Schistosoma japonicum infection induces macrophage polarization
Jingwei Xu,Ho Zhng,Lin Chen,b,Donghui Zhng,b,Minjun Ji,b,Hiwei Wu,b,c,Gunling Wu,b,?
aDepartment of Pathogen Biology and Immunology,Nanjing Medical University,Nanjing,Jiangsu 210029,China;
bJiangsu Province Key Laboratory of Modern Pathogen Biology,Nanjing,Jiangsu 210029,China;
cCenter for International Health Research,Rhode Island Hospital,Providence,Rhode Island,USA.
Schistosoma japonicum infection induces macrophage polarization
Jingwei Xua,Hao Zhanga,Lin Chena,b,Donghui Zhanga,b,Minjun Jia,b,Haiwei Wua,b,c,Guanling Wua,b,?
aDepartment of Pathogen Biology and Immunology,Nanjing Medical University,Nanjing,Jiangsu 210029,China;
bJiangsu Province Key Laboratory of Modern Pathogen Biology,Nanjing,Jiangsu 210029,China;
cCenter for International Health Research,Rhode Island Hospital,Providence,Rhode Island,USA.
The role of macrophages(Mφ)as the first line of host defense is well accepted.These cells play a central role in orchestrating crucial functions during schistosomal infection.Thus,understanding the functional diversity of these cells in the process of infection as well as the mechanisms underlying these events is crucial for developing disease control strategies.In this study,we adopted a Mφ polarization recognition system.M1 macrophage was character–ized by expressing CD16/32,IL–12 and iNOS.M2 macrophage was characterized by expressing CD206,IL–10 and arg–1.In vivo(mouse peritoneal macrophages of different infection stages were obtained)and in vitro(different S. japonicum antigens were used to stimulate RAW264.7)were characterized by using the above mentioned system. NCA and ACA stimulated RAW264.7 express significantly higher levels of IL–12 while significantly higher levels of IL–10 were detected after soluble egg antigen(SEA)stimulation.The results showed that dramatic changes of antigen in the microenvironment before and after egg production led to macrophage polarization.Furthermore, through TLR blocking experiments,the TLR4 signaling pathway was found to play a role in the process of macro–phage polarization toward M1.Our data suggest that macrophage polarization during S.japonicum infection had significant effects on host immune responses to S.japonicum.
macrophage,polarization,schistosome japonicum,TLRs
INTRODUCTION
Schistosomiasis is a zoonotic parasitic diseases and poses a serious hazard to human health[1].Schistosoma japonicum invades mammalian hosts and triggers com–plex immune responses.Researchers are gradually uncovering the complex interplay between this helminth and its appropriate hosts including humans.It has been reported that specific cellular immunity plays a vital role in the immune response of schistosomiasis.Th1 type immune response plays a dominant role at the early stage of S.mansoni and S.japonicum infection[8],while Th2 type immune response predominates after egg production.A large number of studies have summarized the characteristics of host immune response after S. japonicum infection:in the initial 2 to 4 weeks after infection,under the stimulation of migrating schistoso–mula,the host shows Th1 type(IFN–γ,TNF)response; once egg production begins,Th1 type response drops rapidly,and Th2 type(IL–4,IL–13,IL–10,IL–5) response starts[2–8].Therefore,host cytokine environ–ment changes before and after egg production.But so far,the mechanism underlying this immune phenom–enon has not been fully elucidated.Thus,it is very important to continue to explore and understand the immune responses induced by S.japonicum so asto manipulate host immune responses toward the direction that will facilitate human immunity against schistosomiasis.
Macrophages(Mφ)occupy a unique position in the immune system.They function in both non–specific defense(innate immunity)as well as help initiate spe–cific defense mechanisms(adaptive immunity)of ver–tebrate animals.It has been found that macrophage are highly heterogeneous cells that can differentiate into distinct subtypes in response to local microenviron–mental signals[9].There are two major subtypes of macrophage:M1 and M2.M1 is classically activated and phagocytoses and destroys pathogens and malig–nant cells.It also activates adaptive immune responses by secretion of proinflammatory cytokines and che–mokines and acting as professional antigen–presenting cell.M2 is alternatively activated macrophages induced by GM–CSF,IL–4,–13,–10,glucocorticoid, TGF–beta,vitamin D3,PGE2 and etc.M2 has low antigen presentation capacity and can down–regulate adaptive immune responses by secretion of IL–10, CCL17,CCL18 and TGF–β[10–17].Obviously,M1 and M2 can lead to either positive or negative regulatory signals to the immune responses,respectively.It is important to determine whether macrophage polariza–tion may be linked to the change of microenvironments before and after egg production during schistosome infection and then regulates the development of adap–tive immune responses,including Th polarization shift. The aim of this study was to find the molecular evi–dence of Mφ polarization associated with the change of antigen microenvironments before and after egg production during schistosome infection.We carried out in vitro(macrophage cell line RAW264.7)and in vivo(experimental infection of mice with S.japonicum)experiments.Our study may enrich our knowl–edge of host immune responses during S.japonicum infection,and add experimental evidence of immune suppression induced by egg antigens.We also discuss possible strategies of S.japonicum escape from host immune responses,which may provide insight into developing anti–schistosomiasis vaccines and more effective anti–schistosome drugs.Our study points to the possibility of enhancing anti–schistosome immune responses by skewing in vivo macrophage polarization as part of vaccination strategy.
MATERIALS AND METHODS
Experimental mice and parasites
Six to eight–week–old C57BL/6J(B6)mice(female, 120)were purchased from the Animal Research Center of Nanjing University(Nanjing,China).All mice were maintained and bred under specific pathogen–free con–ditions.All experiments were undertaken with the approval of Nanjing Medical University Animal Ethics Committee.Schistosoma japonicum(S.japonicum,a Chinese mainland strain)cercariae were main–tained in Oncomelania hupensis snails as the intermediate host,which were purchased from Jiangsu Institute of Parasitic Disease(Wuxi,China).
Infection withS.japonicumand isolation of peritoneal macrophages
C57BL/6J(B6)mice were percutaneously infec–ted with 20±2 S.japonicum cercariae through their shaved abdomen.All mice were sacrificed at dif–ferent S.japonicum infection stages(at 0,3,6 and 9 weeks after infection)to obtain murine peritoneal macrophages according to the literature[18].
