Antioxidant and antimicrobial properties of Litsea elliptica Blume and Litsea resinosa Blume (Lauraceae)
Mui-Hung Wong, Li-Fang Lim, Fasihuddin bin Ahmad, Zaini bin Assim
1Faculty of Applied Sciences, Universiti Teknologi MARA, Samarahan Campus, Sarawak Branch, 94300 Kota Samarahan, Sarawak, Malaysia
2Faculty of Engineering, Computing and Science, Swinburne University of Technology, Sarawak Campus, 93350 Kuching, Sarawak, Malaysia
3Department of Chemistry, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia
Antioxidant and antimicrobial properties of Litsea elliptica Blume and Litsea resinosa Blume (Lauraceae)
Mui-Hung Wong1*, Li-Fang Lim2, Fasihuddin bin Ahmad3, Zaini bin Assim3
1Faculty of Applied Sciences, Universiti Teknologi MARA, Samarahan Campus, Sarawak Branch, 94300 Kota Samarahan, Sarawak, Malaysia
2Faculty of Engineering, Computing and Science, Swinburne University of Technology, Sarawak Campus, 93350 Kuching, Sarawak, Malaysia
3Department of Chemistry, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia
PEER REVIEW
Peer reviewer
Associate Professor Dr. Ling Teck Yee Tel: +6082-583040
E-mail: tyling@frst.unimas.my
Comments
Results of this study are important contributions to the body of knowledge. It has been shown that the methanol extracts of root and stem of both L. elliptica and L. resinosa showed significant scavenging activity and L. resinosa showed strong antibacterial activities compared to L. eliptica and less polar extracts such as hexane and dichloromethane extracts showed significant activities as compared to methanol extracts which is polar. Essential oil of both species also showed great potential in inhibiting fungus F. oxysporum.
Details on Page 391
Objective:To investigate antioxidant and antimicrobial activities of two plant species, Litsea elliptica (L. elliptica) and Litsea resinosa (L. resinosa).
Litsea elliptica, Litsea resinosa, Antioxidant, Antifungal, Antibacterial, EC50
1. Introduction
Litsea, which is an important genus from the Lauraceae family, is frequently found in regions such as tropical and subtropical Asia, Australia, and from North America to subtropical South America[1]. Indigenous plants like this have been widely utilized as traditional medicine in maintaining human health[2]. The increase in pervasiveness of multi-drug resistant microorganisms has raised the interest in natural product discovery from ethnomedicinal plants[3]. Thus, this species might have the potential to fulfill the increasing demands of antibiotics globally.
Secondary metabolites are produced by plants when they respond to environmental stress. Their production might also be a defense mechanism towards plant diseases[4]. They are selected by nature for specific biological interactions and possess drug-like properties. These metabolites usually retained antimicrobial characteristicand can be roughly classified into several classes. Major clusters of antimicrobial compounds including alkaloids[5], butanolide[6], flavonoids[7], lignans[8], sesquiterpenes, fatty acids[9] and essential oils[10] have been discovered through phytochemical investigations performed towardsLitseaspp. These compounds have shown significant activities including antimicrobial[11], antitumor[12], anticancer[13], antioxidant[14], anti-inflammatory, wound healing[15], antidepressant[16] and anti hyperalgesic[17] properties in studies involvingLitseaspp.
Litsea elliptica(L. elliptica) andLitsea resinosa(L. resinosa) originated from the Lauraceae family.L. ellipticawas proven to be non-toxic towards female Sprague-Dawley rats in a study carried out by Siti Nor Ainet al.(2011)[18]. On the other hand, there is not much studies published regardingL. resinosaexcept from the large amount of essential oil constituents in it[19]. In fact, most essential oils from plants have been revealed to be vastly effective against food borne pathogens[20].
In determining antioxidant activities of plant compounds,in vitroassay including 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay can be applied. This assay comprises reducing level of DPPH free radical, H2O2scavenging activity by peroxidase/guaiacol and inhibition activity of lipid peroxidation by thiobarbituric acid. Commonly found synthetic antioxidants which are commercially available have been proven to be toxic and carcinogenic[21]. Hence, antioxidants from natural sources will be a considerable substitute to current synthetic antioxidants.
Study from Linet al.(2007) stated that most extracts of plant species from Lauraceae genus showed antioxidant activity and revealed great free radicals of DPPH scavenger properties[22]. Methanol extract and fractions fromLitsea cubebashowed remarkable antioxidant activity, and contained powerful natural antioxidant compounds[14]. However, antioxidant activity of majority ofLitseaspp has not been reported.
