Detection of liver micrometastases from colorectal origin by perfusion CT in a rat model
Caroline Hatwell, Magaly Zappa, Mathilde Wagner, Nicolas Michoux, Valérie Paradis, Valérie Vilgrain, Léon Maggiori and Yves Panis
Clichy, France
Detection of liver micrometastases from colorectal origin by perfusion CT in a rat model
Caroline Hatwell, Magaly Zappa, Mathilde Wagner, Nicolas Michoux, Valérie Paradis, Valérie Vilgrain, Léon Maggiori and Yves Panis
Clichy, France
BACKGROUND:Some patients with colorectal carcinoma have liver metastases (LMs) which cannot be detected by conventional imaging. This study aimed to assess whether hepatic perfusion changes induced by micrometastases can be detected by perfusion computed tomography (CT).
METHODS:LMs were produced in rats by injecting carcinoma cells into the portal vein. Perfusion CT was performed at microscopic (day 10), interval (day 17), and macroscopic stage (day 34). Perfusion parameters were computed using a dualinput one-compartmental model.
RESULTS:Micro and macro LMs presented a mean diameter of 0.5 and 2.6 mm, respectively. Compared to controls, LMs at interval (1.1 mm) and macroscopic stage induced significant perfusion changes: a decrease of 42% (P=0.004) and 41% (P=0.029) in hepatic transit time and an increase of 292% (P=0.073) and 240% (P=0.001) in portal delay, respectively.
CONCLUSIONS:LMs with a mean diameter between 1.1 and 2.6 mm induced significant hepatic perfusion changes, detected by CT. Such detection may help to select patients and propose chemotherapy at the time of primary tumor resection.
(Hepatobiliary Pancreat Dis Int 2014;13:301-308)
liver circulation;
colorectal liver micrometastasis;
perfusion imaging;
computed tomography
Introduction
Colorectal cancer is one of the most frequently diagnosed cancers. Colorectal cancer liver metastases (LMs) develop in more than onethird of patients but are accessible to potentially curative resection in only 15% to 30% of patients.[1]Resection of the colorectal primary tumor with resection of LM is warranted, because this is the sole strategy with curative potential.[2]
In some patients with colorectal cancer, LMs develop very early after colorectal resection, suggesting the presence of occult micrometastases at the time of primary tumor resection. As it was suggested in an experimental study,[3]the growth of these "dormant" occult micrometastases is probably facilitated by the immunosuppression after the surgery for primary tumor. It is difficult to propose neoadjuvant chemotherapy in all patients with colorectal cancer because such preoperative regimens induce side effects and aim to increase the survival in only one-third of patients.[4]In this context, a diagnostic tool, which could identify patients at risk of occult LM development, would be beneficial in guiding therapeutic strategy.
The solution may lies in the accuracy of liver imaging regarding early detection of micro LM. Approximately 25% of patients have occult LMs called micro LMs which are impalpable at the time of initial surgery.[5]Modern imaging system can detect lesions of 8 mm in diameter but screening of such lesions and differentiation between benign or malignant lesionsremain unclear.[6-8]
Liver hemodynamic changes have been observed in patients with LMs with dynamic colloid scintigraphy,[8-10]Doppler ultrasound[11]and perfusion computed tomography (CT).[11,12]Although an association between macro LM and abnormal liver blood flow is well established, suggesting that the hepatic perfusion measurement could be a prognostic criterion,[13]the fact that perfusion changes can detect occult liver deposits accurately remains controversial.[12,14]To our knowledge, only one group has reported changes in hepatic perfusion caused by occult colorectal LM in a rat model.[15,16]
The aim of this experimental study was to assess whether hepatic perfusion changes induced by the growth of LM can be detected by perfusion CT in a rat model of liver colorectal metastasis.
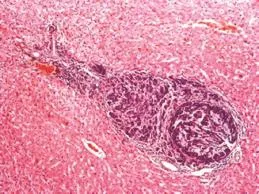
Fig. 1.Hematoxylin and eosin examination on a rat liver showing an occult metastasis or micrometastases induced at day 10 by injection of DHDK12 cells in the portal vein (original magnification ×20).
