Reclassification of Oligodon ningshaanensis Yuan, 1983 (Ophidia: colubridae) into a New Genus, Stichophanes gen. nov. with Description on Its Malacophagous Behavior
*
1chengdu Institute of Biology, chinese Academy of Sciences, chengdu 610041, Sichuan, china
2Department of Biological and Environmental Sciences, Alabama A and M University, Normal 35801, Alabama, USA
3Institute of Zoology, chinese Academy of Sciences, Beijing 100101, china
Reclassification of Oligodon ningshaanensis Yuan, 1983 (Ophidia: colubridae) into a New Genus, Stichophanes gen. nov. with Description on Its Malacophagous Behavior
Xiaohe WANG1, Kevin MESSENGER2, Ermi ZHAO1*and chaodong ZHU3*
1chengdu Institute of Biology, chinese Academy of Sciences, chengdu 610041, Sichuan, china
2Department of Biological and Environmental Sciences, Alabama A and M University, Normal 35801, Alabama, USA
3Institute of Zoology, chinese Academy of Sciences, Beijing 100101, china
The complete mitochondrial cytb gene and the partial nuclear c-mos gene of Oligodon ningshaanensis Yuan, 1983 were sequenced and used for reconstructing the phylogenetic relationship of this taxon amongst alethinophidian snakes. Its strong affinity to the New World subfamily Dipsadinae and the Old World species Thermophis baileyi was inferred. Hemipenial morphology found by authors conflicts with the original description and its similarity with those of the dipsadid snakes is in accordance with our molecular results. Feeding tests show that O. ningshaanensis is a malacophagous predator, which is another matchless character for this species. This behavior is described and compared with other known slug- and snail-feeding snakes. The discovery of the particular position of our subject indicates that erecting a new genus is necessary accommodate this unique species.
Stichophanes gen. nov. ningshaanensis, Dipsadinae, molecular phylogeny, hemipenial morphology, mollusks-predator
1. Introduction
There has been much recent progress on higher-level snake phylogenies based on different molecular datasets (Slowinski and Lawson, 2002; Lawson et al., 2005; Vidal et al., 2007, Vidal and Hedges, 2009; Wiens et al., 2012; Pyron et al., 2011, 2013; Pyron and Burbrink, 2012). Molecular work has rapidly produced new phylogenies of colubridae, the largest and most speciose group among snakes. Approximately half of the known snake species have already been sequenced and incorporated in phylogenetic reconstructions. While most subgroupshave already been included, it is possible to employ the known molecular inferences to elucidate the phylogenetic position for some of the lesser-known species when the subject presents many unique characters.
Hemipenial morphology has functioned to obtain traditional morphological character, with its importance in the systematics of snakes already acknowledged by the end of 19th century (cope, 1894, 1895). It fits the widely accepted biological species concept and does not correlate with ecological factors or behavior. Dowling (1967) considered the hemipenial features to be more reliable than other morphological characteristics. Before modern methods, trying to diagnose monophyletic subgroups within colubridae was based primarily on the morphology of the hemipenes. Along with fast developing molecular research, Zaher et al. (2009) subsequently reworked the classification of caenophidian snakes as a higher-level taxonomic category. Hemipenial features continue to be used in describing, defining and diagnosing new species and genera (Zaher, 1999; Myers, 2003; Sawaya and Sazima,
2003; Passos et al., 2005; Myers and McDowell, 2014).
Oligodon ningshaanensis Yuan, 1983 was described from one adult and two subadult specimens collected in Huoditang, Ningshan county, Shaanxi Province, People’s Republic of china. It was assigned to the genus Oligodon based on some diagnostic characters, and was distinguished from “the closely related O. catenata” by: 1) presence of a pair of internasals; 2)prefrontals not in contact with second supralabial; 3) less ventrals; and 4) more subcaudals (Yuan, 1983). The genus Oligodon Fitzinger 1826, the Kukri snakes, also known as the Smallheaded snakes in chinese, currently contains 74 smallto-medium sized species, most of which are nocturnal. Oligodon’s feeding preference for eggs is recorded in detail (Minton, 1963; Hu and Zhao, 1987; coleman et al., 1993; Zhao et al., 1998). Their diet relies on the strongly recurved hind teeth, which resemble kukri knives. Given their wide distribution throughout Southeast Asia, most studies only involve a few species of the genus. Even though there are an increasing number of investigations that target the phylogeny among snake species, affinities of the neglected O. ningshaanensis (Yuan, 1983) remain mysterious amongst all previous work looking at congeners (Green et al., 2010; Lawson et al., 2005; Pryon et al., 2011, 2013).
Little work has been done on this species since its initial description, with the exception of examining the microstructure of the skin, which revealed a canaliculatedtype microstructure (Li and Liang, 2007), a trait that is shared with many different subfamilies of colubridae (Prince, 1982). Since 2006, a series of field surveys in Shennongjia, Hubei province, discovered large number of O. ningshaanensis. This material represented the first documentation of large population of the species since its discovery, as well as a new record for this province (Yang et al., 2009). During these surveys, we found a lot of characters conflicting with the designation of this species within Oligodon (e.g., slim body shape, lack of an upturned snout, lack of the “kukri” teeth, etc.). Its prey made of terrestrial snails and slugs is different from most colubrid snakes, and especially Oligodon. compared to the number of species that prey on vertebrates, malacophagous behavior is seldom seen among snakes (Laporta-Ferreira and Salom?o, 2004). All of these observations brought an ineluctable investigation on the basic information of this neglected and previously “rare”species. Here we use the mitochondrial and nuclear DNA to reconstruct its phylogenetic relationship. We are also using morphological data to supplement its distinction from Oligodon.