The present study focused on the antioxidant activity of extracts fromL. ellipticaBlume andL. resinosaBlume by DPPH radical scavenging assay as well as antimicrobial properties through agar well diffusion method and mycelial radial growth assay.
2. Materials and methods
2.1. Plant materials
L. ellipticaBlume andL. resinosaBlume were collected from the forest around Universiti Malaysia Sarawak, Kota Samarahan, Sarawak.
2.2. Sample preparation
Samples collected were washed, sorted out by different parts of the plants, shade air-dried, cut and grinded prior to sequential solvent extraction.
2.3. Crude extraction
The samples in powder form were used for solvent extraction following the polarity sequence fromn-hexane, dichloromethane, chloroform, ethyl acetate to methanol (Merck EMSURE?ACS, ISO, Reag. Ph Eur) at room temperature. The sample was macerated in solvent in 5 L conical flasks at ambient temperature for 3 d, swirled three to four times per day. The extract was filtered with Advantec No. 1 filter paper and residue was kept for the subsequent extraction. Extraction of each solvent was carried out trice before the next solvent was used. Filtrates from the triplicate extractions were combined and concentrated using rotary evaporator (Heidolph Hei-VAP Advantage) at 40 °C. The concentrated filtrates were transferred into a pre-weighed beaker and left in a fume hood to completely dry out the solvent. Dried crude extracts were wrapped with foil and kept in freezer before use. The extraction yield was obtained in percentage using the weight of the extracts collected. The whole process was repeated and applied to different plant parts.
2.4. Essential oil extraction
Essential oils were extracted using hydrodistillation on a Clevenger-type apparatus. Samples were separated (root bark, root, inner bark of root, stem bark, twig, inner bark of stem and leaf) and cut into small pieces. Approximately 100-150 g of fresh samples was transferred into a 2 L flat-bottom round flask before 1.5 L of distilled water was added. The samples were hydrodistilled for 6 h continuously with the distillation rate of 1-2 drops per second. The essential oils were collected and left to cool to room temperature. The oil was separated from water and dried over anhydrous sodium sulphate. The hydrodistillation was repeated twice for each plant part. The essential oils were kept in vials and placed in 4 °C before analysis. The percentage yields were calculated.
2.5. Antioxidant assay-DPPHradical scavenging assay
DPPH radical scavenging antioxidant assay was modified from Wanget al.(2002)[23]. Samples of each extract including standard butylated hydroxytoluene (BHT) were prepared at different concentrations (0, 1, 10, 100 and 1 000 mg/L) in triplicates using methanol. Methanol (negative control) and 2 mL of reaction reagent were used as controls. Reaction reagent was methanolic solution of DPPH prepared by dissolving 5.9 mg of DPPH (Sigma-Aldrich) in 100 mL of methanol. Exactly 1 mL of each sample was mixed with 2 mL of the reaction reagent. The mixture was shaken thoroughly and left to stand in the dark for about 30 min at room temperature. Radical scavenging activity of the samples against DPPH free radical was measured according to the transmission of absorbance using UV-vis (ultraviolet visible) spectrophotometer (Jasco V-630). Distilled water (without reagent) was used as blank (solvent blank) in a cuvette for baseline. BHT was used as standard or positive control of this assay. Resultants absorbance of the controls and sample-reagent mixtures were recorded respectivelyin cuvettes (Type 1Q, 10 mm, quartz cell 3.5 mL) at 517 nm. The percentage of DPPH radical scavenging activity (RSA) is obtained using the following equation:
RSA (%) = [(Ac- As)/Ac] × 100
Where, Acis mean value of absorbance of the control, Asis mean of absorbance values obtained in three replicates from reaction mixture of DPPH-methanol reagent and each sample.
2.6. Antimicrobial assay
2.6.1 Antibacterial assay-agar well diffusion assay
Antibacterial activities of extracts were evaluated using agar well diffusion assay modified from Tanet al.(2008) [23]. Plant extracts were dissolved in methanol to a final concentration of 10 000 mg/L. Nutrient broth was used for the culturing of 6 different test strains including yeast strain which consist ofEscherichia coli(E. coli),Bacillus subtilis(B. subtilis),Bacillus megaterium(B. megaterium),Pseudomonas aeruginosa(P. aeruginosa),Staphylococcus aureus(S. aureus), andSaccharomyces cerevisiae(S. cerevisiae). Test strains cultured for 24 h were spread on the surface of nutrient agar. Equidistant well was bored into the agar using sterile cork borer (5 mm) followed by the addition of 50 μL diluted extracts. The plates were incubated at 37 °C for 24 h. Benzylpenicillin potassium (10 000 mg/mL), tetracycline (30 000 mg/L) and thymol (10 000 mg/L) were used as positive controls while methanol as the negative control. Antibacterial activity was obtained by measuring the diameter of zone of inhibition of the triplicates.