Culture and stimulation of murine macrophage cell line(RAW264.7)
The RAW264.7 murine macrophage cell line(TIB–71;American Type Culture Collection,Manassas, VA)wasmaintainedat37°Cin5%CO2in10–cmdishes with Dulbecco′s modified Eagle′s medium(DMEM) (Invitrogen,Carlsbad,CA,USA)supplemented with antibiotics and 10%fetal bovine serum(FBS).Cells in passages 5 to 8 were used in the experiments.
For stimulation,at 37°C in 5%CO2for 48 hours, classic M1 inducer IFN–γ(2 ng/μL)(PeproTech, USA),lipopolysaccharide(LPS)(5 ng/mL)(Sigma, St.Louis,MA,USA)and M2 inducer IL–4(10 ng/ μL)(PeproTech,USA)were used.The normal cercaria antigen(NCA)(40 ng/μL),attenuated cercaria antigen (ACA)(40 ng/μL),soluble egg antigen(SEA)(40 ng/ μL)and soluble worm anti–gen(SWAP)(40 ng/μL) were prepared as before[19].The concentrations of anti–gens were assayed using Bicinchoninic Acid Protein Assay Kit(Pierce,Rockford,IL,USA).
Measurement of M1/M2 cytokine levels in the macrophage culture supernatants
Stimulated RAW264.7 cells or isolated peritoneal macrophages from different S.japonicum infection stages cultivated at 37°C in 5%CO2 for 48 hours. Next,the supernatants were collected for the M1/M2 cytokine assay.IL–12,IL–10,TNF–α and TGF–β1 levels were examined by enzyme–linked immuno sor–bent assay(ELISA)kits(eBioscience,USA).
Flow cytometry method
Percentage of M1 and M2 was determined by two–colorstainingwithFITC–andPECy5–labeledantibodies.For each staining,one antibody for lineage markers (CD16/32 or CD206)were used with one antibody for macrophage markers(F4/80).Totally,4×105cells were incubated with staining antibodies for 15 minutes at room temperature in the dark.Cells were washed three times with PBS before the expression of surface molecules was examined by flow cytometry on a FACSCaliburTM(BD Biosciences,Heidelberg, Germany).A total of 10,000 events were analyzed.Data analysis was performed by FCS Express(De Novo Software,Los Angeles,CA,USA).
Quantitative real-time PCR(qRT-PCR)
Total RNA was extracted from RAW264.7 stimu–lated by different stimuli and mouse peritoneal macro–phages obtained from mice with different S.japonicum infection stages.Total RNA was transcribed to cDNA by using a commercially available reverse transcription kit(Epicentre Technologies,Madison,WI,USA).The cDNA was employed as a template in real–time PCR using the ABI 7900 real–time PCR system(ABI, USA).Primers specific for gapdh,iNOS(inos)and Arg–1(arg1)are shown inTable 1.Reactions were performed using 2 μL of cDNA in a 10 μL reaction volume and the following thermal cycle profile: 10 minutes of denaturation at 95°C,40 cycles of dena–turation at 95°C for 15 seconds and then 60 seconds of extension at 60°C.PCR amplification of gapdh was performed to allow normalization between samples.
Antibody blocking assay
Antibody blocking experiment was performed to understand the effect of TLR2 and TLR4 in S.japonicum antigen induced macrophage polarization.RAW 264.7 cells were incubated in 96–well flat–bottom plates(2.0×105 cells/well).For experiments blocking TLR4,adherent RAW 264.7 cells were treated with 50 μg/mL of anti–mouse TLR4 antibody or isotype control antibody IgG2a for 30 minutes at 37°C. Following incubation with antibodies,NCA,SEA, and SWAP(40 μg/mL),were respectively added,and cells were incubated for 48 hours at 37°C,5%CO2. Then,these cells were collected and assayed for line–age markers(CD16/32 or CD206).The culture super–natants were collected and assayed for IL–12 and IL–10 production.Cells cultured with medium alone were used as controls.
Statistical analysis
The data were presented as mean±SEM.ANOVA followed by the Newman–Keuls test was used for the analysis of differences among multiple groups. Student′s t–test was used for comparisons between the two groups.All statistic analyses were performed with SPSS 16.0 software(SPSS Inc.,Chicago,IL, USA).Significant difference was established when P was<0.05.
RESULTS
Reading criteria foracrophage polarization
We used flow cytometry to detect CD16/32 and CD206 expression.The results showed that upon IFN–γ/LPS stimulation,CD16/32 expression increased significantly(P<0.01)in RAW264.7 with no signif–icant changes in CD206 expression.Following IL–4 stimulation,CD206 expression increased significantly in RAW264.7 compared to unstimulated cells(P<0.01)while CD16/32 expression did not change (Fig.1A1Band ).The ELISA assay results indicated that RAW264.7 secreted significantly higher levels of IL–12 following IFN–γ/LPS stimulation compared to cells without stimulation,but almost with no detectable level of IL–10;however,IL–4 stimulated RAW264.7 barely secreted IL–12 but significantly higher levels of IL–10(P<0.01)(Fig.1C).Compared to unsti–mulated RAW264.7,IFN–γ/LPS stimulated cells expressed significantly higher levels of iNOS gene encoded fragments,but almost no expression of Arg–1 gene coding fragments(P<0.01);However,IL–4 stimulated RAW264.7 expressed higher levels of Arg-1 gene encoded fragments with significance(P<0.01),while there was almost no expression of iNOS gene encoded fragments(Fig.1D).