Methods
Cell line and culture
DHDK12 is a chemically induced rat colon carcinoma cell line from BDIX rats. The DHDK12 cells were previously grown to confluent monolayer in Dulbecco's Modified Eagle's Medium and Ham's F-10 medium (1:1, Gibco, Gaithersburg, MD, USA) containing 10% of fetal calf serum. All media were complemented with 4 mmol/L of L-glutamine, 100 IU/mL of penicillin and 100 μg/mL of streptomycin. They were cultured in 150 cm2Falcon flasks at 37 ℃ and 5% CO2.
Model of LM in rats
Male BDIX rats (Charles River, France) with an average weight of 190 g and with normal livers were used. The care given to the animals and all of the surgical procedures performed were complied with institutional guidelines. The study was approved by the local ethics committee for animal care and use (protocol #2011-14/773-0057). The rats were anesthetized with an intraperitoneal injection of pentobarbital (0.001 mL/ g). LMs were induced by injecting a suspension of 1 ×106DHDK12 cells directly into the portal vein using a 30-gauge needle through a mini-laparotomy incision.
In vivoexperimental protocol and pathological examination
Rats were randomly divided into the control group including animals with saline portal vein injection with no tumor cells and the studied group i.e. rats with portal DHDK12 cells injection. This latter group was divided into 3 groups: "micro LM" group (perfusion CT at day 10), "interval LM" or stage with micro and small macro lesions (perfusion CT at day 17), and "macro LM" group (perfusion CT at day 34). The dates of perfusion CT were chosen according to the results of preliminary studies, showing that occult LM or micro LM (with a size around 1 mm) appeared at day 10 (Fig. 1), and macro LM at day 20 (with a size around 3 mm).
After each CT session, all rats were sacrificed by an intraperitoneal injection of pentobarbital (0.01 mL/g). Immediately after death of the rats, livers were harvested, fixed in 10% formaldehyde, embedded, sectioned, stained with hematoxylin-eosin-safran, and analyzed with computer-assisted optical microscopy. Each LM was then identified and their maximal diameter was measured using the HistoLab software (Microvision Instruments, Evry, France).
Perfusion CT examination
After an overnight fast, the rats were anesthetized using the same protocol described above. A polyurethane catheter was placed directly in the vena cava, through a short midline laparotomy, to achieve a venous access. CT scan was performed on a clinical system (LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA), with the following parameters: 80 kV, 160 mA, and DFOV 15. A total of 0.4 mL iodinated contrast agent (Visipaque, 270 mg/mL, GE Healthcare, Ireland) was injected manually and 4 sequential CT slices (thickness=5 mm) centered on the liver hilum were acquired every 0.5 second during 30 seconds and then every 4 seconds during 270 seconds. The acquisition began 3 seconds before the injection.
Image processing
CT images were transferred to a workstation (Advantage Windows, GE Healthcare, Bux, France) for data analysis by a senior radiologist (Zappa M). Three regions of interest (ROIs) were drawn: one ROI (1.5 mm2) in the aorta, one ROI in the portal vein(1.5 mm2) and one ROI in the liver parenchyma (in the right median lobe) (20 mm2), but the part with the large vessels was avoided (Fig. 2). ROIs were drawn on one section chosen for anatomic reasons and were automatically transferred into the section over time in order to extract CT attenuation over time and to obtain attenuation curves (Fig. 3). A manual motion correction was performed during perfusion CT, as ROIs were recentered in case of significant motion.
A dual-input one-compartmental model[17]was used to fit the data of the attenuation-time curves. This model was described below. With this model, the following parameters were calculated: arterial and portal blood flow (mL/min/100 g); mean transit time (time for the contrast agent to cross the capillary system of the liver parenchyma, reported in second); portal delay (time between the injection of contrast agent and the tissue enhancement, reported in second); arterial delay; and distribution volume of contrast agent (percentage).