2.6.2 Antifungal assay-mycelial radial growth inhibition assay
The antifungal activities of extract were examined using mycelial radial growth assay[24]. Extracts of volume of 250 mg were dissolved in 2.5 mL of methanol prior added into 250 mL sterile potato dextrose agar at 60 °C. Approximately 15 mL of the mixed medium was poured into Petri dish and the final concentration of extract in media was 1 000 mg/L. For negative control, blank methanol was added instead of the extracts. Cycloheximide of concentration 10 mg/L and 1 000 mg/L as well as thymol of concentration 10 mg/L were preferred as positive controls. Upon carrying out the assay, a 5 mm plug with fungus strain was placed in the centre of the media before incubating at 25 °C. The results of assay were obtained after 7 d forFusarium oxysporum(F. oxysporum). The average measurements of the triplicates were used to calculate the percentage of mycelial radial growth (MRG) inhibition by applying the formula below:
% of MRG Inhibition = [(Dc-Ds)/Dc] × 100
Where, Dcis the average diameter of fungal strain in negative control, Dsis the average diameter of fungal strain in media with extract samples.
3. Results
Percentage yields of the essential oils ofL. ellipticaandL. resinosaas shown in Table 1 ranged from 0.11% to 2.28% (vol/ wt of dry materials). The leaf oil ofL. elliptica[(2.28±0.09)%] and the stem bark oil ofL. resinosa[(2.27±0.38)%] gave the highest yields.
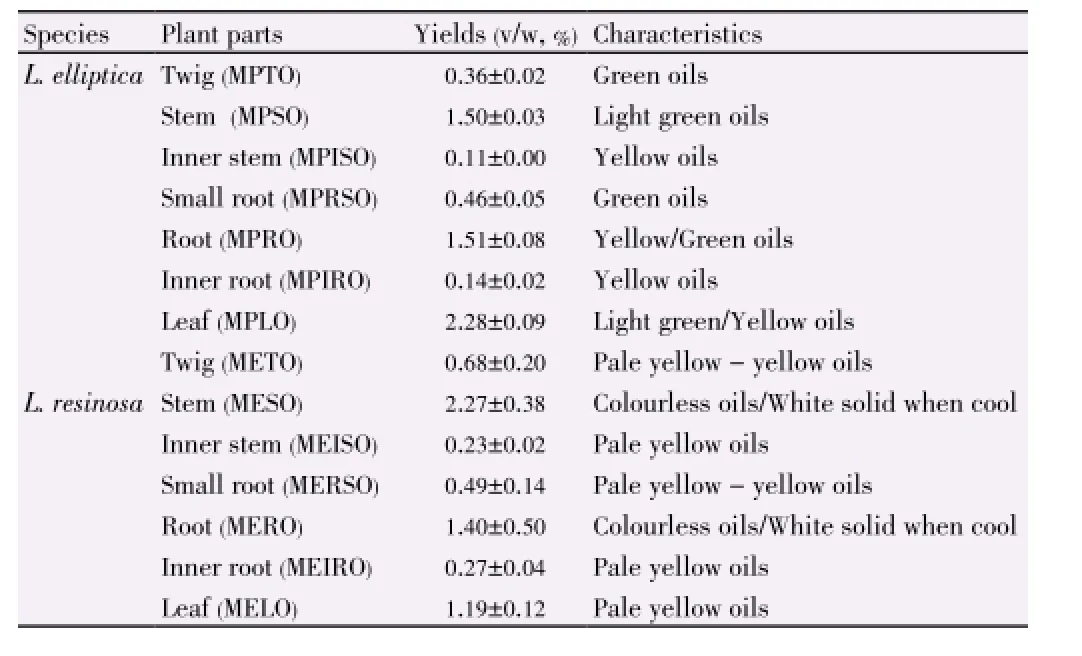
Table 1 Percentage yields and characteristics of the essential oils of L. elliptica and L. resinosa.
EC50values of the extracts (Table 2) showed that methanol extracts from root and stem of bothL. ellipticaandL. resinosaexhibited the highest antioxidant activity among of the samples studied, with EC50(50% DPPH free radical scavenging and effectiveness concentration) values of 23.99, 41.69, 11.22 and 35.48 mg/L respectively. The methanol extracts from both the root ofL. resinosaas well asL. ellipticashowed stronger scavenging activity with EC50values lower than that of the standard BHT with EC50value of 28.18 mg/L.