2. Material and Methods
2.1 The material used in this studyNAFU071227: adult male, collected from type locality, Huoditang, Ningshan, Shaanxi Province without known date, fixed and preserved in Northwest Agriculture and Forestry University, Yangling, Shaanxi Province.
cIB100191: adult female, collected from type locality in 2008, fixed and preserved in chengdu Institute of Biology, chinese Academy of Sciences, chengdu, Sichuan Province.
cIB100192–100200: newborn specimens in 2009, from two clutches laid by females from Shennongjia, Hubei Province, deposited in chengdu Institute of Biology, chinese Academy of Sciences.
2.2 DNA extraction, amplification, and sequencingWe extracted DNA from alcohol fixed muscle tissue from cIB100191 and cIB100192 using the QIAGEN DNeasy Blood & Tissue Kit according to the manufacturer’s instructions. The entire mitochondrial cytochrome b gene (cytb) was amplified by using the primers L14910 (de Queiroz et al., 2002) and H16064 (Burbrink et al., 2000). The partial oocyte maturation factor gene (c-mos) was amplified and sequenced by using the primers S77 and S78 (Lawson et al., 2005). PcR was performed with the 50 μL system (2 μL template, 1 μL of each primers, 25 μL dNTPs mix and 21 μL ddH2O) and the following reaction parameters: predenaturation at 94°c for 4 min, followed by 40 cycles of 30 s denaturation (94°c), 30 s annealing (55°c–60°c), and a 1 min extension (72°c) with a final extension at 72°c for 7 min. Then the PcR products were sent to a sequencing company for purification and sequencing (ABI PRISM 3730xl Genetic Analyzer). The sequences generated in this study have been deposited in GenBank (Accession Numbers: KJ638715–KJ638718).
2.3 Phylogenetic analysesFor our analyses, we mainly follow the dataset used by Lawson et al. (2005). For most colubroid families, subfamilies, and some important taxa, the cytb and c-mos sequences of Sibynophis subpunctatus (Genebank Accession Numbers: Kc347471, Kc347411) and Thermophis baileyi (EU496923, EU496922) were added. The translated protein sequences were aligned by using MUScLE (Edgar, 2004) in MEGA6 (Tamura et al., 2013) with default settings. Phylogenetic reconstruction was conducted on the combined cytb and c-mos genes by using Maximum Likelihood (ML) method on RAxMLBlackbox (http://embnet.vital-it.ch/ raxml-bb/) (Stamatakis et al., 2008) and RAxML7.2.5 (Stamatakis, 2006). cylindrophis ruffus was employed as
the outgroup. The alignment was partitioned by the codon position of different genes and the GTRcAT strategy was used by all six partitions on the web server for obtaining a best-scoring tree. This initial tree was passed to the RAxML 7.2.5 for an additional search with 1000 standard bootstrap replicates. The bipartition information was overlaid on the best-scoring tree based on multiple bootstrap trees.
2.4 Hemipenis preparationThe hemipenis from the topotypic specimen NAFU071227 was prepared. We followed the method presented by Pesantes (1994), but modified by Zaher (1999) and Jiang (2010) to prepare the hemipenis from preserved specimens. Removal of the right hemipenis was carefully done, and the organ was placed into 3% KOH solution for six hours in a 25°c water bath. The capsule and muscles were gradually taken away during this period as the tissue gets softer. When the hemipenis became as flexible as a fresh organ, it was incised to enlarge opening at the base of asulcate side. Then roundtip-forceps were used to evert the whole structure. Instead of gelatin or agar, the organ was filled with absorbent cotton. The terminology follows Zaher (1999).
2.5 clearing and StainingThe adult female specimen (cIB100191) was used in this section. After dehydrating the snake without skin, gastrointestinal tract or gonads in 100% alcohol for two days, the specimen was placed into a stain solution made from 40 mL acetic acid, 60 mL 100% ethanol and 20 mg alcian blue for 40 hours. Then a series of alcohol solution (25%, 50%, 75% and 100%) were used to wash the stain gradually away for one day each, respectively, until the muscle became white. Then the specimen was carefully moved into a mixture of 100 mL of 0.3 mol/L KOH solution and 5 mg alizarin red for 20 hours for coloring the bones. Later, the colored specimen was placed into a gradient glycerol solution (25%, 50%, 75% and 100%) for one day each, respectively, until the bones were transparent. The preserved specimen was then placed in a 0.1% phenol solution in glycerol (chen and Wang, 2002).
2.6 Feeding Behavior ObservationcIB100191–cIB 100200 were involved in this simple behavioral test. The specimens were kept in separate boxes (30 × 18 × 10 cm3for adults and 10 × 10 × 10 cm3round for newborn specimens) with water dishes and shelters. Different vertebrate and invertebrate preys were offered, including mice (various sizes), fish, frogs (small species and tadpoles), lizards (skinks and geckos), insects (crickets, moth and moth larva), mollusks (terrestrial and autopotamic), earthworms, and planaria.
3. Results
3.1 Phylogenetic position ofO. ningshaanensis As we chose one of the henophidian species to be outgroup, the tree estimated from the aligned cytb and c-mos genes presents a higher-level caenophidian phylogeny (Figure 1). Though there was limited taxon sampling, the members of the well-known main snake groups were clustered together, respectively. However, the topological structure among the main clades of our bestscoring ML tree is not well supported by the overlaid bootstrap values (BS < 70% on some nodes). The tree is approximately congruent with many previous molecular studies on different datasets (Lawson et al., 2005; Pyron and Burbrink, 2012; Pyron et al., 2011, 2013; Vidal et al., 2007; Vidal and Hedges, 2009; Wiens et al., 2012; Zaher et al., 2009) except the position of small subfamilies of the colubridae (calamariinae, Pseudoxenodontinae and Sibynophiinae).