The solution of this kinetic model can be written:
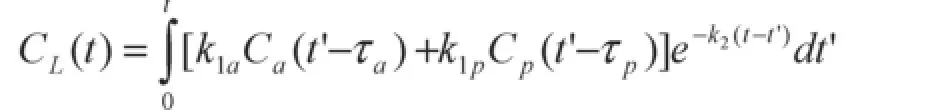
whereCaandCp(g/mLplasma) are the concentrations of the contrast media in the plasma of the hepatic artery and the portal vein;CL(g/mLliver) is the concentration of the contrast media in the liver;k1aandk1p(mLplasma/ s/mLliver) represent the aortic and portal venous plasma inflow, respectively; andk2(/s) represents the outflow rate;τaandτprepresent the transit times from the aorta and portal vein regions to the liver ROI. The arterial delayτawas fixed to the delay between the first nonzero value of theCa(t) curve and the first nonzero value of theCL(t) curve. Therefore, the model contains four free parameters to fit,k1a,k1p,k2andτp. To obtain the coefficientsk1aandk1pexpressed in (mL/min/100 mL), the coefficients expressed in (mLplasma/s/mLliver) were multiplied by 60 s/min and by 100. Two derived parameters, the fractional distribution volumevd(%)of the contrast media in the liver calculated as 100k1/k2-1and the hepatic mean transit time (s) calculated ask2-1, were also estimated. The optimization of the fit was achieved with a weighted nonlinear least squares fit based on the Levenberg-Marquardt method combined with a four-dimensional grid of starting parameter values to find the solution corresponding to the true global minimum of the error function.[18]Initial values fork1aandk1pranged from 0.6 to 1200 mL/min/100 g, fork2from 0.6 to 6000 mL/min/100 g, forτpfrom 0.5 toτmaxseconds [whereτmaxis measured as the time to peak of theCL(t) curve minus the time of the first nonzero value of theCp(t) curve]. All the parameters were constrained to be positive andvdwas constrained to be inferior to 65%.
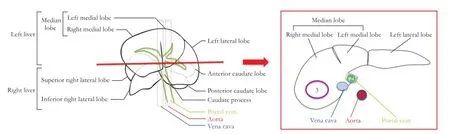
Fig. 2.Rat liver segmentation (front view) and liver schematic axial slice. Three ROIs were drawn: one in the aorta (dark blue ROI 1), one in the portal vein (light blue ROI 2) one in the liver parenchyma (in the right medial lobe) (purple ROI 3).
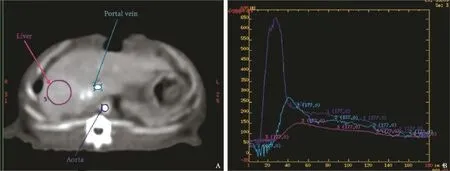
Fig. 3.Example of perfusion CT scan.A: axial slice centered on the liver hilum; three ROIs were drawn: one in the aorta (dark blue), one in the portal vein (light blue), one in the liver parenchyma (in the right medial lobe) (purple).B: the corresponding attenuation curves.
Statistical analysis
All calculations were done with Matlab (v.6.5, rel 13, Mathworks, Natick, MA, USA). Results were expressed as mean±standard deviation (SD). The perfusion parameters were compared between the four groups of rats (control, micro, interval and macro LM) with the Kruskal-Wallis test, followed by two by two comparisons with the Mann-WhitneyUtest. The diagnostic performances of arterial blood flow, mean transit time, and portal delay were assessed with non-parametric receiver operating characteristic (ROC) curves. Cutoff values of pure diffusion were chosen by maximizing the Youden index on the estimated curves. APvalue less than 0.05 was considered statistically significant.
Results
Thirty-six rats were included, nine in each group. Two rats in the control group, 4 in the micro LM group, 2 in the interval LM group died during the anesthesia induction or the catheter introduction. Thus, 28 rats were evaluated: 7 rats in the control group, 5 rats in the micro LM group, 7 rats in the interval LM group, and 9 rats in the macro LM group.