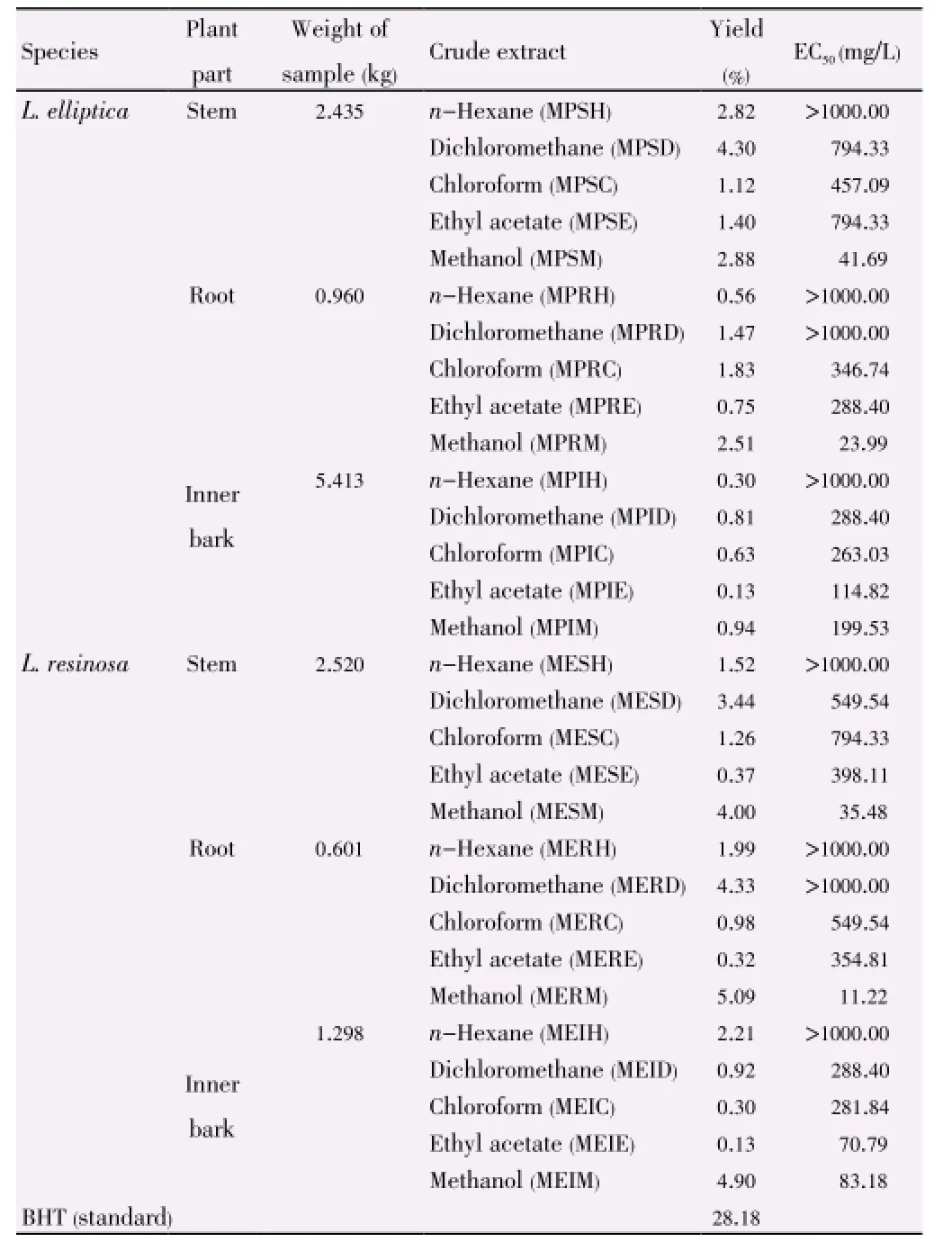
Table 2 Extraction yield and EC50values of L. elliptica and L. resinosa.
In comparison of the different parts of plant, theinhibitory activities of both plants decreased from stem, root to inner bark and extracts from L. elliptica showed better inhibitory result as compared to that of L. resinosa. The inhibition zones support the results achieved through well diffusion assay. Plant extracts showed the strongest antimicrobial activities towards skin pathogen P. aeruginosa, followed by food-borne pathogens E. coli and B. subtilis (Table 3). The inhibition activities of the three positive controls were evaluated as shown in Table 4.
