FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelialmesenchymal transition
Jun Yu, Wu Zhang, Feng Gao, Yuan-Xing Liu, Zhi-Yun Chen, Long-Yu Cheng, Shang-Fen Xie and Shu-Sen Zheng
Hangzhou, China
FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelialmesenchymal transition
Jun Yu, Wu Zhang, Feng Gao, Yuan-Xing Liu, Zhi-Yun Chen, Long-Yu Cheng, Shang-Fen Xie and Shu-Sen Zheng
Hangzhou, China
BACKGROUND:FBW7 is a tumor suppressor which regulates a network of proteins with central roles in cell division, cell growth and differentiation. This study aimed to evaluate the role of FBW7 in chemosensitivity and epithelial-mesenchymal transition (EMT) in different hepatocellular carcinoma (HCC) cell lines and to investigate the relevant underlying mechanisms.
METHODS:Different human HCC cell lines (Hep3B, Huh-7, and SNU-449) were cultured. The cell viability was evaluated by cell counting kit-8, and FBW7 mRNA transcription and protein expression were quantitated by real-time PCR and Western blotting. Expressions of vimentin (mesenchymal biomarker) and E-cadherin (epithelial biomarker) were evaluated by Western blotting and immunocytochemistry. Cell invasion was assayed by Transwell migration, and FBW7 plasmid or siRNA was used to evaluate the effect of FBW7 overexpression or silencing on cell chemosensitivity.
RESULTS:FBW7 expression affected tumor cell chemosensitivity to doxorubicin and tumor cell invasive capacity in different HCC cell lines. FBW7hi(high FBW7 expression) Hep3B and FBW7mi(median FBW7 expression) Huh-7 cells were more sensitive to doxorubicin and lower in invasive capacity than FBW7lo(low FBW7 expression) SNU-449 cells. Silencing of FBW7 in Huh-7 and Hep3B cells induced the resistance to doxorubicin and enhanced cell invasion, whereas overexpression of FBW7 in SNU-449 cells restored the sensitivity to doxorubicin andsignificantly reduced invasive capacity. Furthermore, doxorubicin induced EMT toward mesenchyme in HCC cells. Downregulation of FBW7 in Huh-7 and Hep3B cells or upregulation of FBW7 in SNU-449 cells altered the direction of EMT.
CONCLUSIONS:The level of FBW7 expression impacted the tumor resistance to doxorubicin and the invasion capability of HCC cells. FBW7 therefore may be a potential target for the chemotherapy of HCC through the regulation of EMT.
(Hepatobiliary Pancreat Dis Int 2014;13:184-191)
FBW7;
hepatocellular carcinoma;
doxorubicin;
epithelial-mesenchymal transition;
drug resistance
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common solid tumor worldwide. Based on the GLOBOCAN2008 estimates, there were 10.8 cases and 9.9 deaths per 100 000 people.[1]About 80%-90% of HCCs were not resectable when diagnosed and therefore, chemotherapy might be the only option for some of these patients. However, the chemotherapy is far from satisfaction due to the development of multidrug resistance (MDR). Some proteins, such as F-box and WD repeat domain-containing 7 (FBW7), play important roles in chemosensitivity.
FBW7, the substrate recognition component of the SKP1-CUL1-F-box (SCF) ubiquitin ligase complex, is an evolutionarily conserved member of the F-box protein family. FBW7 regulates a network of signal proteins functioning in cell division, cell growth and differentiation.[2]FBW7 binds to substrates after they have been phosphorylated within conserved phosphodegron motifs, called the Cdc4 phospho-degrons(CPD). SCF complexes bind to protein substrates, targeting them for ubiquitylation and subsequent degradation by proteasomes.[3]Fully phosphorylated FBW7 substrates may facilitate cells to rapidly and coordinately downregulate cell growth and division in response to antiproliferative signals.[2]Although FBW7 mutation, which results in dysregulation of apoptosis and chemosensitivity, may be associated with MDR,[4]the mechanism is still unclear.
Epithelial-mesenchymal transition (EMT) is also associated to the development of MDR. EMT describes the process by which cells gradually lose typical epithelial characteristics and acquire mesenchymal traits.[5]EMT can pathologically lead to carcinoma progression, invasion and metastasis, and may thereby play a key role in enabling carcinoma cells to evade chemotherapy.[6]Epithelial functions that are lost during EMT include disordered expression and distribution of proteins, such as β-catenin and E-cadherin, which mediate loss of cellcell, cell-matrix contacts and cytoskeletal organization responsible for normal epithelial polarity. The concurrent gain of mesenchymal characteristics includes the ability to migrate and invade the surrounding matrix, through expression of proteins such as vimentin and fibronectin.[5,7]Epithelial-type HCC cells are more chemosensitive than mesenchymal cells;[8]when HCC cells undergoing EMT re-express E-cadherin, they regain chemosensitivity.[5]The present study was to evaluate the relationship between doxorubicin sensitivity and FBW7 expression in HCC cells, to investigate whether FBW7 mutation interfered with EMT, and to explore the underlying mechanisms.