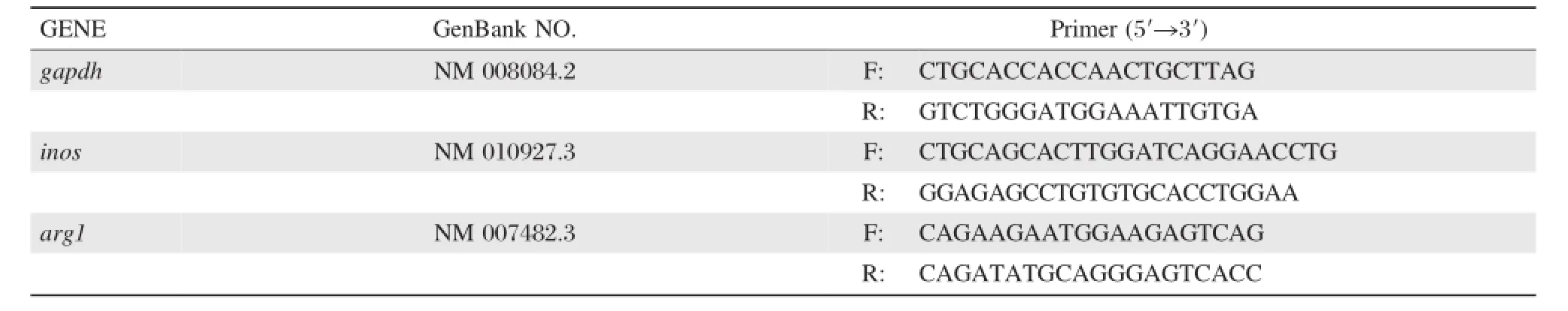
Table 1qRT-PCR primers
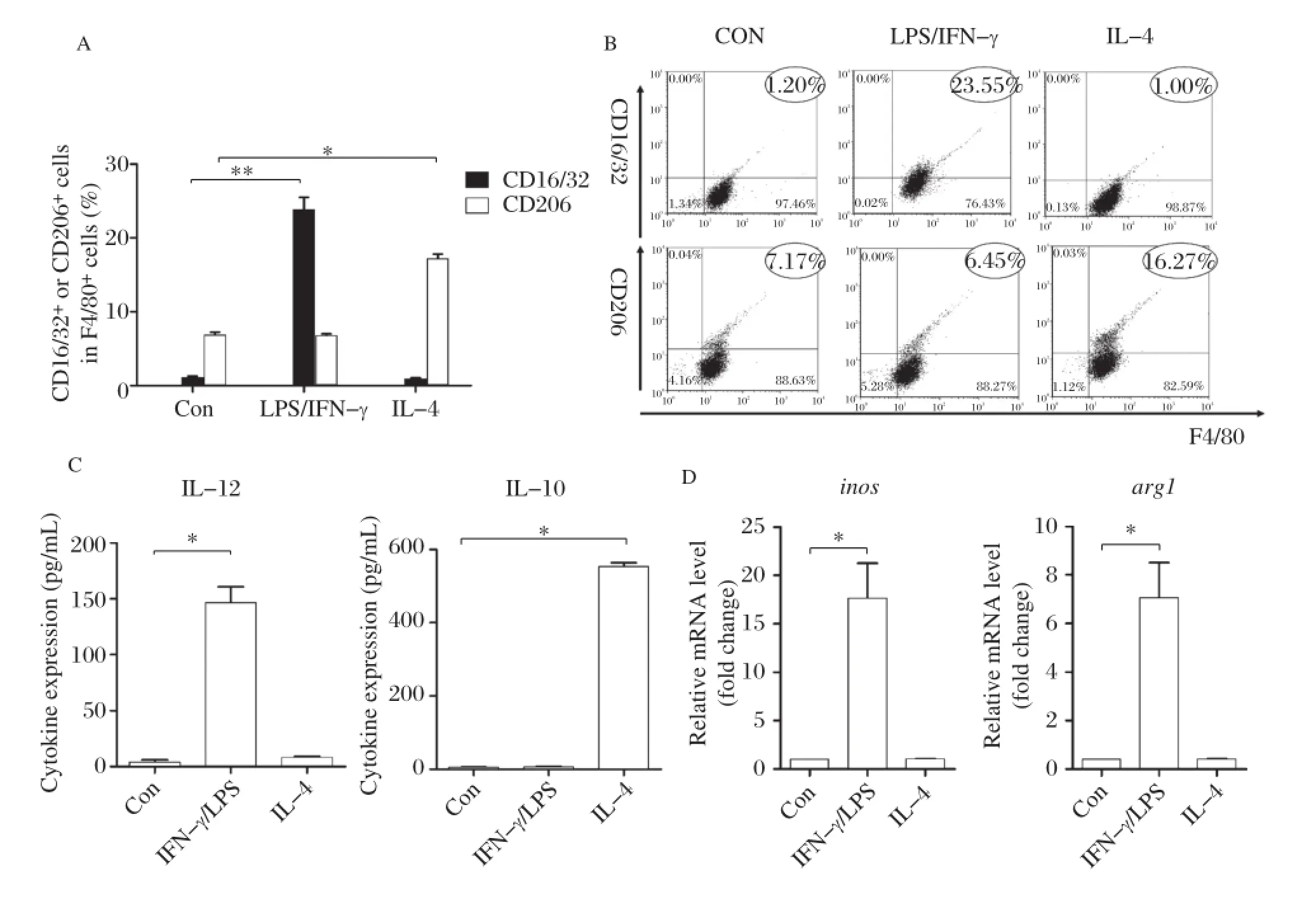
Fig.1Mφpolarization recognition system adopted in this study.A and B:Percentage of CD16/32+or CD206+cells among F4/80+cells as determined by FACS after IFN–γ/LPS and IL–4 stimulation(compared with unstimulated cells as the control group,*P<0.01).C:IL–12 and IL–10 cytokine levels in the supernatant of RAW264.7 cultures by ELISA after IFN–γ/LPS and IL–4 stimulation(compared with the control group,*P<0.01). D:Relative transcription level of inos and arg1 level in RAW264.7 by real–time PCR after IFN–γ/LPS and IL–4 stimulation(compared with the control group,*P<0.01).The results are shown as mean of three independent experiments.
Different antigens ofS.japonicuminduced Mφ polarization
CD16/32 expression increased significantly in RAW264.7 with NCA and ACA stimulation while CD206 expression showed an insignificant difference. CD206 expression increased significantly in RAW264.7 SEA stimulation compared to unstimulated cells(P<0.01)while CD16/32 expression showed an insignificant difference.SWAP stimulated RAW264.7 expressed both high levels of CD16/32 and CD206(Fig.2A2Band ).RAW264.7 secreted significantly higher levels of IL–12 as shown by ELISA with NCA and ACA stimulation compared to cells without stimulation,but almost with no IL–10. However,SEA stimulated RAW264.7 secreted signif–icantly high levels of IL–10 with low levels of IL–12. SWAP stimulated RAW264.7 secreted both high levels of IL–12 and IL–10(P<0.01)(Fig.2C).Compared to unstimulated RAW264.7,NCA and ACA stimulated cells expressed significantly high levels of iNOS gene encoded fragments,but almost no expression of Arg-1 gene encoded fragments(P<0.01).However, SEA stimulated RAW264.7 expressed high levels of Arg-1 gene encoded fragments with significance(P<0.01),while almost no expression of iNOS gene encoded fragments.SWAP stimulated RAW264.7 expressed both high levels of iNOS and Arg-1 gene encoded fragments(Fig.2D).