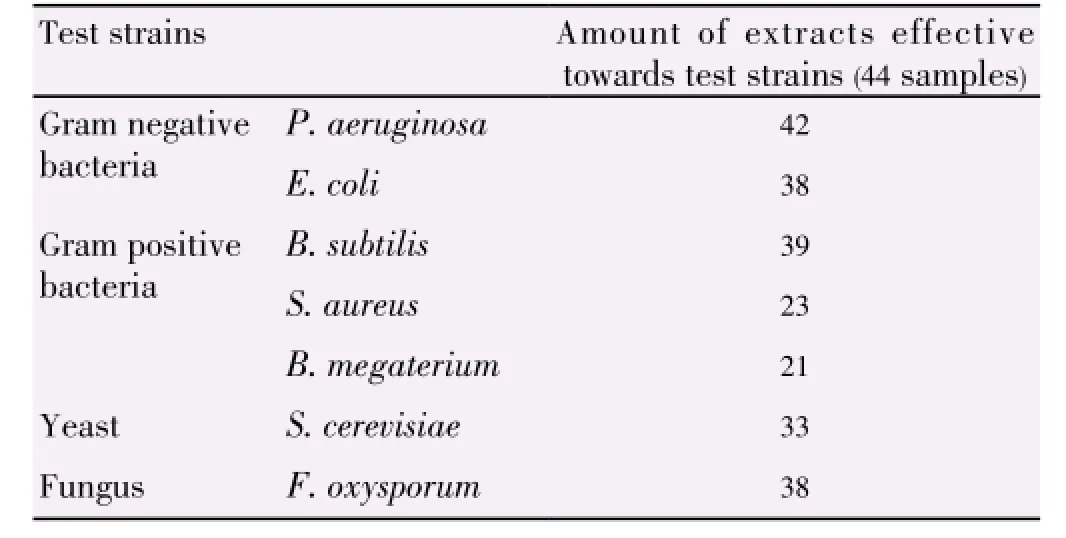
Table 3 Summary of antimicrobial activities of sample extracts.
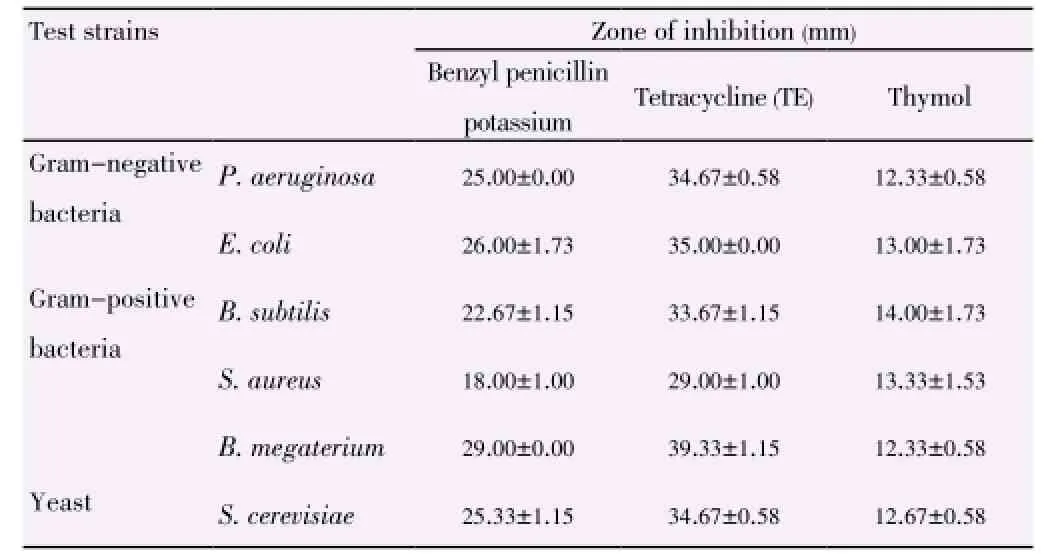
Table 4 Inhibition of positive controls towards test strains.
Extracts from L. resinosa demonstrated stronger antibacterial properties than L. elliptica especially in inhibiting Gram negative bacteria as shown in Table 5 and Table 6. Hexane extract from stem of L. resinosa presented the largest inhibition in Gram-negative E. coli [(19.33±1.15) mm] while chloroform extract from inner bark of L. resinosa showed major inhibition [(15.33±3.21) mm] towards Gram-positive B. subtilis. Major crude extracts from L. resinosa showed higher efficacy towards E. coli with inhibition zones larger than standard thymol [(13.00± 1.73) mm].