Among colubridae, which contains the species Oligodon cinereus and O. ningshaanensis, three clades (colubrinae, Dipsadinae and Natricinae), each with a large number of species separate, are clearly separated from each other. The clade ((Dipsadinae + Pseudoxenodontinae) + Sibynophiinae) and Natricinae form one main clade of colubridae. calamariinae clusters with (colubrinae + Grayiinae) to form another. Oligodon cinereus nested within colubrinae. However, two specimens from different localities of O. ningshaanensis are placed within the subfamily Dipsadinae as the sister group of all the New World dipsadid species. Thermophis baileyi stays as a basal branch of Dipsadinae; several other molecular works confirm this position (He et al., 2009; Pyron et al., 2011, 2013). The support value on the node between Dipsadinae and Pseudoxenodontinae is low (BS 56%), but on the basis of the bootstrap value (BS 100%) of the 50% majority-rule consensus tree, this node is firmly supported.

Figure 1 The best-scoring maximum-likelihood tree derived from cytb and c-mos genes. The support values (≥0.50 on the overlaid best tree / ≥0.70 on the 50% majority-rule consensus tree) are left above nodes. The Oligodon cinereus is labeled with a diamond and two O. ningshaanensis specimens were labled with triangles. The subfamilies of colubridae and the families of caenophidia are labeled beside trees by vertical bars.
3.2 Hemipenial morphologyWhen retracted, the hemipenes of O. ningshaanensis reach the 12thsubcaudal and are forked at level of the the 9th. The lobes twist slightly to the central line of tail. After complete eversion, the total length of the right hemipenis reaches 25 mm. The hemipenis is bilobed, semicapitate and semicalyculate. The lobes are not equally long on both hemipenes. The sulcus spermaticus is bifurcated at around the proximal one-third of the hemipenial body and centrolineal along both lobes. Outside sulcus spermaticus both lobes are almost covered with identical reticular-arranged lobular calyces, the areas of which are gradually enlarged towards the proximal region and disappeared at half of the hemipenial body. Laterally, the outer reticular calyces increase in height for merging into the body calyces on the asulcate side and large spines at the merging region. The intrasulcar calyces are almost latitudinally shaped with a small longitudinal area near the fork. The asulcate side is nude medially and has irregularly arranged body calyces on the lobes. The first pair of lateral large papillae appear with a noncapitulate basal region of both lobes. Nearly symmetrical vertically directed conspicuous large spines cover the main body of asulcate hemipenis and spread to the proximal sulcate surface to form two slender thorny triangles pointing to the apex (Figure 2).
3.3 Feeding behavior ofO. ningshaanensis Though many prey types were offered, only terrestrial slugs and snails were accepted. When earthworms and planaria
were offered, the snake showed some interest with tongue flicking and actively staring at the prey item. However, none were attacked, and after several seconds the snake stopped showing interest and moved away. No ontogenetic change was found on their preference or hunting behavior. The mollusk species accepted were as follows, slugs: Deroceras laeve and Phiolomycus bilineatus; snails: Bradybaena fruticola, B. fruticum, B. ravida, B. similaris, Euphaedusa sp., and Laeocathaica prionotropis
For hunting a slug (Figure 3), when the prey is noticed, the snake moves closer and targets the slug’s head with the neck in an arch and constant tongue flicking. Once it grasps the slug, the snake lifts the slug off the ground and starts to ingest immediately. After ingestion, the snake rubs the mucosa from the sides of its mouth on nearby substrate.

Figure 2 The right hemipenis of Oligodon ningshaanensis. A and c: Lateral views; B: Asulcate view; D: Sulcate view with scale.

Figure 3 Sequence of slug-preying behavior by a hatchling Oligodon ningshaanensis specimen. A: Gazing at the slug with tongue flickering; B: Targeting at the anterior end of the slug; c: Biting the head of slug.
The process of preying on a snail is initially very similar with that of a slug – the snake targets the animal, bites into the anterior portion and lifts it off the ground. Typically the snake will then try to find a crack, or anchor point, in which to attempt to pull the snail out of its shell.
If it cannot find anything in the surrounding habitat, the snake may hold the snail in the air for quite some time (more than 20 min). There was no mandible insertion observed, as seen in other snail specialists (Figure 4). The entire snail or most of the soft body was ripped out and the shell left behind.
It is worth noting that the different species of snails in this test were diversified in shape. Euphaedusa sp., with a quite slender helical shell, got attacked immediately by the adult specimen cIB100191, but the snail was too slender for the snake to find any snag for pulling it out of its shell. Eventually, the snake gave up and the snail was released alive. Laeocathaica prionotropis, whose shells are left-handed, rotating chirally from Bradybaena sp., were taken successfully by the snakes. It is difficult to find a sufficient sample size of chiral snail species for a feeding experiment with statistical significance, but anecdotally, no difference was noticed on the level of success during hunting B. ravida and L. prionotropis.
3.4 DentitionThe specimen examined (cIB100191) has 5 teeth on the maxillary subequal in size. On the pterygoid 16 teeth decrease in size posteriorly, and 12 on the dentary do likewise. The 10 teeth on palatine increase slightly in size posteriorly. All teeth point backward. No significant asymmetry was observed in either size or density of the teeth (Figure 5).
3.5 Mental grooveEither a shallow or no mental groove were met in the examined specimens of O. ningshaanensis between two pairs of chin-shield. In NAFU071227, the chin-shields touch each other tightly and their inward angles do not contact each other in the central line of the lower jaw. The paired chin shields are not completely bilaterally symmetrical either in the shallow-groovepresent specimens (Figure 6). This partial loss of mental groove in O. ningshaanensis is very similar with Sibon dimidiatus (Günther, 1872) and S. nebulatus (Linnaeus, 1758) from South and Middle America (Peters, 1960).