Methods
Cell culture and reagents
Human HCC cell lines (Hep3B, Huh-7, and SNU-449) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained according to ATCC guidelines. Hep3B cells were cultured in minimum essential medium (Gibco), Huh-7 cells in Dulbecco's modified Eagle's medium (Gibco) and SNU-449 cells in RPMI 1640 medium (Gibco). All of these media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were maintained at 37 ℃ with 5% CO2. Doxorubicin was purchased from Sigma (St. Louis, MO, USA)
Western blotting analysis
HCC cells were seeded into 6-well plates (2×105cells/well) and incubated overnight until they were adherent. After 72-hour doxorubicin treatment, the cells were harvested in 100 μL of cell lysis buffer (Cell Signaling, Danvers, MA, USA) containing protease inhibitors (Sigma). Then, cell lysates were separated by 10% SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Membranes were then blocked by 5% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 and followed by incubation overnight with primary antibodies (E-cadherin, vimentin and FBW7; Cell Signaling). After incubation with secondary antibody conjugated to goat anti-mouse horseradish peroxidase (GE Healthcare, Piscataway, NJ, USA) for 2 hours, membranes were washed, and immunoreactive bands were detected by ECL (GE Healthcare) and visualized by autoradiography (X-ray film; Kodak, Rochester, NY, USA). Protein loading was normalized against GAPDH (Cell Signaling).
FBW7 mRNA expression
The HCC cell lines were cultured as described above. Total RNA was extracted with Trizol (Invitrogen), and cDNA was synthesized using a cDNA synthesis kit (Takara, Japan) following the manufacturer's instructions. The mRNA levels of FBW7 were measured by real-time PCR using the following gene-specific primers (Takara): forward, 5'-CAC TCA AAG TGT GGA ATG CAG AGA C-3'; reverse, 5'-GCA TCT CGA GAA CCG CTA ACA A-3'. The relative expression of FBW7 mRNA was normalized to β-actin using the β-actin gene-specific primers (Takara): forward, 5'-GGA GAT TAC TGC CCT GGC TCC TA-3'; reverse, 5'-GAC TCA TCG TAC TCC TGC TTG CTG-3'.
Cell viability assays
A cell counting kit-8 kit (CCK-8; Dojindo, Kumamoto, Japan) was used to evaluate the relative cell viability. In brief, the cells were seeded into a 96-well microplate (5×103cells/well) and incubated overnight until they were adherent. After 24 hours of culture in a serumfree medium, HCC cells were treated with doxorubicin at different concentrations for 72 hours. Then, 10 μL CCK-8 solution was added into the medium and incubated for another 2 hours. The absorbance of the medium was measured at 450 nm using an MRX II microplate reader (Dynex, Chantilly, VA, USA).
Silencing FBW7: transfection with FBW7 siRNA
HCC cell lines were transfected with FBW7 siRNA (Santa Cruz Biotechnology) diluted in lipofectamine 2000 (10 μmol/mL; Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After 12-hour transfection, the medium was replaced witha complete medium and HCC cells were incubated for specified times. The effects of transfection on cell viability were tested by CCK-8.
Overexpression of FBW7: transfection with FBW7 plasmid
Recombinant overexpression plasmid designed in our lab (named as "pLVX-FBW7-IRES-ZsGreen1") was transfected into the HCC cell lines using lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. All experiments were performed in 12 hours after transfection, and repeated for three times. The effects of transfection on cell viability were tested by CCK-8.
Immunocytochemistry
The HCC cell lines were seeded into 24-well plates and treated as described above. HCC cells were fixed with 4% formaldehyde for 15 minutes, washed with PBS, and incubated with 5% BSA for 30 minutes at room temperature (RT). Then, the HCC cells were stained with mouse anti-human vimentin or anti-human E-cadherin primary antibodies (Cell Signaling) at 4 ℃ overnight. The cells were subsequently incubated with goat anti-mouse IgG-FITC (vimentin) or IgG-Cy5 (E-cadherin) secondary antibodies (Abcam, Cambridge, MA, USA) in PBS for 2 hours at 4 ℃. The cells were incubated for 2 minutes at RT with 4, 6-diamidino-2-phenylindole (DAPI) (Sigma) after staining, washed twice with phosphate buffered saline (PBS), and visualized under an inverted fluorescence IX81 microscope (Olympus, Tokyo, Japan).