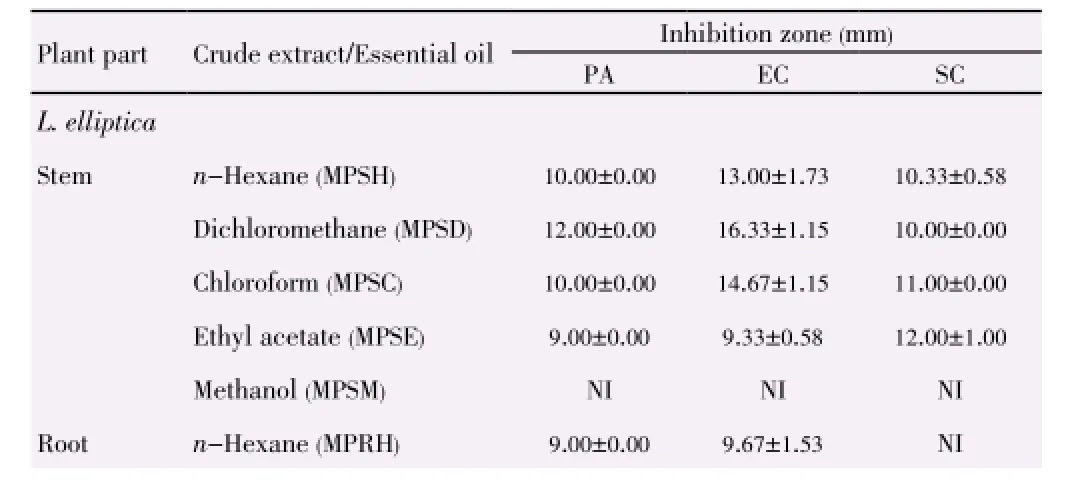
Table 5 Inhibition zones of L. elliptica and L. resinosa towards Gram-negative bacteria strains and yeast strain.
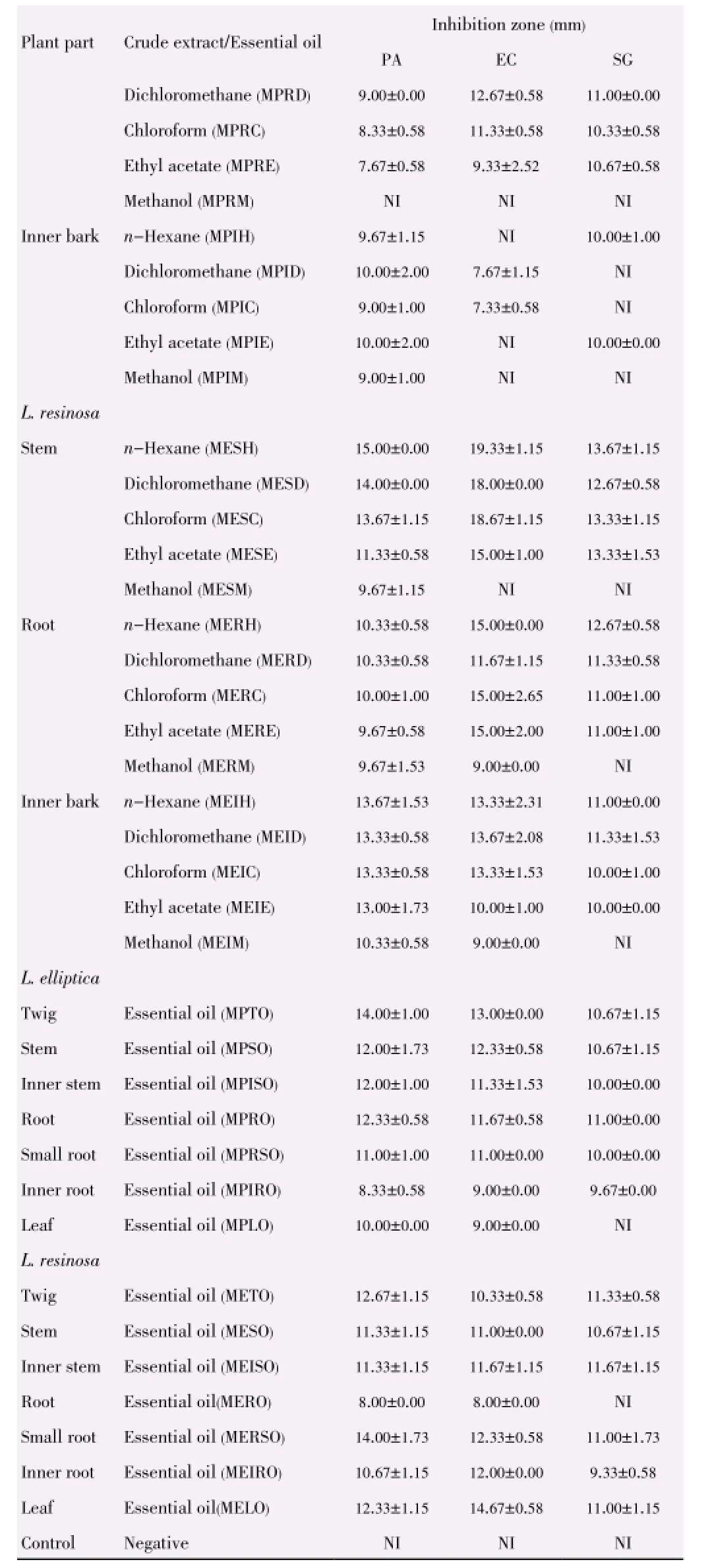
Table 5 Inhibition zones of L. elliptica and L. resinosa towards Gram-negative bacteria strains and yeast strain.