Pathological findings
The mean number of LM in each lobe was 4±1. Liver pathological assessment showed LM with a meandiameter of 0.5±0.2 mm at day 10 (micro LM), 1.1±0.3 mm at day 17 (interval LM) and 2.6±1.4 mm at day 34 (macro LM).
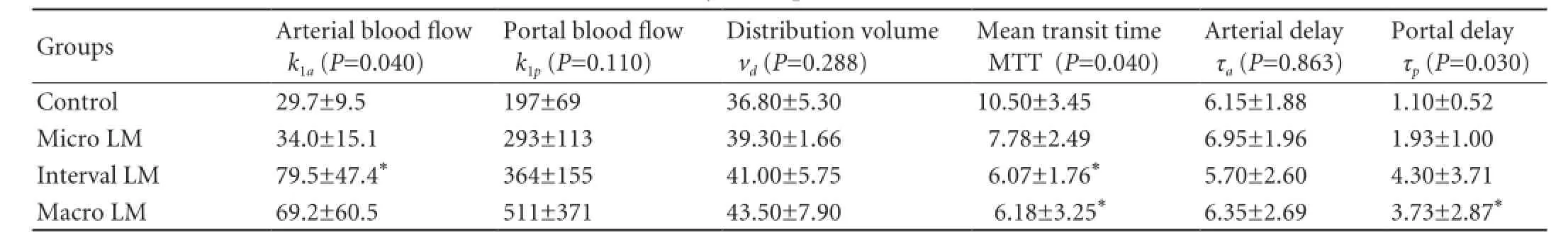
Table 1.Hemodynamic parameters (mean±SD)
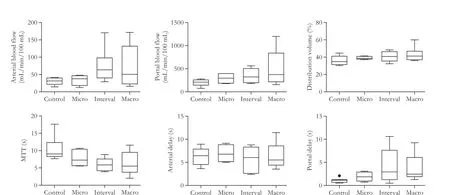
Fig. 4.Box and Whisker plots of perfusion parameters in the control, micro, interval, and macro LM groups.
Perfusion CT findings
Perfusion CT was available and analyzed for the 28 rats. Comparison of the 4 groups showed no obvious visual difference between the enhancement behaviors. For all groups, the quality of the fits of the attenuationtime curves was good. Hemodynamic parameters are summarized in Table 1 and Fig. 4.
There were significant differences in the mean transit time, portal delay, and arterial blood flow between the 4 groups (P=0.040,P=0.030 andP=0.040, respectively). But no difference was found in the portal blood flow, arterial delay and distribution volume between the 4 groups (P=0.110,P=0.863, andP=0.288, respectively).
Compared to the control group, none of the perfusion parameters was altered significantly in the micro LM group. However, a trend toward a decrease in the hepatic mean transit time (7.78±2.49 in the micro LM group vs 10.50±3.45 in the control group,P=0.315), an increase in the portal delay (1.93±1.00 in the micro LM group vs 1.10±0.52 in the control group,P=0.109) and an increase in the portal blood flow (293±113 in the micro LM group vs 197±69 in the control group,P=0.412) were observed.
Compared to the control group, interval LM induced significant hepatic perfusion changes and showed a lower mean transit time (P=0.004) and a higher arterial blood flow (=0.007). The portal delay (P=0.073) and portal blood flow (P=0.053) were higher in the interval LM group than in the control group.
The macro LM group showed a lower mean transit time (P=0.029), a higher portal delay (P=0.001) and a higher portal blood flow (P=0.029) than the control group. There was an increase of the arterial blood flow (P=0.121).
The areas under the ROC curves of the arterial blood flow, the mean transit time and the portal delay in differentiating between the control group and micro LM group, between the control group and interval LM group, and between the control group and macro LM group are presented in Table 2 and Fig. 5.