Cell invasion assay
HCC cells seeded into the upper chambers of polycarbonate membrane treated Transwell? compartment (Corning Incorporated, Corning, NY, USA) pre-coated with basement membrane matrix (BD Bioscience, Bedford, MA, USA) were cultured in serum-free medium. The lower receiver well was added with 10% FBS as chemoattractant. After 48-hour incubation, the invaded cells were fixed and stained by crystal violet. The number of stained cells was counted in five randomly selected fields within each insert under an inverted fluorescence IX81 microscope, and the invasive rates were calculated by dividing the number of invaded cells and the number of cells seeded in each insert.
Statistical analysis
Statistical analysis was made using Prism 5 software (GraphPad, San Diego, CA, USA). Data were presented as the mean±SD. Two-way ANOVA was used to compare the difference in the inhibitory effects of doxorubicin and combined therapy after the Bonferronipost-hoctest. For all the tests, statistical significance was set atP<0.05.
Results
FBW7 expression and doxorubicin chemosensitivity in HCC cells
FBW7 protein and mRNA expressions were significantly higher in Hep3B and Huh-7 cells compared with those in SNU-449 cells (FBW7lo) (Fig. 1A, B). Hep3B cells had the highest expression of FBW7 (Hep3B: FBW7hi; Huh-7: FBW7mi). CCK8 assay after 72-hour treatment with doxorubicin in three HCC cells revealed that increased doses of doxorubicin reduced cell viability in the three HCC cell lines. However, FBW7hiHep3B and FBW7miHuh-7 cells were more sensitized than FBW7loSNU-449 cells (Fig. 1C). Mean IC50 values for doxorubicin in Hep3B, Huh-7 and SNU-449 cells were 0.1267 μg/mL (0.1136-0.1398 μg/mL; 95% CI), 0.8160 μg/mL (0.7185-0.9648 μg/mL; 95% CI), and 1.2700 μg/mL (1.1110-1.4280 μg/mL; 95% CI), respectively (Table).
FBW7 upregulation increasing doxorubicin chemosensitivity in HCC cells
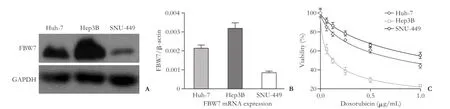
Fig. 1.A: Western blotting analysis showing FBW7 expression levels in three HCC cell lines (Huh-7, Hep3B and SNU-449); GAPDH was used as a control.B: real-time PCR results showing FBW7 mRNA expression levels in the three cell lines; β-actin was used as a control.C: cell viability CCK-8 assay results showing doxorubicin chemosensitivity to the three cell lines.
To further confirm the relationship between FBW7 expression and doxorubicin chemosensitivity in HCC cells, FBW7 siRNA was used to silence FBW7 expression in all three HCC cell lines and the interference efficiency was measured by Western blotting (Fig. 2). The dosedependent viability assay showed that silencing FBW7 expression led to downregulation of doxorubicin sensitivity in the cell lines (Fig. 2). FBW7 siRNA co-treatment significantly attenuated doxorubicin cytotoxicity in Hep3B and Huh-7 cells (two-way ANOVA;P<0.001) with concurrent increases in IC50 values of doxorubicin 0.5440 μg/mL (0.4230-0.6650 μg/mL; 95% CI), and 3.0230 μg/mL (1.6990-4.3460 μg/mL; 95% CI), respectively. These values were similar to those in SNU-449 cells. The IC50 value of doxorubicin was 7.6350 μg/mL (2.9990-12.2700 μg/mL; 95% CI) in FBW7 siRNA transfected cells (Table).
Transfection with FBW7 plasmid (pLVX-FBW7-IRES-ZsGreen1) was used to upregulate FBW7 expression in the HCC cell lines. Expression levels were also measured by Western blotting (Fig. 2), and the effect on chemosensitivity was verified by cell viability assays as described above. The results showed that FBW7 plasmid transfection significantly increased doxorubicin-induced cytotoxicity in Hep3B, Huh-7 and SNU-449 cells (Fig. 2), with concurrent decreases in IC50 values of doxorubicin 0.0828 μg/mL (0.0729-0.0928 μg/mL; 95% CI), 0.4514 μg/mL (0.3980-0.5049 μg/mL; 95% CI), and 1.0950 μg/ mL (0.8816-1.3090 μg/mL; 95% CI), respectively (Table).