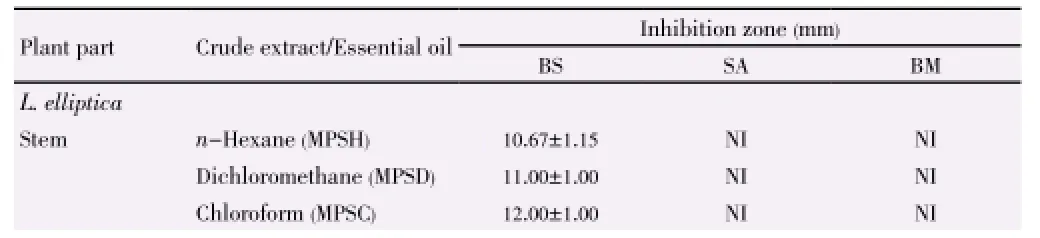
Table 6 Inhibition zones of L. elliptica and L. resinosa towards Gram-positive bacteria strains.
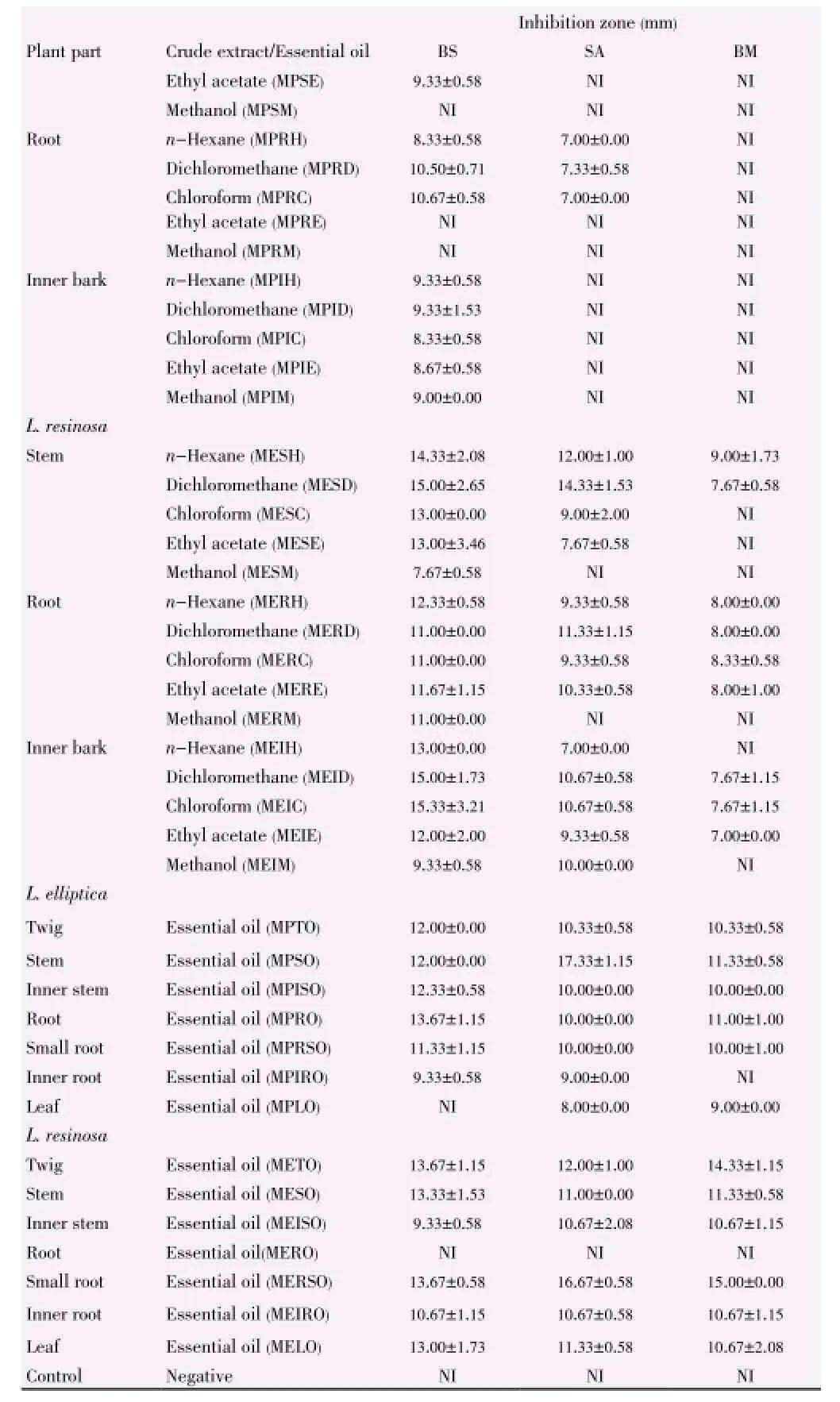
Table 6 Inhibition zones of L. elliptica and L. resinosa towards Gram-positive bacteria strains.