Mφpolarizationinducedbyschistosomalinfection
The highest expression of M1 surface marker CD16/ 32 appeared at 3 weeks after infection,and gradually declined from 6 weeks after infection(P<0.01). M2 surface marker CD206 expression increased at 3 weeks after infection,reaching to the highest level at 9 weeks after infection(Fig.3A3Band ).We also detected IL–12 and IL–10 secretion,and iNOS and Arg–1 mRNA expression to identify polarization prop–erties.The results showed that mouse macrophages secreted high levels of IL–12 and before the productionof worm egg,and then gradually decreased to a lower level after egg production,while IL–10 levels signif–icantly increased at the time of egg deposition, which is 6 weeks after infection.iNOS mRNA expression reached the highest level at 3 weeks after infection,Arg-1 mRNA expression significantly increased after egg production(Fig.3C3Dand ). These results showed that M1 polarization domi–nated before egg production while M2 dominated after egg production.
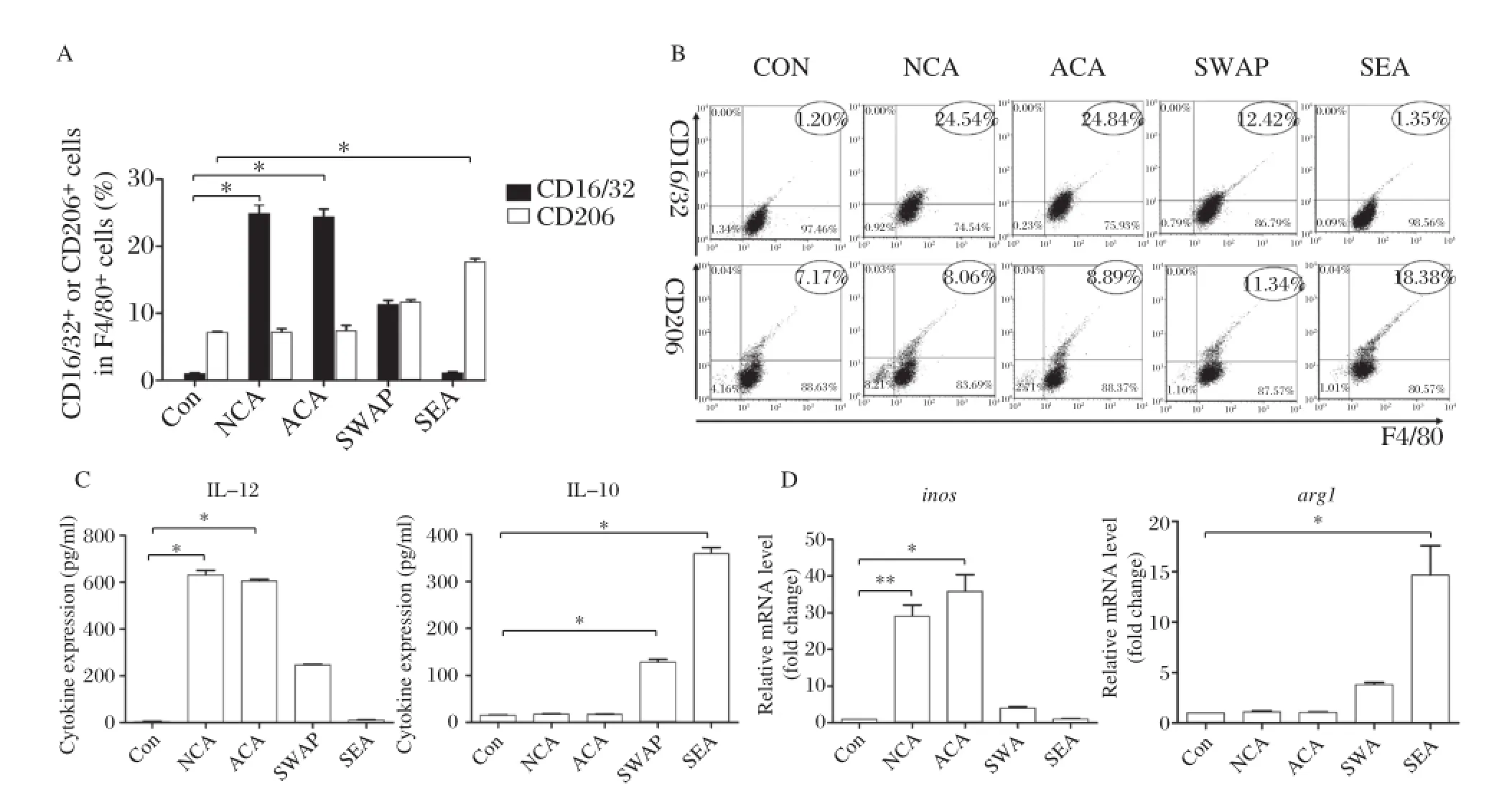
Fig.2Different antigens ofS.japonicuminduced Mφpolarization.A and B:Percentage of CD16/32+or CD206+cells among F4/80+cells determined by FACS after NCA,ACA,SWAP and SEA(antigens from different stages of S.japonicum)stimulation compared with unstimulated cells as(*P<0.01).C:IL–12 and IL–10 cytokine levels in the supernatant of RAW264.7 cultures by ELISA after NCA,ACA,SWAP and SEA stimulation compared with unstimulated cells(*P<0.01).D:Relative transcription level of inos and arg1 level in RAW264.7 by RT–PCR after NCA,ACA, SWAP and SEA stimulation compared with unstimulated cells(*P<0.01).The results are shown as mean of three independent experiments.
To investigate the Th1/Th2 cytokine levels of polar–ized macrophages,we used schistosome antigens of different developmental stages as stimuli.The ELISA assay results showed that compared to week 0,TNF–α secretion of peritoneal macrophages reached the highest level at 3 weeks(P<0.01),and gradually declined from 6 weeks after infection;TGF–β expres–sion increased at 3 weeks after infection,reaching the highest level at 9 weeks after infection(Fig.4Aand4B).As the cytokine levels of peritoneal macro– phage without stimulation were very low,we also used R264.7 cell line to verify the results of in vivo experi–ment.The results showed that TNF–α expression was significantly increased with NCA stimulation com–pared to SEA stimulated(P<0.01);TGF–β expres–sion was significantly increased following SEA stimulation compared to the NCA stimulated group, which was consistent with the in vivo results(Fig. 4C4Dand ).