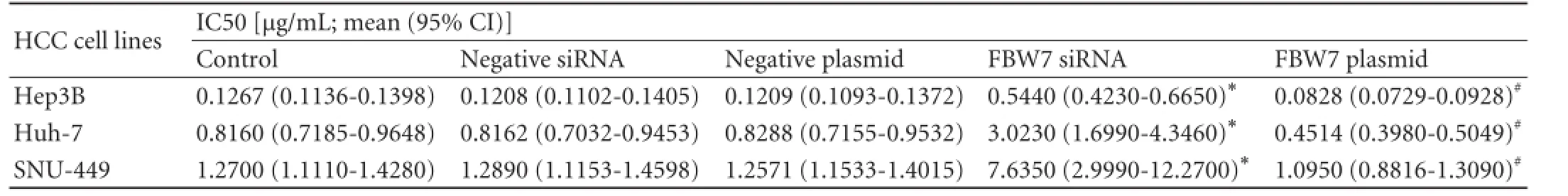
Table.IC50 values and statistical analyses of doxorubicin treatments in the three HCC cell lines after FBW7 downregulation or upregulation

Fig. 2.Effects of FBW7 siRNA interference or FBW7 plasmid transfection on FBW7 expression levels (Western blotting analyses; left panels) and chemosensitivity (cell viability assays; right panels) in HCC cell lines: (A,B) Hep3B; (C,D) Huh-7; (E,F) SNU-449.
Epithelial phenotype being more sensitive to doxorubicin than mesenchymal phenotype in HCC cells
To ascertain whether the disparity in response to doxorubicin between Huh-7/Hep3B cells and SNU-449 cells was phenotype dependent, Western blotting and immunocytochemistry were used to measure the expression of phenotype markers in HCC cells. Western blotting revealed that doxorubicin-resistant FBW7loSNU-449 cells showed the lowest E-cadherin expression, but the highest vimentin expression. In contrast to FBW7hiHep3B and FBW7miHuh-7 cells (Fig. 3A), the results were similar in immunocytochemistry, with FBW7loSNU-449 cells displaying an almost complete absence of E-cadherin signal (red), but high level of vimentin signal (green), compared to FBW7hiHep3B and FBW7miHuh-7 cells (Fig. 3B).
Doxorubicin reducing expression of FBW7 and inducing EMT in HCC cells
After doxorubicin treatment (IC50 concentration) for 72 hours, the three HCC cell lines showed reduced expression of FBW7 (Fig. 4), accompanied with significantly increased levels of vimentin and decreased levels of E-cadherin. The effect was more obvious in FBW7hiHep3B and FBW7miHuh-7 cells (Fig. 5A, C, E). Immunocytochemistry showed similar results, i.e., lower expression of E-cadherin and higher expression of vimentin in doxorubicin treated cells (Fig. 5B, D, F)