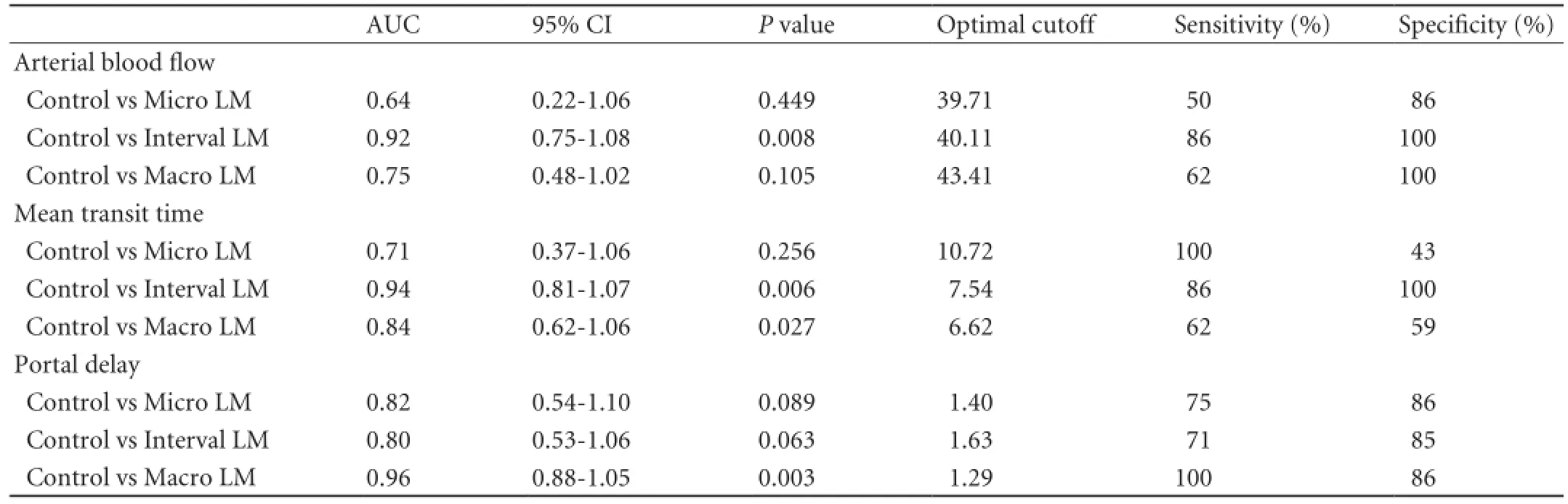
Table 2.ROC analysis for the arterial blood flow, the mean transit time and the portal delay

Fig. 5.ROC curves of arterial blood flow, mean transit time and portal delay were used to differentiate the control group from micro LM, interval LM and macro LM groups.
Discussion
In this experimental study, significant hepatic perfusion changes were induced by LM at stages of "macro LM" (mean diameter 2.6 mm) and "interval LM" (mean diameter 1.1 mm). The latter lesions were detected by perfusion CT which demonstrated, compared to control rats, a decrease in the hepatic mean transit time and an increase in the arterial blood flow.
In surgical management, resection of colorectal primary tumor with combined or staged resection of synchronous LM is warranted because this is the sole strategy with curative potential.[19]Therapeutic strategy is commonly influenced by two criteria: the symptoms pattern of the colorectal primary lesion and the potential resectability of LM.[20]So, it is crucial to have an accurate preoperative diagnosis of all liver lesions so as to choose the best treatment. Ambiru et al[21]have reported that micro LM appeared in 31% of the patients who underwent curative hepatectomy for LM and thus contributed substantially to treatment failure. Furthermore, micro LMs have been shown to be present around a macro LM in up to 50% of the cases, leading to an increased rate of incomplete resection or local destruction.[22]Other authors[23]showed that micro LM could be detected by immunohistochemistry in about two-third of the resected specimens and suggested that these lesions may be associated with intrahepatic recurrence. The accuracy of currently available techniques for asymptomatic LM remains widely and challenging. LMs are present but usually not detected in up to one-third of the patients who undergo apparently curative excision of primary colorectal cancer.