4. Discussion
4.1 Indication from the molecular resultsThe tree obtained in this work figures out the phylogenetic position of O. ningshaanensis clearly, though the position of Sibynophiinae and its relationship with Pseudoxenodontinae and Dipsadinae conflict with the previous molecular and morphological research (Zhang et al., 1984; Zaher, 1999; Pyron and Burbrink, 2012; chen et al., 2013; Pyron et al., 2013). Interestingly, O. ningshaanensis is not a sister species of Thermophis baileyi, but that of the American Dipsadinae, with Thermophis being their most common ancestor in the Old World. The close relationship between Thermophis and Dipsadinae (including the former Xenodontinae, following Vidal et al., 2010 and the works later on) is well known based on several molecular works (He et al., 2009; Huang et al., 2009; Pyron et al., 2011) and morphological investigation(Malnate, 1953; Zaher, 1999; Zhang et al., 1984). Eventually this genus was moved into the subfamily Dipsadinae by Pyron et al. (2013). Oligodon cinereus, a typical member of the genus Oligodon (see Green et al., 2010; Pryon et al., 2013), is clustering with the colubrinae species. The far relationship between O. ningshaanensis and Oligodon requires a necessary
reclassification of the former species.

Figure 4 The position of holding different snails by the adult specimen. The contained snails are A: Bradybaena ravida; B: Laeocathaica prionotropis.
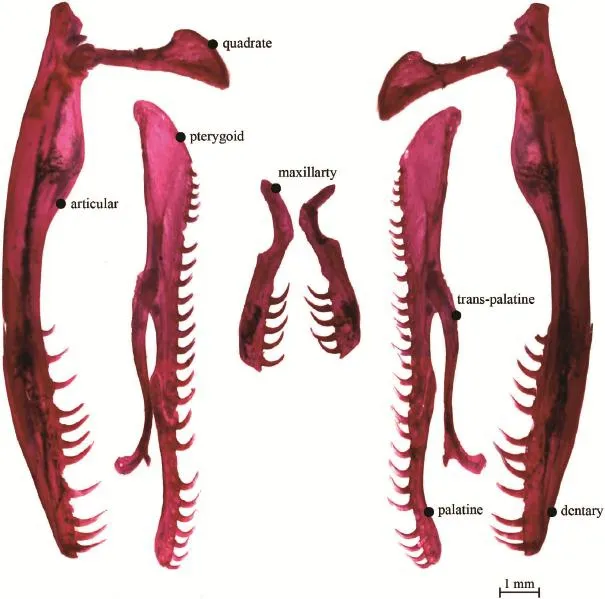
Figure 5 Dentition of Oligodon ningshaanensis.
Vidal et al. (2000) suggested an Asian-North American origin of xenodontines (meaning differently from dipsadines) in the early Tertiary. This result is also mentioned by He et al. (2010) that the divergence time of Thermophis from other xenodontine snakes is ~10–28 mya. The topologies of our results may agree with an Asian origin of the American Dipsadinae. While the phylogenetically controversial position of Pseudoxenodon, as a group in Dipsadinae according to Zhang and Huang (2013), entered or returned to the Old World from the New World across the Bering Land Bridge during the early Tertiary and the warm mid-Miocene. Different from the dispersal of Natricinae at the similar time (Guo et al., 2008), the distributions of O. ningshaanensis and Thermophis, the central montane region and the northwest plateau of china respectively, are separated by large geographically diversed territory in North Asia from the New World. Whether these two mysterious relict groups of snakes present the ancestor or descendant of the American dipsadines remains unknown from the limited data.

Figure 6 comparision of Mental groove between Oligodon ningshaanensis and Orthriophis moellendorffi. Dorsal view of head in NAFU071227 (A), cIB100192 (B), Orthriophis moellendorffi adult specimen (c) and new born specimen (D) from private collection.
4.2 conflicts with the original hemipenial descriptionThe hemipenis we examined is very different from the original description (Yuan, 1983) and those quoted later on (Zhao, 2006; Zhao et al., 1998). The text of the original description is translated as: “proximal region thin, smooth, cylindrical, taken 1/4 of the total length; middle region enlarged, sulcus apparent; distal region bifurcate, sulcus spermaticus reaches the node; on the asulcate side, two rows of little spines located symmetrically from the middle body of hemipenis to the top of both lobes.”According to Yuan (per. comm.), the type specimens were in the collection of Northwest Agriculture and Forestry University. More than 20 years absence of herpetological reseach in this university brought huge losses for the preserved specimens. We visited their museums in 2013 but found no specimens left except NAFU071227 without identification. Staff of the university provided information about broken bottles labeled “Oligodon”. The type specimens appear to be lost, thus, we were unable to get the original material for re-examination. Although some cases for intraspecific variation in hemipenes have been recorded, the differences found were not particularly different in these cases, at least among specimens that share same/close locality (e.g. shape [T-shaped or Y-shaped, Keogh, 1999, Zaher and Prudente, 1999; lobes wider or thinner, Zaher et al., 2005], length [cole and Hardy, 1981; Keiser, 1974] and ornamentation [cole and Hardy, 1981; Keogh, 1999; Prudente and Passos, 2010]). The only exception we are aware of is calamaria lumbricoidea, which has been found with single or forked hemipenis from different geographic distributions (Inger and Marx, 1962). But a reconsideration of the status of these populations seems to be necessary. Nonetheless, no intraspecific variation on sulcus spermaticus division or orientation was observed for any documented species. Therefore, we trend to treat the original description as a possible mistake.