FBW7 regulating EMT in HCC cells
To confirm whether FBW7 is involved in the doxorubicin-induced EMT in HCC cells, we transfected mesenchymal FBW7loSNU-449 cells with FBW7 plasmid and epithelial FBW7hiHep3B and FBW7miHuh-7 cells with siRNA, respectively. Western blotting analysis showed that SNU-449 cells showed a higher expression of E-cadherin and a lower expression of vimentin after transfection with FBW7 plasmid (Fig. 6A), suggesting that these cells were transformed to epithelial phenotype after FBW7 upregulation. Immunocytochemistry showed that SNU-449 cells displayed a higher expression of E-cadherin (red) anda lower expression of vimentin (green) after FBW7 plasmid transfection (Fig. 6B).
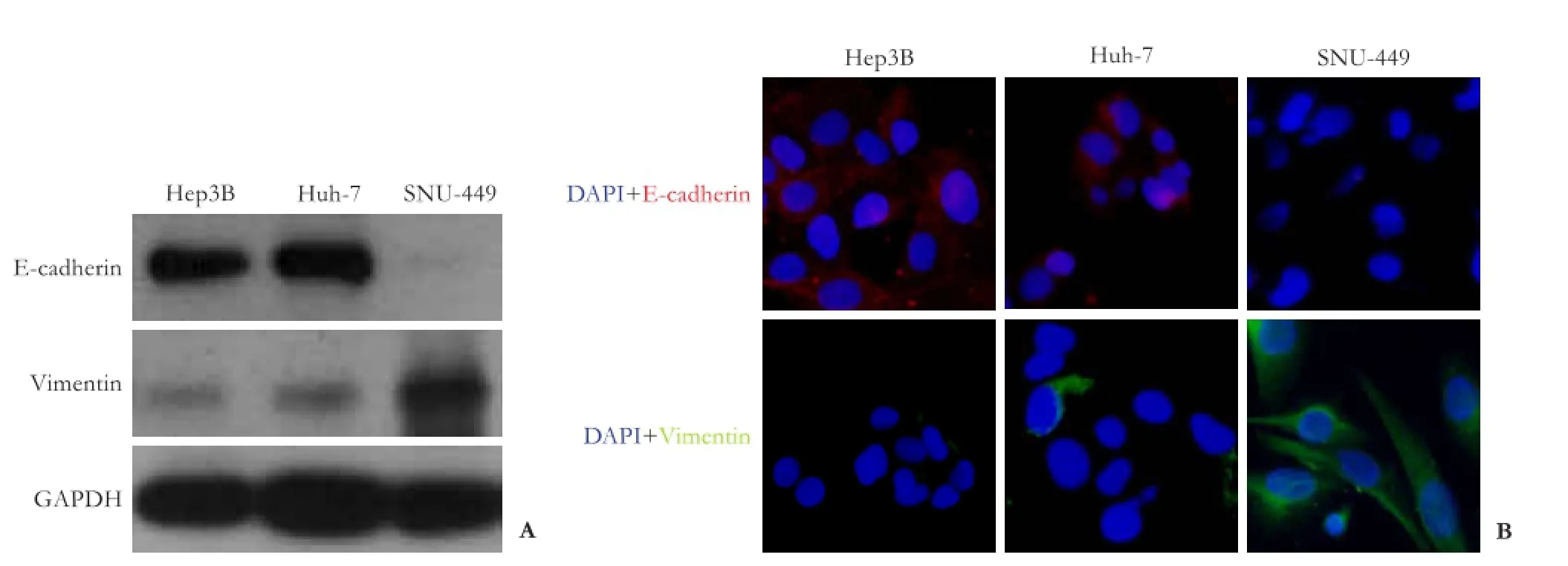
Fig. 3.Western blotting analyses (A) and immunocytochemistry (B) showing that Hep3B and Huh-7 cells expressed a higher level of epithelial marker proteins (E-cadherin) but a lower level of mesenchymal marker proteins (vimentin), than SNU-449 cells.

Fig. 4.Doxorubicin treatment reduced the expression level of FBW7 in HCC cell lines. Western blotting analysis of FBW7 in Hep3B (A), Huh-7 (B) and SNU-449 (C) cells after treatment with or without doxorubicin.
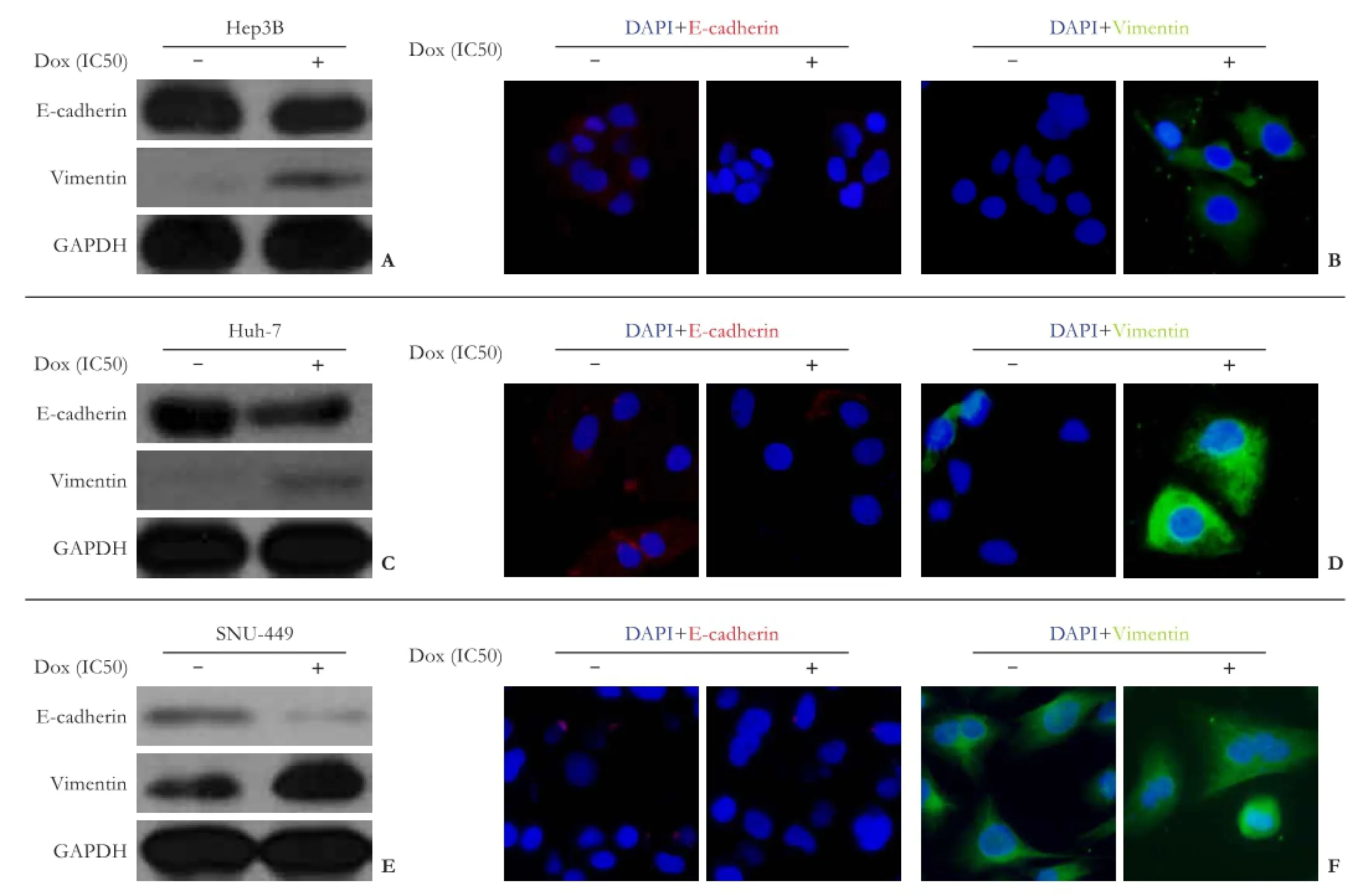
Fig. 5.Influence of doxorubicin treatment on cell phenotype in Hep3B, Huh-7, and SNU-449 cells: (A,C,E) Western blotting analyses and (B,D,F) immunocytochemistry staining showing changes in EMT marker proteins (E-cadherin and vimentin) after doxorubicin treatment.