The TLR4 signaling pathway affected macrophage polarization
Flow cytometry showed that when TLR2 pathway was blocked,there was no significant influence in CD16/32 and CD206 expression in RAW264.7 with NCA,SWAP and SEA stimulation compared to the control group(P<0.01).When the TLR4 pathway was blocked,NCA stimulated Mφexpressed lower levels of CD16/32 compared to the control group(P<0.01),but with no significant difference in CD206 expression after SWAP and SEA stimulated(Fig. 5A5Band ).When the TLR2 pathway was blocked, there was no significant difference in the levels of IL–12 and IL–10 secretion by RAW264.7 with NCA, SWAP and SEA stimulation stimulated compared to the control group(P<0.01).When the TLR4 pathway was blocked,NCA stimulated Mφsecreted lower levels of IL–12 compared to the control group(P<0.01),but with no significant difference in IL–10 expression after SWAP and SEA stimulation(Fig.5C).When the TLR2 pathway was blocked,there was no significant influence on inos and arg1 expression in RAW264.7 with NCA,SWAP and SEA stimulation compared tothe control group(P<0.01)When the TLR4 path–way was blocked,NCA stimulated Mφ expressed lower levels of inos compared to the control group (P<0.01),but with no significant difference in arg1 expression after SWAP and SEA stimulation (Fig.5D).
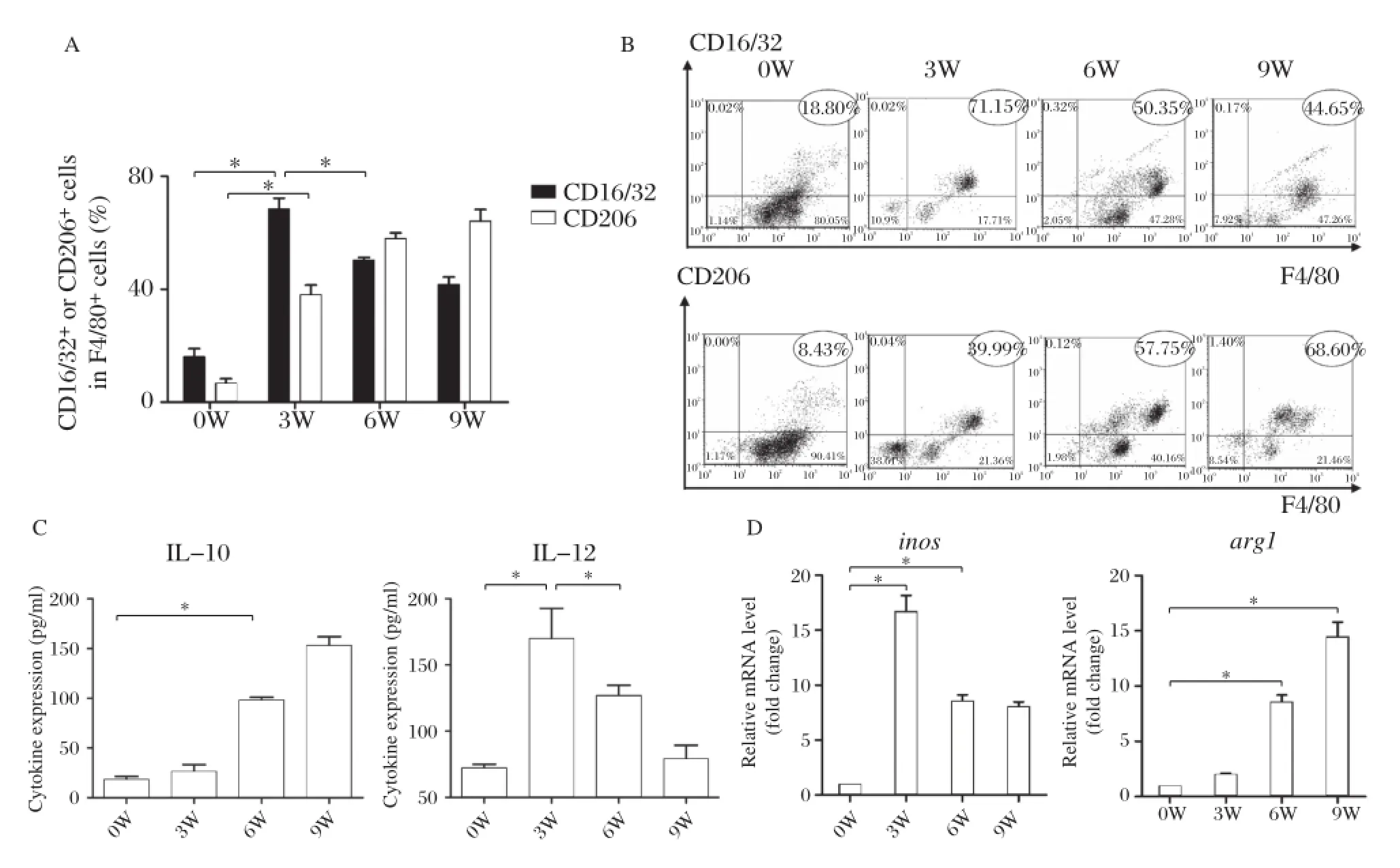
Fig.3Mφpolarization during the process of schistosomal infection.A and B:Percentage of CD16/32+or CD206+cells among F4/80+cells according to FACS of peritoneal macrophages isolated at 0,3,6 and 9 weeks after infection(one–way analysis of variance(ANOVA),*P<0.01).C: Dynamics of IL–12 and IL–10 cytokine levels according to ELISA of sera harvested at 0,3,6 and 9 weeks after infection(one–way ANOVA,*P<0.01).D:Dynamics of relative transcription level of inos and arg1 level according to RT–PCR of RNA isolated at 0,3,6 and 9 weeks after infection (one–way ANOVA,*P<0.01).Data are representative of two independent experiments.
DISCUSSION
S.japonicum experiences several developmental stages in the host.S.japonicum antigens derived from different stages may induce macrophage polarization and regulate immune responses.In this study,in vitro and in vivo experiments demonstrated the relationship between the stimulation of S.japonicum antigens and macrophage polarization,and provided molecular evi–dence of macrophage differentiation.
Firstly,we built a macrophage polarization recogni–tion system,through which to observe the influence of S.japonicum antigens on macrophage polarization. Although there were functional differences between M1 and M2,their phenotypes were similar.According to the previous study[20],we chose the arginine metabolic pathway(inos,arg–1),surface markers(CD16/32, CD206)and cytokine secretion(IL–12,IL–10)to com–pose the macrophage polarization recognition system. As classic M1 inducer IFN–γ/LPS and M2 inducer IL–4 can stimulate M1/M2 polarization,we stimulated RAW264.7(M0)with IFN–γ/LPS and IL–4.The result showed that the system was successfully reproduced under our experimental conditions.