Actually, magnetic resonance imaging (MRI) is the most sensitive technique for LM detection, especially for the LM smaller than 10 mm, as suggested by a recent meta-analysis by Niekel et al.[24]Despite imaging progress and accuracy increase, with diffusion-weighted imaging and hepatospecific magnetic resonance contrast agent, imaging performance for LM smaller than 10 mm remains limited with a low detection rate, and new imaging technique is mandatory to decrease detection size threshold.[25-27]However, even in the small lesions, these techniques might give valuable indications, which may help to individually manage patient treatment.
It has been suggested that micro LM could induce liver changes in blood flow similar to those caused by macro LM.[13,15]Changes of blood supply are usually caused by liver diseases, and it seems logical to evaluate the hemodynamic changes to discover occult lesions. Two main reasons are given to explain liver flow alterations in patients with LM. The first one refers to the concept of angiogenesis due to tumor development with the growth of new vessels.[13]Consequences include intrahepatic resistance caused by obstruction and compression of the hepatic sinusoidal capillaries.[15]These intrahepatic shunts associated with neoangiogenic vascular networks could explain the increase of hepatic blood flow. The second refers to the secretion of vasoconstrictor agents by metastatic cells with constriction of the arterial vasculature, which leads to a reduction in the blood flow of the portal vein, not observed in the present study.[28]Such changes have been observed in the liver of patients with LM with dynamic colloid scintigraphy,[8-10]Doppler ultrasound[11]and perfusion CT.[12,13]The significant changes detected in the liver microcirculation suggest the role of perfusion CT as a diagnostic tool of occult lesions in the liver. To allow such early detection, a baseline examination should be performed in order to assess perfusion modification. However, this strategy leads to a significant increase of additional irradiation.
However, studies on perfusion changes showed variable results and perfusion modifications due to LM are not clear. Indeed, in animal studies, both increase and decrease of hepatic or portal blood flow and mean transit time were reported. A reduction of portal flow prior to growth of visible LM was shown by Kruskal et al.[28]Cuenod et al[15]agreed with those findings and found a decrease of the portal blood flow and an increase of the mean transit time in case of micro LM. However, Jiang et al[13]showed a decrease of the mean transit time, as in our study. They also reported an increase of the hepatic blood flow.
In clinical studies, CT measurements of hepatic perfusion in 27 patients showed a correlation between increased arterial perfusion and LM, whereas reduced portal perfusion indicated a progressive disease.[14]Using the hypothesis that patients with micro LM had a reduction of the portal blood flow, a recent study suggested the possibility that the liver with such occult lesions decreased in size before metastatic tumor growth.[29]
The differences in time (of the natural history of LM) in which the tumor microcirculation is observed may partly explain the discrepancy of the results in the literature. Based on in vivo microscopy, Liu et al[30]have proposed four stepwise patterns to describe the changes (during the tumor growth) in the morphologic characteristics of intratumoral microvessels and microcirculation of the tumor and the surrounding hepatic sinusoids. The mean diameter of metastases (0.8-3.0 mm) reported in our study is consistent with the patterns III to IV of large metastases supplied mainly by the hepatic artery, whereas the mean diameter(0.5±0.3 mm) of the metastases is consistent with the earlier pattern II of smaller metastases with an important portal blood flow.[15]Differences in the models used for assessing the perfusion parameters are also of importance. Some authors used a semi-quantitative model based on the geometrical characteristics of the attenuationtime curves.[14]Others preferred a distributed-parameter model using a single arterial input, thus neglecting the portal contribution.[13,31]Nevertheless, Cuenod et al[15]used a dual-input deconvolution model. Though close to this model, the dual-input kinetic model used in our study exhibits two major differences. First, we added two delaysτaandτpto account for the bolus arrival time from the aorta and portal vein regions to the liver ROI. Indeed, it has been shown that neglecting such delays leads to inaccurate estimates of the perfusion parameters.[32]Second, in using two distinct parametersk1aandk1p, the arterial or portal blood flood was estimated independently and not derived from each other via the hepatic perfusion index α. Further analysis should address the accuracy of these two representations in model fitting.