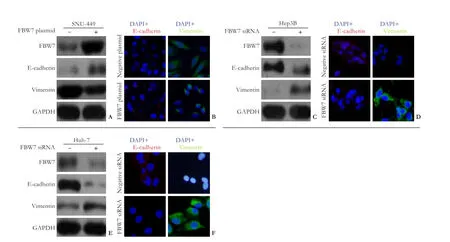
Fig. 6.A-B: Western blotting analysis (A) and immunocytochemistry (B) showing changes in EMT marker proteins after FBW7 upregulation in SNU-449 cells.C-D: Western blotting analysis (C) and immunocytochemistry (D) showing changes in EMT marker proteins after FBW7 downregulation in Hep3B cells.E-F: Western blotting analysis (E) and immunocytochemistry (F) showing changes in EMT marker proteins after FBW7 downregulation in Huh-7 cells.
In contrast, after transfection of FBW7hiHep3B and FBW7miHuh-7 cells with FBW7 siRNA to silence FBW7 expression, both Western blotting analysis and immunocytochemistry revealed a decreased expression of epithelial marker protein E-cadherin and an increased expression of mesenchymal marker protein, vimentin (Fig. 6C-F).
In addition, matrigel invasion assay showed that mesenchymal FBW7loSNU-449 cells had higher invasive capacity than epithelial FBW7hiHep3B and FBW7miHuh-7 cells (Fig. 7). The transfection of FBW7 plasmid significantly reduced invasion in SNU-449 cells. In line with this, transfection of FBW7 siRNA significantly increased invasive capacity in Hep3B and Huh-7 cells (Fig. 7).
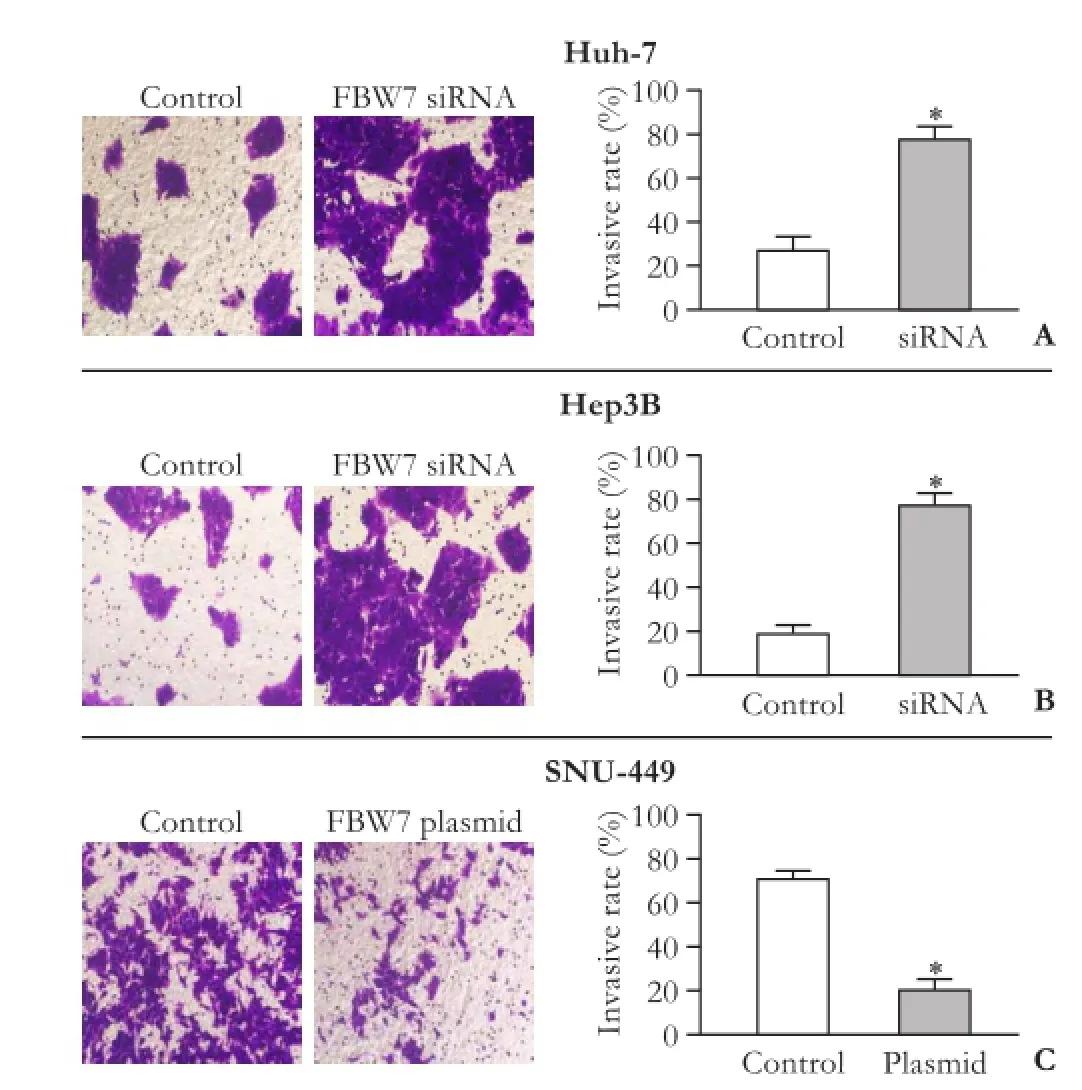
Fig. 7. The effects of FBW7 on the invasive capacity of HCC cells. Control HCC cells and FBW7 siRNA/plasmid transfected HCC cells (A, Huh-7; B, Hep3B; C, SNU-449) were seeded into the upper chambers of Transwell compartment insert. Invaded cells were stained with crystal violet and viewed with a microscope in five randomly selected field. The invasive rates were calculated by dividing the number of invaded cells by the number of cells seeded in each insert; *: P<0.001, vs control.
Discussion
As a serious problem in carcinoma treatment,[9]MDR is hindering the clinical use of common chemotherapeutic agents such as doxorubicin and cisplatin.[10,11]Resistance to doxorubicin leads to a lower response rate and the use of high doses of drugs, giving rise to severe adverse effects.