4.3 On the hemipenial morphologyOligodon, as one of the largest snake genera, presents lots of variation in the morphology of the hemipenes. Some Oligodon members, which cluster and firmly belong to colubrinae from the molecular results (Green, 2010; Pryon et al., 2011, 2013), present deeply forked hemipenes, which are relatively rare for this subfamily. Excluding O. ningshaanensis, 16 species possess the forked hemipenes (Green, 2010; Zhang et al., 1984; Zhao, 1998), and most are deeply forked. The hemipenes of O. dorsalis and O. taeniolatus are 1/3 and 2/5 bifurcate, respectively, but these two species were not included in the molecular sampling to elucidate their position between the single and forked groups. According to the presence of single sulcus spermaticus (with some exception), of well-developed calyces present on the distal hemipenis (characters for colubrinae, Zaher, 1999; Zaher et al., 2009), and of the nearly symmetric ornamentation on the sulcus and asulcus sides (character for Oligodon, Jiang, unpublished data), the hemipenial morphology of O. ningshaanensis does not agree with the morphology that is associated with Oligodon, and other colubrinae species.
compared with other subfamilies of colubridae, the hemipenis of O. ningshaanensis can be easily distinguished from calamariinae (nude hemipenial body), Natricinae (hemipenial calyces absent), Pseudoxenodontinae (spinulate lateral and sulcate surfaces) and Sibynophiinae (a sharply curved U-shaped convolution of the sulcus spermaticus). characters like “bifurcate sulcus spermaticus, armed with welldeveloped spines, which are developed from the marginal papollae of calyculi” described by cope (1894) for Xenodontinae, “a row of enlarged lateral spines on each side of the hemipenis, and hemipenial lobes with distinct differentially ornamented regions (a sulcate capitulum and an asulcate nude or weakly calyculate region)” by Zaher (1999) are obvious in O. ningshaanensis.
From large number of descriptions on hemipenial morphology by Zaher (1999) for the Xenodontinae and Dipsadinae, that of O. ningshaanensis is seemingly similar with that of Arrhyton vittatum and A. taeniatum from cuba. Independent evidence demonstrates the close relationship between the American subfamily Dipsadinae and this Old World species.
4.4 Erection of a new genus,Stichophanes, gen. nov.The relationships depicted by our analyses on both molecular and morphological data support the position of Oligodon ningshaanensis in the subfamily Dipsadinae (at least incertae sedis in the Dipsadinae as advised by Zaher et al. [2009]). A new genus is required for this unique species, which we name here as Stichophanes gen. nov.
Etymology:From the Greek στ?χo? (stichos, a row or a line of a book) + the suffix -φαν?? ( -phanes, appearing, conspicuous), alluding both to the slenderness of the body and its straight dorsal lines throughout the trunk and tail. Gender masculine.
common English Name:line-shaped snake.
common chinese Name:線形蛇屬.
Type Species:Stichophanes ningshaanensis [Ningshan line-shaped snake (寧陜線形蛇)].
content:Monotypic.
Stichophanes ningshaanensis(Yuan, 1983) new comb. Definition and Diagnosis:No similarity with any other Old World colubridae species. Small terrestrial colubrid. Head blunt, pupil of eye round, neck distinguishable from head, body slender. All 9 large shields present on the dorsal head, loreal absent, preocular 1, postocular 2, temporal 1+2 (Figure 7 B), rostral broad, supralabial 6, 3rd and 4th entering the eye, infralabial 6, 4th the largest, sublingual 2 pairs, mental groove inconspicuous. Body scales smooth, 13 dorsal rows throughout the trunk, ventrals 159–170, anal divided, subcaudals 64–73 pairs.
The species exhibits sexual dimorphism, both in size and color. Males are shorter as adults with a longer tail (24% to 27% of the total body length), and dorsal color olive to brown in life. Females are longer with a shorter tail (21% of the total body length), and dorsal color is yellowish-brown in life (Figure 7 A). Preserved specimens are dark bluish brown in formalin. Head darker, from which originate two dark lines extending on 4throws of dorsals (D4) throughout the body and tail, other two thin dark lateral lines from neck to vent located between D1 and D2. At the posterior head a large elongated dark spot fades along the central dorsal row (Figure 7 A and D). Belly pale yellow with two lines of black little dots forming ventral dashed stripes (Figure 7 c).
Hemipenis bilobed, semicapitate and semicalyculate, with deeply forked sulcus spermaticus and large lateral
spines. Maxillary teeth 5 or 6. This species is oviparous, laying between 8–9 eggs in a clutch.

Figure 7 Stichophanes ningshaanensis in life. A: Dorsal view of anterior body demonstrating sexually dimorphic coloration; B: Lateral view of head; c: Ventral dots; D: full-body photo displaying the dorsal stripes; E: a female specimen sitting among leaf litter.
Distribution:Shaanxi Province: Huoditang, Ningshan county; Hubei Province: Muyu, Pingqian and Guogongping, Shennongjia Nature Reserve.
Habit and Habitat:Stichophanes ningshaanensis is a diurnal leaf litter dweller feeding on terrestrial gastropods. Living specimens were found among dead leaves near roads and trails, and usually in proximity to water (Figure 7 E). The species lives in moderately high elevations (1550 to 2022 m) and are most active in late June and early July.
Note:Type specimens are lost.
4.5 comparison of hunting skillsApproximately 180 known snake species consume mollusks as their diet (Laporta-Ferreira and Salom?o, 2004). Among colubroidea (revised after Pyron et al., 2011, 2013), except the well-known family Pareatidae and many species from Dipsadinae (Peters, 1960), species in Lamprophiidae (Duberria, Gans, 1975) and Natricinae (Storeria, Judd, 1954, Rossman and Myer, 1990; Thamnophis, Britt et al., 2006, 2009;) were documented as malacophagous predators. Although all genera mentioned above include species able to hunt slugs, preying on snails is not handled by all of them. The exclusive snail-eater Pareas developed high specialization on extracting the snail from the gastropod helical shell (Gotz, 2002; Hoso and Hori, 2008; Hoso et al., 2007). In the Dipsadinae, inserting mandibles are also employed by Dipsas (Gans, 1975; Peters, 1960; Sazima, 1989) and Sibynomorphus (Agudo-Padrón, 2013; Laporta-Ferreira and Salom?o, 2004), but the morphological and behavioral asymmetry in Pareatidae for efficient handling dextral shell has not been documented. Beside this, “snag and drag” strategy is another method used by Sibon and Tropidodipsas (Sheehy, 2012), and now it is known from S. ningshaanensis. Similarly, the “snag” is also used by Storeria dekayi (Rossman and Myer, 1990) — “substrate”and “torsion.” The long time holding and searching of S. ningshaanensis was very similar as for Storeria when no snag was offered: “the snakes pushed the snails around the terrarium for more than 30 min before abandoning the feeding attempt”.