Next,we found that S.japonicum antigens derived from different developmental stages could induced Mφ polarization.M1 marker(CD16/32,IL–12 and inos)expression was significantly increased in RAW264.7 following NCA and ACA stimulation(P<0.01)while M2 marker(CD206,IL–10,arg1) expression was significantly increased in RAW264.7 following SEA stimulation compared with the control group(P<0.01).These results showed that in vitro ACA and NCA(before laying eggs)induced M1 polarization,while SEA(after laying eggs)induced M2 polarization.In addition,SWAP could induce both M1 and M2 due to its complicated ingredients(con–taminated with SEA).
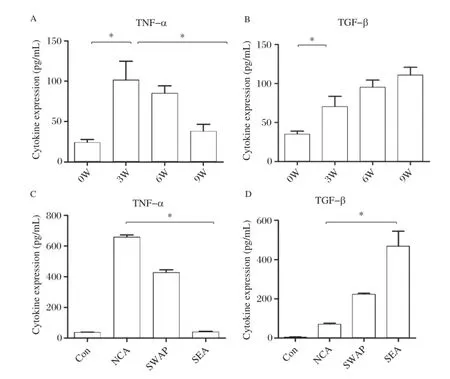
Fig.4TNF-a and TGF-b levels detected in different time points and antigents stimulation.A and B:TNF–α and TGF–β cytokine levels according to ELISA of sera harvested at 0,3,6 and 9 weeks after–infection(one–way ANOVA,*P<0.01).C and D:TNF–α and TGF–β cytokine levels in the supernatant of RAW264.7 cultures by ELISA after NCA,ACA,SWAP and SEA(antigens from different stages of S.japonicum)stimulation (one–way ANOVA,*P<0.01).The results are shown as mean of three independent experiments.
Further experiments were performed in vivo to detect Mφ polarization shift during S.japonicum natural infection.By using the macrophage polariza–tion recognition system,we detected M1 markers (CD16/32,IL–12 and inos)and M2 markers(CD206, IL–10,arg1)of mouse peritoneal macrophages from different stages of S.japonicum infection.The results showed that M1 polarization dominated before egg production while M2 dominated after egg production. Interestingly,during 3 to 9 weeks after infection,we observed that peritoneal cells were divided into two groups according to F4/80 fluorescence intensity.A small proportion of peritoneal cells did not stain positive for F4/80,and were likely not macrophages.Therefore, we compared macrophage polarization markers within those F4/80–positive cells.We found that a higher per–centage of macrophages were positive for CD16/32 at the third week,but gradually decreased to less than 50%at the ninth week.On the other hand,CD206–posi–tive macrophages increased through the course of infec–tion,reaching 76%of all macrophages at the ninth week.This observation in peritoneal macrophages is consistent with macrophage polarization shift observed in vitro.
At the same time,we noticed that macrophages pre–dominantly secreted Th1 cytokines(TNF alpha,IL–12) before egg production,but mainly Th2 cytokines(IL–10,TGF–beta)after egg production,which hypothe–sized that macrophages polarization regulated immune responses after S.japonicum infection.The results of in vivo and in vitro studies above showed that S.japonicum antigens derived from different developmental stages could induce macrophage polarization and reg–ulate immune microenvironment through secreting cytokines,thus contributing to host immune responses after S.japonicum,such as Th cell shift in cellular immunology.
TLR2 and TLR4 are mainly expressed on cells that are related to the first line defense of the host[21–26],including dendritic cells,macrophages,mononuclear cells and neu–trophils.Many studies have demonstrated that TLR2 and TLR4 play an important role in infections by viruses, bacteria,fungi and protozoa,and the functions of TLR2 or TLR4 vary according to the nature of pathogens[27–30].
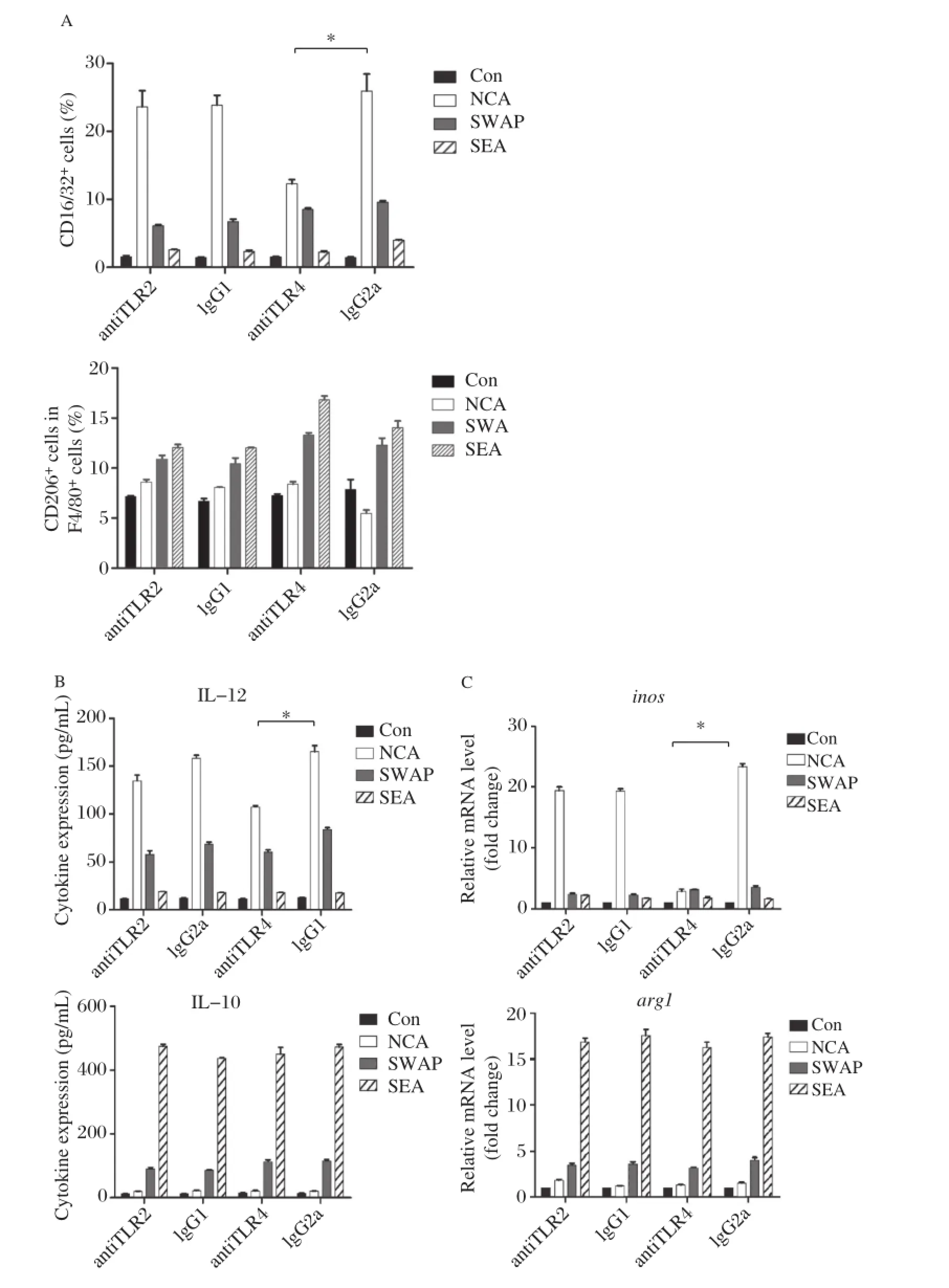
Fig.5The effect of TLR2 and TLR4 on Mφpolarization induced by differentS.japonicumantigens.A:Percentage of CD16/32+or CD206+cells among F4/80+cells as determined by FACS after NCA,SWAP and SEA stimulation when the TLR2 or TLR4 pathway was blocked (compared with the isotype control,*P<0.01).B:IL–12 and IL–10 cytokine levels in the supernatant of RAW264.7 cultures by ELISA after NCA, SWAP and SEA stimulation when the TLR2 or TLR4 pathway was blocked(compared with the isotype control,*P<0.01).C:Relative transcription level of inos and arg1 level in RAW264.7 by RT–PCR after NCA,SWAP and SEA stimulation when the TLR2 or TLR4 pathway was blocked (compared with the isotype control,*P<0.01).The results are shown as mean of 3 independent experiments.