[12]In our study, FBW7 was associated with doxorubicin sensitization in HCC cells. FBW7hiHep3B cells and FBW7miHuh-7 cells displayed a higher level of chemosensitivity to doxorubicin than FBW7loSNU-449 cells. However, FBW7 siRNA inhibited the sensitivity to doxorubicin in all three cell lines. In comparison, the upregulation of FBW7 expression by transfecting FBW7 plasmid enhanced the sensitivity of doxorubicin. This finding indicated that FBW7 expression in HCC cells was correlated with the chemosensitivity to doxorubicin. Upregulation of FBW7 significantly enhanced the cytotoxicity of doxorubicin in HCC cells. As a tumor suppressor, FBW7 regulated a network of proteins with central roles in cell division, cell growth and differentiation.[2]Studies[13-17]reported FBW7 loss-offunction mutations in cell lines from several primary cancers as well as FBW7 mutations in approximately 6% of tumors.[2]In recent studies,[2,4]FBW7 mutations were found to be linked to MDR. FBW7-deficient T-ALL cells were particularly resistant to the BCL2 antagonist ABT-737 and primary tumors enriched for FBW7 inactivation were resistant to anti-tubulin chemotherapeutics. Although these findings highlight the role of FBW7 in drug resistance and subsequent oncogenesis,[16]its precise mechanisms are not clear. In the present study, doxorubicin resistance in FBW7loHCC cells was associated with EMT processes. EMT biomarker patterns demonstrated that FBW7loSNU-449 and FBW7hiHep3B or FBW7miHuh-7 cells belonged to two different phenotypes, and that FBW7hiHep3B and FBW7miHuh-7 cells displayed more epithelial characteristics (E-cadherin) than FBW7loSNU-449 cells. Similarly, the expression of mesenchymal biomarker (vimentin) was higher in FBW7loSNU-449 cells than in FBW7hiHep3B or FBW7miHuh-7 cells, indicating that epithelial HCC cells are more sensitive to doxorubicin therapy than mesenchymal cells. Furthermore, our study showed that knockdown of FBW7 altered EMT patterns in FBW7hiHep3B and FBW7miHuh-7 cells, namely, upregulation of mesenchymal biomarkers and downregulation of epithelial biomarkers.