Our study had some limitations. First, the LM diameter in each rat is not homogeneous and the presence of LM with different size may bias the results. Second, the number of rats was low. Third, we didn't compare perfusion CT performance with another validated imaging technique as MRI, or diagnostic CT. Finally, despite the motion correction, the perfusion CT examinations might have been biased by the breathing motion of the animals, as this is anin vivostudy.
In conclusion, this study, assessing the performance of perfusion CT to detect liver colorectal metastasis in a rat model, is of interest because it might yield the opportunity to identify, prior to surgery, patients with metastatic disease undetectable with any other means of investigation.
Contributors:VV and PY proposed the study. HC, ZM, WM and ML performed research and wrote the first draft. HC, ZM, MN, PV and ML collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. PY is the guarantor.
Funding:None.
Ethical approval:The study was approved by the local ethics committee for animal care and use (protocol #2011-14/773-0057).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-259.
2 Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-1016.
3 Panis Y, Ribeiro J, Chrétien Y, Nordlinger B. Dormant liver metastases: an experimental study. Br J Surg 1992;79:221-223.
4 Andre T, de Gramont A; Study Group of Clinical Research in Radiotherapies Oncology, Oncology Multidiciplinary Research Group. An overview of adjuvant systemic chemotherapy for colon cancer. Clin Colorectal Cancer 2004;4:S22-28.
5 Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg 1986;73:732-735.
6 Koh DM, Berry J. Critical questions in the imaging of colorectal hepatic metastases. Cancer Imaging 2008;8:S69-78.
7 Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology 2005;237:123-131.
8 Rappeport ED, Loft A. Liver metastases from colorectal cancer: imaging with superparamagnetic iron oxide (SPIO)-enhanced MR imaging, computed tomography and positron emission tomography. Abdom Imaging 2007;32:624-634.
9 Leveson SH, Wiggins PA, Giles GR, Parkin A, Robinson PJ. Deranged liver blood flow patterns in the detection of liver metastases. Br J Surg 1985;72:128-130.
10 Huguier M, Maheswari S, Toussaint P, Houry S, Mauban S, Mensch B. Hepatic flow scintigraphy in evaluation of hepatic metastases in patients with gastrointestinal malignancy. Arch Surg 1993;128:1057-1059.
11 Leen E, Angerson WJ, Wotherspoon H, Moule B, Cook TG, McArdle CS. Detection of colorectal liver metastases: comparison of laparotomy, CT, US, and Doppler perfusion index and evaluation of postoperative follow-up results. Radiology 1995;195:113-116.
12 Warren HW, Gallagher H, Hemingway DM, Angerson WJ, Bessent RG, Wotherspoon H, et al. Prospective assessment of the hepatic perfusion index in patients with colorectal cancer. Br J Surg 1998;85:1708-1712.
13 Jiang HJ, Zhang ZR, Shen BZ, Wan Y, Guo H, Shu SJ. Functional CT for assessment of early vascular physiology in liver tumors. Hepatobiliary Pancreat Dis Int 2008;7:497-502.
14 Leggett DA, Kelley BB, Bunce IH, Miles KA. Colorectal cancer: diagnostic potential of CT measurements of hepatic perfusion and implications for contrast enhancement protocols. Radiology 1997;205:716-720.
15 Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, et al. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology 2001;218:556-561.
16 Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, Tran PL, et al. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 2004;14:2125-2133.
17 Van Beers BE, Materne R, Annet L, Hermoye L, Sempoux C, Peeters F, et al. Capillarization of the sinusoids in liverif brosis: noninvasive assessment with contrast-enhanced MRI in the rabbit. Magn Reson Med 2003;49:692-699.
18 Ahearn TS, Staff RT, Redpath TW, Semple SI. The use of the Levenberg-Marquardt curve-fitting algorithm in pharmacokinetic modelling of DCE-MRI data. Phys Med Biol 2005;50:N85-92.
