-4
 Hepatobiliary & Pancreatic Diseases International2014年2期
Hepatobiliary & Pancreatic Diseases International2014年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Improved anterior hepatic transection for isolated hepatocellular carcinoma in the caudate
- Instrumental detection of cystic duct stones during laparoscopic cholecystectomy
- Familial chylomicronemia syndrome related chronic pancreatitis: a single-center study
- Pancreatic fistula after central pancreatectomy: case series and review of the literature
- Multi-visceral resection of locally advanced extra-pancreatic carcinoma
- Pancreatic head cancer in patients with chronic pancreatitis