In the present study, we evaluated the potential role of EMT in doxorubicin resistance associated with low FBW7 expression. In HCC cells, EMT is related to proliferation, invasion, and metastasis in HCC;[18]and potent oncogenicity associated with EMT may be a contributory factor in inefficient drug responses and carcinoma recurrence in current systemic therapies.[19]In addition, there is evidence that reverse EMT, referred to as mesenchymal-epithelial transition (MET), enables HCC cells to regain chemosensitivity.[5]
In conclusion, our study showed that decreased expression of FBW7 leads to doxorubicin resistance in HCC cells, and that the underlying mechanism is associated with regulation of the EMT/MET process. Thus we propose that FBW7, a tumor suppressor, is potentially important in searching for a future chemotherapy for HCC. Targeting FBW7 is capable of eliminating drug resistance in chemotherapy of HCC.
Contributors:ZSS proposed the study. YJ, ZW, GF, LYX, CZY, CLY and ZSS performed research and wrote the first draft. YJ and XSF collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Funding:This study was supported by grants from National High-Tech Research and Development Projects (863 Program) (2012AA021002), Organ Transplantation Key Technology Project (863 Program) (2012AA022409) and Zhejiang Province Natural Science Foundation (LQ12H03002 and LY12H03010).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008;8:83-93.
3 Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev 1996;10:1979-1990.
4 Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011;471: 104-109.
5 Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology 2009;50:2007-2013.
6 Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009;27:2059-2068.
7 Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-746.
8 Hu QD, Chen W, Yan TL, Ma T, Chen CL, Liang C, et al. NSC 74859 enhances doxorubicin cytotoxicity via inhibition of epithelial-mesenchymal transition in hepatocellular carcinoma cells. Cancer Lett 2012;325:207-213.
9 Miracco C, Maellaro E, Pacenti L, Del Bello B, Valentini MA, Rubegni P, et al. Evaluation of MDR1, LRP, MRP, and topoisomerase IIalpha gene mRNA transcripts before and after interferon-alpha, and correlation with the mRNA expression level of the telomerase subunits hTERT and TEP1 in five unselected human melanoma cell lines. Int J Oncol 2003;23:213-220.
10 van Oosterwijk JG, Herpers B, Meijer D, Briaire-de Bruijn IH, Cleton-Jansen AM, Gelderblom H, et al. Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann Oncol 2012;23:1617-1626.
11 Lukyanova NY, Rusetskya NV, Tregubova NA, Chekhun VF. Molecular profile and cell cycle in MCF-7 cells resistant to cisplatin and doxorubicin. Exp Oncol 2009;31:87-91.
12 Thomas MB, O'Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol 2008;15:1008-1014.
13 Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 2001;413:311-316.
14 Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001;413:316-322.
15 Lee JW, Soung YH, Kim HJ, Park WS, Nam SW, Kim SH, et al. Mutational analysis of the hCDC4 gene in gastric carcinomas. Eur J Cancer 2006;42:2369-2373.
16 Kemp Z, Rowan A, Chambers W, Wortham N, Halford S, Sieber O, et al. CDC4 mutations occur in a subset of colorectal cancers but are not predicted to cause loss of function and are not associated with chromosomal instability. Cancer Res 2005;65:11361-11366.
17 Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 2007;67:9006-9012.
18 Ding W, You H, Dang H, LeBlanc F, Galicia V, Lu SC, et al. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology 2010;52:945-953.
19 Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. J Clin Invest 2010;120:1031-1034.
Received July 19, 2013
Accepted after revision January 19, 2014
Author Affiliations: Department of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine (Yu J, Zhang W, Gao F, Liu YX, Chen ZY and Zheng SS); Key Laboratory of Multi-Organ Transplantation of Ministry of Public Health (Cheng LY, Xie SF and Zheng SS), Hangzhou 310003, China
Shu-Sen Zheng, MD, PhD, FACS, Department of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine; Key Laboratory of Multi-organ Transplantation of Ministry of Public Health, Hangzhou 310003, China (Tel/Fax: 86-571-87236601; Email: zyzss@zju.edu.cn)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60029-1
 Hepatobiliary & Pancreatic Diseases International2014年2期
Hepatobiliary & Pancreatic Diseases International2014年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Improved anterior hepatic transection for isolated hepatocellular carcinoma in the caudate
- Instrumental detection of cystic duct stones during laparoscopic cholecystectomy
- Familial chylomicronemia syndrome related chronic pancreatitis: a single-center study
- Pancreatic fistula after central pancreatectomy: case series and review of the literature
- Multi-visceral resection of locally advanced extra-pancreatic carcinoma
- Pancreatic head cancer in patients with chronic pancreatitis