4.6 The characters associated with the feeding behaviorSlender teeth are required for grasping the mollusks with efficiency in spite of their slimy secreta. This character is very common among the malacophagous snake species as a morphological homoplasy but is missing in the slug-eater Thamnophis ordinoides and T. elegans terrestris (Britt et al., 2009). The snail-eating pareatid species have more teeth on the right mandible than the left for the dextral (clockwise) snail houses which are predominant in nature (Hoso et al., 2007). It is difficult to provide any suitable comparison with a near sister group as Britt et al. (2009) used for Thamnophis to prove if the dentition of S. ningshaanensis is more slender than that of the non-malacophagous predator. However, the symmetry is noticeable, as in most other snail or slug eaters in Dipsadinae, and reflects that the feeding behavior developed lately (not earlier than the other dipsadid malacophagous species).
Mental groove absence is a very important character in the Pareatidae (Guo et al., 2011) and the genus Dipsas (Peters, 1960). According to Peters (1960) loss of the mental groove adds rigidity for the slug-eating snakes. And the rigidity perhaps compensates in part for the increased mobility of all the toothed bones. Though Dipsas species do not consume mollusks exclusively (Ray et al., 2012), their common diet with Pareatidae displays their convergence to some extent. Given the distance between S. ningshaanensis and the Dipsadini species (Figure 1), phylogeny reconstructed by many previous works (Pinou et al., 2004; Sheehy, 2012; Vidal et al., 2010; Zaher et al., 2009; Pyron et al., 2013), S. ningshaanensis might have obtained this diet and the related characters independently in the Dipsadinae.
AcknowledgementsWe are grateful to Huoditang Forestry Station and Shennongjia Nature Reserve for allowing us to collect specimens. We would like to thank Xianhua TIAN, Lei PAN, Junhai XU, Yang YU and Shanzhong GONG, Dong XIE, and Linsen YANG for their great help in the field work; cong WEI and Hong HE for their effort in searching the preserved specimens in NAFU; Guanfu WU, Ke JIANG, Fang YU, chen WANG, Wenqing ZHOU, Xiuwei LIU and Tianjuan SU for the help with experimental skill and laboratorial technique; Jiatang LI and Robert MURPHY for their advice on the structure of this article; Sergey RYABOV for collecting and identifying different mollusks. We thank Yujia ZHANG and Robert ScHLEIcHER for their kindly revision on the language of this manuscript. We also thank the anonymous reviwers for their precious comments and suggestion to improve the quality of the paper.
Agudo-Padrón A. I.2013. Snail-eating snakes ecology, diversity, distribution and alimentary preferences in Brazil. J Environ Sci Wat Resour, 2(8): 238–244
Britt E. J., clark A. J., Bennett A. F.2009. Dental morphologies in gartersnakes (Thamnophis) and their connection to dietary preferences. J Herpetol, 43(2): 252–259
Britt E. J., Hicks J. W., Bennett A. F.2006. The energetic consequences of dietary specialization in populations of the garter snake, Thamnophis elegans. J Exp Biol, 209(16): 3164–3169
chen B., Wang Y.2002. Introduction a method of clearing and Staining. Bull Biol, 37(4): 57 (In chinese)
chen X., Huang S., Guo P., colli G. R., Nieto Montes de Oca A., Vitt L. J., Pyron R. A., Burbrink F. T.2013. Understanding the formation of ancient intertropical disjunct distributions using Asian and Neotropical hinged-teeth snakes (Sibynophis and Scaphiodontophis: Serpentes: colubridae). Mol Phylogenet Evol, 66(1): 254–261
cole c. J., Hardy L. M.1981. Systematics of North American colubrid snakes related to Tantilla planiceps (Blainville). Bull AMNH, v. 171, article 3
coleman K., Rothfuss L. A., Ota H., Kardong K. V.1993. Kinematics of egg-eating by the specialized Taiwan snake Oligodon formosanus (colubridae). J Herpetol, 27(3): 320–327
consul A., Eger S., Kwet, A.2009. The Grass Snake, Natrix natrix natrix (Squamata: colubridae), as a predator of the Great Ramshorn Snail, Planorbarius c. corneus (Gastropoda: Planorbidae). Salamandra, 45(1): 50–52
cope E. D.1894. The classification of snakes. Am Nat, 28: 831–844
cope E. D.1895. The classification of the Ophidia. Trans Am Philos Soc, 28: 186–219
de Queiroz A., Lawson R., Lemos-Espinal J. A.2002. Phylogenetic relationships of North American Garter Snakes (Thamnophis) based on four mitochondrial genes: How much DNA sequence is enough? Mol Phylogenet Evol, 22(2): 315–329
Dowling H. G.1967. Hemipenes and other characters in colubrid classification. Herpetologica, 138–142
Edgar R. c.2004. MUScLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res, 32(5): 1792–1797
Forstner M. R., Davis S. K., Arévalo E.1995. Support for the hypothesis of anguimorph ancestry for the suborder Serpentes from phylogenetic analysis of mitochondrial DNA sequences. Mol Phylogenet Evol, 4(1): 93–102
Gans c.1975. Reptiles of the world. New York: Bantam Books, 159pp
Gotz M.2002. The feeding behavior of the snail-eating snake Pareas carinatus Wagler 1830 (Squamata: colubridae). Amphibia Reptilia, 23(4): 487–494
Green M.2010. Molecular phylogeny of the snake genus Oligodon (Serpentes: colubridae), with an annotated checklist and key. M.Sc. Thesis. University of Toronto. 169pp