19 Adam R. Colorectal cancer with synchronous liver metastases. Br J Surg 2007;94:129-131.
20 Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009; 27:1829-1835.
21 Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum 1999;42:632-639.
22 Seidensticker M, Wust P, Rühl R, Mohnike K, Pech M, Wieners G, et al. Safety margin in irradiation of colorectal liver metastases: assessment of the control dose of micrometastases. Radiat Oncol 2010;5:24.
23 Yokoyama N, Shirai Y, Ajioka Y, Nagakura S, Suda T, Hatakeyama K. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer 2002;94:1642-1647.
24 Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/ or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-684.
25 Donati OF, Fischer MA, Chuck N, Hunziker R, Weishaupt D, Reiner CS. Accuracy and confidence of Gd-EOB-DTPA enhanced MRI and diffusion-weighted imaging alone and in combination for the diagnosis of liver metastases. Eur J Radiol 2013;82:822-828.
26 Macera A, Lario C, Petracchini M, Gallo T, Regge D, Floriani I, et al. Staging of colorectal liver metastases after preoperative chemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy. Eur Radiol 2013;23:739-747.
27 Soyer P, Boudiaf M, Placé V, Sirol M, Pautrat K, Vignaud A, et al. Preoperative detection of hepatic metastases: comparison of diffusion-weighted, T2-weighted fast spin echo and gadolinium-enhanced MR imaging using surgical and histopathologic findings as standard of reference. Eur J Radiol 2011;80:245-252.
28 Kruskal JB, Thomas P, Kane RA, Goldberg SN. Hepatic perfusion changes in mice livers with developing colorectal cancer metastases. Radiology 2004;231:482-490.
29 Ishizawa T, Yamamoto T, Sekikawa T. The diagnostic values of measuring the liver volume in detecting occult hepatic metastases from colorectal cancer. Hepatogastroenterology 2007;54:514-517.
30 Liu Y, Matsui O. Changes of intratumoral microvessels and blood perfusion during establishment of hepatic metastases in mice. Radiology 2007;243:386-395.
31 Sahani DV1, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology 2007;243:736-743.
32 Kershaw LE, Buckley DL. Precision in measurements of perfusion and microvascular permeability with T1-weighted dynamic contrast-enhanced MRI. Magn Reson Med 2006;56: 986-992.
Received August 20, 2013
Accepted after revision December 9, 2013
AuthorAffiliations:Department of Colorectal Surgery (Hatwell C, Maggiori L and Panis Y), Department of Radiology (Zappa M, Wagner M, Michoux N and Vilgrain V), and Department of Pathology (Paradis V), Beaujon Hospital (AP-HP), 100 boulevard du Général Leclerc, 92118 Clichy, France; Research Unit Bichat-Beaujon, INSERM U773, Université Paris VII (Denis Diderot), Paris, France (Hatwell C, Wagner M, Michoux N, Paradis V, Vilgrain V, Maggiori L and Panis Y)
Professor Yves Panis, MD, PhD, Service de Chirurgie Colorectale, P?le des Maladies de l'Appareil Digestif, H?pital Beaujon – Assistance Publique des H?pitaux de Paris (AP-HP), Université Paris VII (Denis Diderot), 100 boulevard du Général Leclerc, 92118 Clichy, France (Tel: 33-01-40874547; Fax: 33-01-40874431; Email: yves.panis@bjn. aphp.fr)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60043-6
Published online March 27, 2014.
 Hepatobiliary & Pancreatic Diseases International2014年3期
Hepatobiliary & Pancreatic Diseases International2014年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Letters to the Editor
- PossibIe benefit of spIenectomy in Iiver transpIantation for autoimmune hepatitis
- Validation of the moderate severity category of acute pancreatitis defined by determinant-based cIassification
- Emergency cholecystectomy vs percutaneous cholecystostomy plus delayed cholecystectomy for patients with acute cholecystitis
- Sodium butyrate protects against toxin-induced acute liver failure in rats
- HepG2 cells recovered from apoptosis show altered drug responses and invasiveness