Our previous study found that TLR2 and TLR4 deficiencies could lead to opposite results during S.japonicum infection[34].The enhanced cytokine milieu in TLR2 deficien background was unfavorable for the parasite.On the contrary,TLR4 might be involved in the protection against the infection.
To understand the effect of TLR2 and TLR4 macro–phage polarization induced by different S.japonicum antigens,we performed antibody blocking experiment. When TLR2 was blocked,there were no significant changes in both M1 marker(CD16/32,IL–12 and inos) and M2 marker(CD206,IL–10 and arg1)expression after antigen stimulation.However,when TLR4 was blocked, NCA stimulated Mφexpressed significantly lower levels of M1 marker(CD16/32,IL–12 and inos)compared with the isotype control group(P<0.01),indicating that the TLR4 pathway may be related to M1 polarization,which is in accordance with our previous study[31]that the TLR4 signalingpathwayplayedaprotectiveroleinS.japonicum infection.Cheng et al.[32]also found that macrophages were mainly regulated by S.japonicum eggs through the TLR4 signal pathway according to the analysis of micro–array data.Recent studies showed that M2 macrophage polarization were related processes orchestrated by p50 nuclear factor kβ through the inhibition of NF–kB–driven, M1–polarizingandIFN–βproduction[33].Wespeculatethat the TLR4 signaling pathway may affect Mφpolarization through this downstream molecules–p50 NF–kB,which needs further exploration.
With the development of genetics and molecular biology study,we more in–depth and comprehensive understanding of M1 and M2.In recent years, researchers made progress in the study of characteristic gene expression of M2.The trypanosoma model set up by Namangala et al.[34]has been proved to have guiding significance in the study of alternative activation of macrophage.In the early stage of trypanosome infec–tion,macrophage polarized into M1,while alterna–tively activated M2 is given priority in the late stage and chronic infection.It explained that macrophages exert both positive and negative effects on immune responses,and this may be related to its polarization. According to immune response shift during S.japonicum infection,we believe that macrophages and schis–tosomiasis antigen interaction have a key influence on the start–up and regulation of host immune responses.
In summary,changes of antigens in the microenviron–ment are the key point to the polarization of macrophages during schistosomal infection.S.japonicum antigens can stimulate macrophages to be polarized into different sub–types;thus,different subtypes of macrophages participate in the regulation of immune response caused by S.japonicum infection.Our study provided new interpretation and experimental evidence to uncover the regularity of complex immune responses after schistosome infection.
REFERENCES
[1] Jia TW,Zhou XN,Wang XH,Utzinger J,Steinmann P, Wu XH.Assessment of the age–specific disability weight of chronic schistosomiasis japonica.Bull World Health Organ 2007;85:458–65.
[2] Ross AG,Bartley PB,Sleigh AC,Olds GR,Li Y, Williams GM,et al.Schistosomiasis.N Engl J Med 2002;346:1212–20.
[3] Gryseels B,Polman K,Clerinx J,Kestens L.Human schistosomiasis.Lancet 2006.368:1106–18.
[4] Doherty JF,Moody AH,Wright SG.Katayama fever:an acute manifestation of schistosomiasis.BMJ 1996;313: 1071–2.
[5] Ross AG,Vickers D,Olds GR,Shah SM,McManus DP.Katayama syndrome.Lancet Infect Dis 2007;7: 218–24.
[6] Pearce EJ,MacDonald AS.The immunobiology of schis–tosomiasis.Nat Rev Immunol 2002;2:499–511.
[7] Ji MJ,Su C,Wu HW,Zhu X,Cai XP,Li CL,et al.Gene expression profile of CD4+T cells reveals an interferon signaling suppression associated with progression of experimental Schistosoma japonicum infection.Cell Immunol 2003;224:55–62.
[8] Ji MJ,Su C,Wang Y,Wu HW,Cai XP,Li GF,et al. Characterization of CD4+T cell responses in mice infected with Schistosoma japonicum.Acta Biochim Biophys Sin (Shanghai)2006;38:327–34.
[9] Gordon S.Macrophage heterogeneity and tissue lipids.J Clin Invest 2007;117:89–93.
[10]Mosser DM.The many faces of macrophage activation.J Leukoc Biol 2003;73:209–12.
[11]Gordon S.Alternative activation of macrophages.Nat Rev Immunol 2003;3:23–35.
[12]Smith P,Walsh CM,Mangan NE,Fallon RE,Sayers JR, McKenzie AN,et al.Schistosoma mansoni worms induce anergy of T cells via selective up–regulation of pro–grammed death ligand 1 on macrophages.J Immunol 2004;173:1240–8.