Green M. D., Orlov N. L., Murphy R. W.2010. Toward a phylogeny of the Kukri snakes, genus Oligodon. Asian Herpetol Res, 1(1): 1–21
Guo P., Liu Q., Xu Y., Jiang K., Hou M., Ding L., Pyron R. A., Burbrink, F. T.2012. Out of Asia: Natricine snakes support the cenozoic Beringian dispersal hypothesis. Mol Phylogenet Evol, 63(3): 825–833
Guo Y., Wu Y., He S., Shi H., Zhao E.2011. Systematics and molecular phylogenetics of Asian snail-eating snakes (Pareatidae). Zootaxa, 1175–5326
He M., Feng J. c., Liu S. Y., Guo P., Zhao E. M.2009. The phylogenetic position of Thermophis (Serpentes: colubridae), an endemic snake from the Qinghai-Xizang Plateau, china. J Nat Hist, 43(7–8): 479–488
He M., Feng J., Zhao E.2010. The complete mitochondrial genome of the Sichuan hot-spring keel-back (Thermophis zhaoermii; Serpentes: colubridae) and a mitogenomic phylogeny of the snakes. Mitochondrial DNA, 21(1): 8–18
Hoso M., Asami T., Hori M.2007. Right-handed snakes: convergent evolution of asymmetry for functional specialization. Biol Lett, 3(2): 169–173
Hoso M., Hori M.2008. Divergent shell shape as an antipredator adaptation in tropical land snails. The Am Naturalist, 172(5): 726–732
Hu S., Zhao E. M.1987. Atlas of china Animals—Amphibians and Reptiles, 2ndedition. Beijing: Science Publishing House, 110 pp (In chinese)
Huang S., Liu S., Guo P., Zhang Y., Zhao E.2009. What are the closest relatives of the hot-spring snakes (colubridae, Thermophis), the relict species endemic to the Tibetan Plateau? Mol Phylogenet Evol, 51(3): 438–446
Inger R. F., Marx H.1962. Variation of hemipenis and cloaca in the colubrid snake calamaria lumbricoidea. Syst Zool, 11(1): 32–3
Jiang K.2010. A Method for Evaginating the Hemipenis of Preserved Snakes. Sichuan J Zool, 29(1): 122–123 (In chinese)
Judd W. W.1954. Observations on the food of the little brownsnake, Storeria dekayi, at London, Ontario. copeia, 1954: 62–64
Keiser E. D.1974. A systematic study of the neotropical vine snake Oxybelis aeneus (Wagler). Texas Memorial Museum, 51pp
Keogh J. S.1999. Evolutionary implications of hemipenial morphology in the terrestrial Australian elapid snakes. Zool J Linn Soc, 125(2): 239–278
Laporta-Ferreira I. L., Salom?o M. D. G.2004. Reptilian predators of terrestrial gastropods. In Barker G. M. (Ed.), Natural enemies of terrestrial molluscs. cABI, 427–482
Li L., Liang G.2007. Microdermatoglyphis patterns of Oligodon ningshanensis. J Xian Univ Art Sci (Nat Sci Ed), 10(3): 66–68 (In chinese)
Lowe T. M., Eddy S. R.1997. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res, 25: 955–964
Lawson R., Slowinski J. B., crother B. I., Burbrink F. T.2005. Phylogeny of the colubroidea (Serpentes): New evidence from mitochondrial and nuclear genes. Mol Phylogenet Evol, 37(2): 581–601
Minton S. A.1963. Feeding habits of the kukri snake, Oligodon taeniolatus. Herpetologica, 19: 147–147
Myers c. W.2003. Rare snakes-five new species from eastern Panama: Reviews of northern Atractus and southern Geophis (colubridae: Dipsadinae). Am Mus Nov, 1–47
Myers c. W., McDowell S. B.2014. New taxa and cryptic species of neotropical snakes (Xenodontinae), with commentary on hemipenes as generic and specific characters. Bull Am Mus Nat Hist, 385(1): 1–112
Pesantes O. S.1994. A method for preparing the hemipenis of preserved snakes. J Herpetol, 28(1): 93–95
Peters J. A.1960. The snakes of the subfamily Dipsadinae. Ann Arbor: University of Michigan, 224pp
Pinou T., Vicario S., Marschner M., caccone A.2004. Relict snakes of North America and their relationships within caenophidia, using likelihood-based Bayesian methods on mitochondrial sequences. Mol Phylogenet Evol, 32(2): 563–574
Passos P., Fernandes R., Zanella N.2005. A new species of Atractus (Serpentes: colubridae) from southern Brazil. Herpetologica, 61(2): 209–218
Price R. M.1982. Dorsal Snake Scale Microdermato-glyphics: Ecological Indicator or Taxonomic Tool? J Herpetol, 16(3): 294–306
Prudente A. L., Passos P.2010. New cryptic species of Atractus (Serpentes: Dipsadidae) from Brazilian Amazonia. copeia, 2010(3): 397–404
Pyron R. A., Burbrink F. T.2012. Extinction, ecological opportunity, and the origins of global snake diversity. Evolution, 66(1): 163–178
Pyron R. A., Burbrink F. T., colli G. R., De Oca A. N. M., Vitt L. J., Kuczynski c. A., Wiens J. J.2011. The phylogeny of advanced snakes (colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol Phylogenet Evol, 58(2): 329–342
Pyron R. A., Burbrink F. T., Wiens J. J.2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMc Evol Biol, 13(1): 93
Ray J. M., Montgomery c. E., Mahon H. K., Savitzky A. H., Lips K. R.2012. Goo-eaters: Diets of the Neotropical snakes Dipsas and Sibon in central Panama. copeia, 2012(2): 197–202
Rossman D. A., Myer P. A.1990. Behavioral and morphological adaptations for snail extraction in the North American brown snakes (genus Storeria). J Herpetol, 434–438
Sazima I. 1989. Feeding behavior of the snail-eating snake, Dipsas indica. J Herpetol, 464–468
Sawaya R. J., Sazima I.2003. A new species of Tantilla (Serpentes: colubridae) from southeastern Brazil. Herpetologica, 59(1): 119–126
Slowinski J. B., Lawson R.2002. Snake phylogeny: Evidence from nuclear and mitochondrial genes. Mol Phylogenet Evol, 24(2): 194–202
Sheehy c. M.2012. Phylogenetic relationships and feeding behavior of neotropical snail-eating snakes (Dipsadinae, Dipsadini). Ph.D. Thesis. University of Texas at Arlington. 135pp
Stamatakis A.2006. RAxML-VI-HPc: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22(21): 2688–2690
Stamatakis A., Hoover P., Rougemont J.2008. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol, 57(5): 758–771
Tamura K., Stecher G., Peterson D., Filipski A., Kumar S.2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol, 30(12): 2725–2729
Utiger U., Helfenberger N., Sch?tti B., Schmidt c., Ruf M., Ziswiler V.2002. Molecular systematics and phylogeny of Old and New World ratsnakes, Elaphe Auct., and related genera (Reptilia, Squamata, colubridae). Russ J Herpetol, 9(2): 105–124
Vidal N., Delmas A. S., David P., cruaud c., couloux A., Hedges S. B.2007. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. c R Biol, 330(2): 182–187
Vidal N., Dewynter M., Gower D. J.2010. Dissecting the major American snake radiation: A molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, caenophidia). c R Biol, 333(1): 48–55
Vidal N., Hedges S. B.2002. Higher-level relationships of caenophidian snakes inferred from four nuclear and mitochondrial genes. c R Biol, 325(9): 987–995
Vidal N., Hedges S. B.2009. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. c R Biol, 332(2): 129–139
Vidal N., Kindl S. G., Wong A., Hedges S. B.2000. Phylogenetic relationships of xenodontine snakes inferred from 12S and 16S ribosomal RNA sequences. Mol Phylogenet Evol, 14(3): 389–402
Vieites D. R., Min M. S., Wake D. B.2007. Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proc Natl Acad Sci, 104(50): 19903–19907
Wiens J. J., Hutter c. R., Mulcahy D. G., Noonan B. P., Townsend T. M., Sites J. W., Reeder T. W.2012. Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol Lett, 8(6): 1043–1046
Yang L., Messenger K., Liao M.2009. Reptiles and Amphibians Diversity of Shennongjia Natational Nature Reserve. Sichuan J Zool, 28(2): 286–291
Yuan H.1983. A new species of the genus Oligodon from Shaanxi, china. Acta Herpetologica Sin, 2: 65–67 (In chinese)
Zaher H.1999. Hemipenial morphology of the South American xenodontine snakes: With a proposal for a monophyletic Xenodontinae and a reappraisal of colubroid hemipenes. Bull AMNH, 240: 1–168
Zaher H., Grazziotin F. G., cadle J. E., Murphy R. W., Moura-Leite J. c. D., Bonatto S. L.2009. Molecular phylogeny of advanced snakes (Serpentes, caenophidia) with an emphasis on South American Xenodontines: A revised classification and descriptions of new taxa. Pap Avulsos Zool, 49(11): 115–153
Zaher H., Prudente A. L. c.2003. Hemipenes of Siphlophis (Serpentes, Xenodontinae) and techniques of hemipenial preparation in snakes: a response to Dowling. Herpetol Rev, 34(4): 302–306
Zaher H., Souza I., Gower D. J., Hingst-Zaher E., Silva Jr. N. J. D.2005. Redescription of Atractus albuquerquei (Serpentes: colubridae: Dipsadinae), with comments on geographical distribution and intraspecific variation. Pap Avulsos Zool, 45(2): 19–32
Zhang F., Hu S., Zhao E.1984: comparative studies and phylogenetic discussions on hemipenial morphology of the chinese colubrinae (colubridae). Acta Herpetologica Sin, N Ser 3(3): 23–44 (In chinese)
Zhang B., Huang S.2013. Relationship of Old World Pseudoxenodon and New World Dipsadinae, with comments on Underestimation of Species Diversity of chinese Pseudoxenodon. Asian Herpetol Res, 4(3): 155–165
Zhao E. M.2006. Snakes of china. Hefei: Anhui Science and
Technology Publishing House. (In chinese)
Zhao E., Huang M., Zong Y., Zheng J., Huang Z., Yang D., LiD.1998. Fauna Sinica: Reptilia, Vol. 3, Squamata Serpentes. Beijing: Science Press (In chinese)
*corresponding author: Prof. Ermi ZHAO, from the Department of Herpetology, chengdu Institute of Biology, chinese Academy of Sciences, chengdu, Sichuan, china, with his research focusing on systematics herpetology; Prof. chaodong ZHU, from the Division of Functional Insect Groups Evolution, Institute of Zoology, chinese Academy of Sciences, Beijing, china, with his research focusing on systematic entomology, evolutionary biology, computational phylogenetics, pollination biology and biological control.
E-mail: zhaoermi@cib.ac.cn (Ermi ZHAO); zhucd@ioz.ac.cn (chaodong ZHU)
Received: 4 April 2014 Accepted: 11 July 2014
 Asian Herpetological Research2014年3期
Asian Herpetological Research2014年3期
- Asian Herpetological Research的其它文章
- First Record of the Gekkonid Genus cnemaspis Strauch, 1887 from Gunung Mulu National Park, Northern Sarawak, East Malaysia may Represent an Undescribed Species
- Microbiological and Histological Examinations of Endangered Neurergus kaiseri Tissues Displaying Red-leg Syndrome
- The Seasonal Acclimatisation of Locomotion in a Terrestrial Reptile, Plestiodon chinensis (Scincidae)
- Phylogenetic Patterns of the Southeast Asian Tree Frog chiromantis hansenae in Thailand
- New Karyological and Morphometric Data on Poorly Known Bufo surdus and Bufo luristanicus in comparison with Data of Diploid Green Toads of the Bufo viridis complex from South of Iran
- Distribution and Environmental Suitability of the Smallscaled Rock Agama, Paralaudakia microlepis (Sauria: Agamidae) in the Iranian Plateau