[13]Mantovani A,Sica A,Locati M.Macrophage polarization comes of age.Immunity 2005;23:344–6.
[14]Lumeng CN,Bodzin JL,Saltiel AR.Obesity induces a phenotypic switch in adipose tissue macrophage polariza–tion.J Clin Invest 2007;117:175–84.
[15]Biswas SK,Gangi L,Paul S,Schioppa T,Saccani A, Sironi M,et al.A distinct and unique transcriptional program expressed by tumor–associated macrophages (defective NF–kappaB and enhanced IRF–3/STAT1 acti–vation).Blood 2006;107:2112–22.
[16]Mantovani A,Sica A,Locati M.New vistas on macro–phage differentiation and activation.Eur J Immunol 2007;37:14–6.
[17]Odegaard JI,Ricardo–Gonzalez RR,Goforth MH,Morel CR,Subramanian V,Mukundan L,et al.Macrophage–specific PPARgamma controls alternative activation and improves insulin resistance.Nature 2007;447:1116–20.
[18]Zhang X,Goncalves R,Mosser DM.The isolation and characterization of murine macrophages.Curr Protoc Immunol.2008;Chapter 14:Unit 14.1.
[19]Zhang M,Gao Y,Du X,Zhang D,Ji M,Wu G.Toll–like receptor(TLR)2 and TLR4 deficiencies exert differential in vivo effects against Schistosoma japonicum.ParasiteImmunol.2011 Apr;33(4):199–209.doi:10.1111/j.1365–3024.2010.01265.x
[20]LiK,GuoQ,WangC,ChenM,XuW,XiongS.Comparative analysis of phenotypes of classically(M1)and alternatively (M2)activated macrophages CURRENT IMMUNOLOLGY 2008;28:177–183.
[21]Murawski MR,Bowen GN,Cerny AM,Anderson LJ, Haynes LM,Tripp RA,et al.Respiratory syncytial virus activates innate immunity through Toll–like receptor 2.J Virol 2009;83:1492–500.
[22]Feng CG,Scanga CA,Collazo–Custodio CM,Cheever AW,Hieny S,Caspar P,et al.Mice lacking myeloid dif–ferentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll–like receptor 2 (TLR2)–and TLR4–deficient animals.J Immunol 2003;171:4758–64.
[23]Pearl JI,Ma T,Irani AR,Huang Z,Robinson WH,Smith RL,et al.Role of the Toll–like receptor pathway in the recognition of orthopedic implant wear–debris particles. Biomaterials 32:5535–42.
[24]Gelani V,Fernandes AP,Gasparoto TH,Garlet TP, Cestari TM,Lima HR,et al.The role of toll–like receptor 2 in the recognition of Aggregatibacter actinomycetem–comitans.J Periodontol 2009;80:2010–9.
[25]Fuse ET,Tateda K,Kikuchi Y,Matsumoto T,Gondaira F,Azuma A,et al.Role of Toll–like receptor 2 in recog–nition of Legionella pneumophila in a murine pneumonia model.J Med Microbiol 2007;56(Pt 3):305–12.
[26]Mun HS,Aosai F,Norose K,Chen M,Piao LX,Takeuchi O,et al.TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection.Int Immunol 2003;15(9):1081–7.
[27]Campos MA,Almeida IC,Takeuchi O,Akira S,Valente EP,Procopio DO,et al.Activation of Toll–like receptor–2 by glycosylphosphatidylinositol anchors from a protozoan parasite.J Immunol 2001;167:416–23.
[28]Oliveira AC,Peixoto JR,de Arruda LB,Campos MA, Gazzinelli RT,Golenbock DT,et al.Expression of func–tional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T.cruzi.J Immunol 2004;173: 5688–96.
[29]Netea MG,Sutmuller R,Hermann C,Van der Graaf CA, Van der Meer JW,van Krieken JH,et al.Toll–like recep–tor 2 suppresses immunity against Candida albicans through induction of IL–10 and regulatory T cells.J Immunol 2004;172:3712–8.
[30]Kurt–Jones EA,Chan M,Zhou S,Wang J,Reed G, Bronson R,et al.Herpes simplex virus 1 interaction with Toll–like receptor 2 contributes to lethal encephalitis.Proc Natl Acad Sci U S A 2004;101:1315–20.
[31]Zhang M,Gao Y,Du X,Zhang D,Ji M,Wu G.Toll–like receptor(TLR)2 and TLR4 deficiencies exert differential in vivo effects against Schistosoma japonicum.Parasite Immunol 33:199–209.
[32]Cheng PC,Lin CN,Peng SY,Li LL,Luo TY,Fan CK, et al.A study of immunomodulatory genes responses to macrophages of Schistosoma japonicum infection during different stages by microarray analysis.Acta Trop 2013;127:251–260.
[33]Porta C,Rimoldi M,Raes G,Brys L,Ghezzi P,Di Liberto D,et al.Tolerance and M2(alternative)macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB.Proc Natl Acad Sci U S A 2009; 106:14978–83.
[34]Namangala B,Brys L,Magez S,De Baetselier P,Beschin A.Trypanosoma brucei brucei infection impairs MHC class antigen presentation capacity of macrophages. Parasite II Immunol 2000;22:361–70.
Received 06 May 2013,Revised 26 July 2013,Accepted 19 November 2013,Epub 29 January 2014
?Corresponding author:Prof.Guanling Wu,Department of Pathogen Biology and Immunology,Nanjing Medical University Hanzhong Road 140,Nanjing,Jiangsu 210029,China.Tel/Fax:+86–25–862793/+86–25–86863187/,E–mail:glwu@njmu.edu.cn.
The authors reported no confilct of interests.
?2014 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.27.20130072
 THE JOURNAL OF BIOMEDICAL RESEARCH2014年4期
THE JOURNAL OF BIOMEDICAL RESEARCH2014年4期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Evaluation of quantitative variation of secondary metabolites in Bergenia ciliata (Haw.)using high performance thin layer chromatography
- Comparing a non-invasive hemodynamic monitor with minimally invasive monitoring during major open abdominal surgery
- Morphological MRI and T2 mapping of cartilage repair tissue after mosaicplasty with tissue-engineered cartilage in a pig model
- Luteolin prevents uric acid-induced pancreatic β-cell dysfunction
- DDX3X regulates cell survival and cell cycle during mouse early embryonic development
- IRE1αknockdown rescues tunicamycin-induced developmental defects and apoptosis in Xenopus laevis