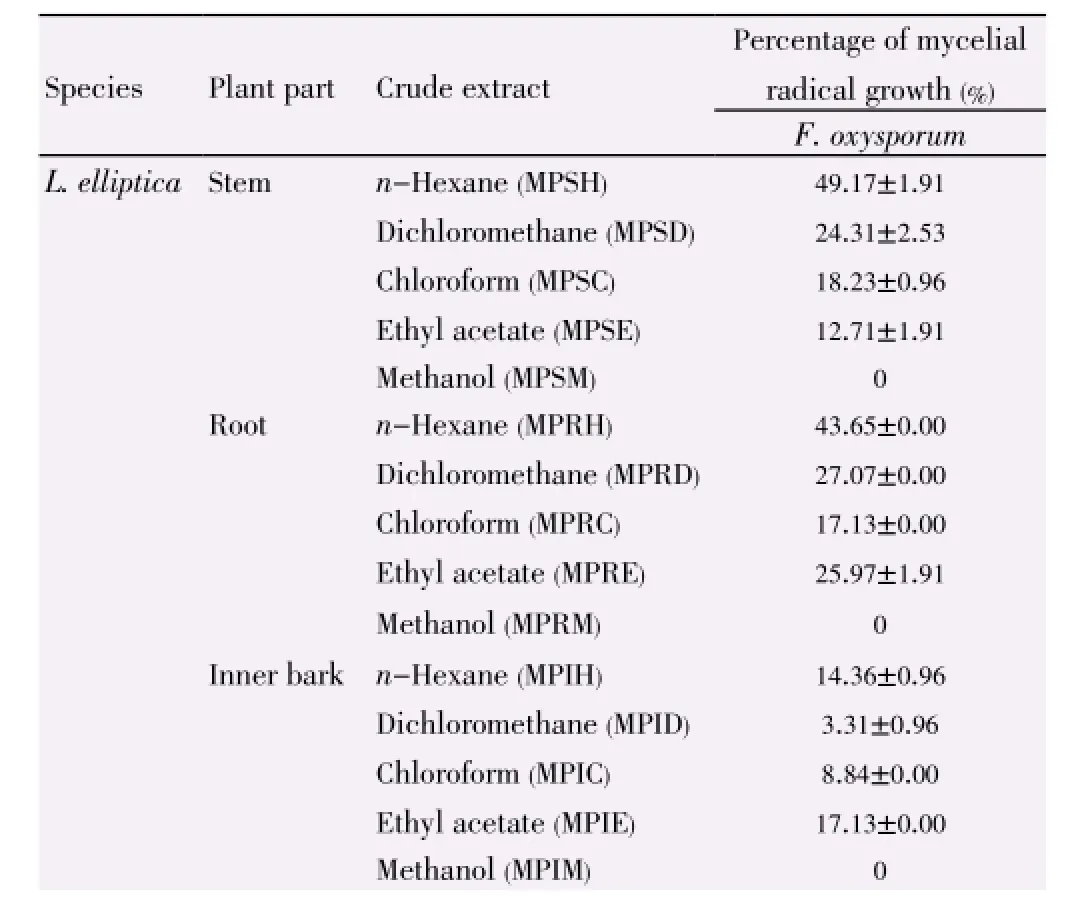
Table 7 Antifungal activity of L. elliptica and L. resinosa towards F. oxysporum.
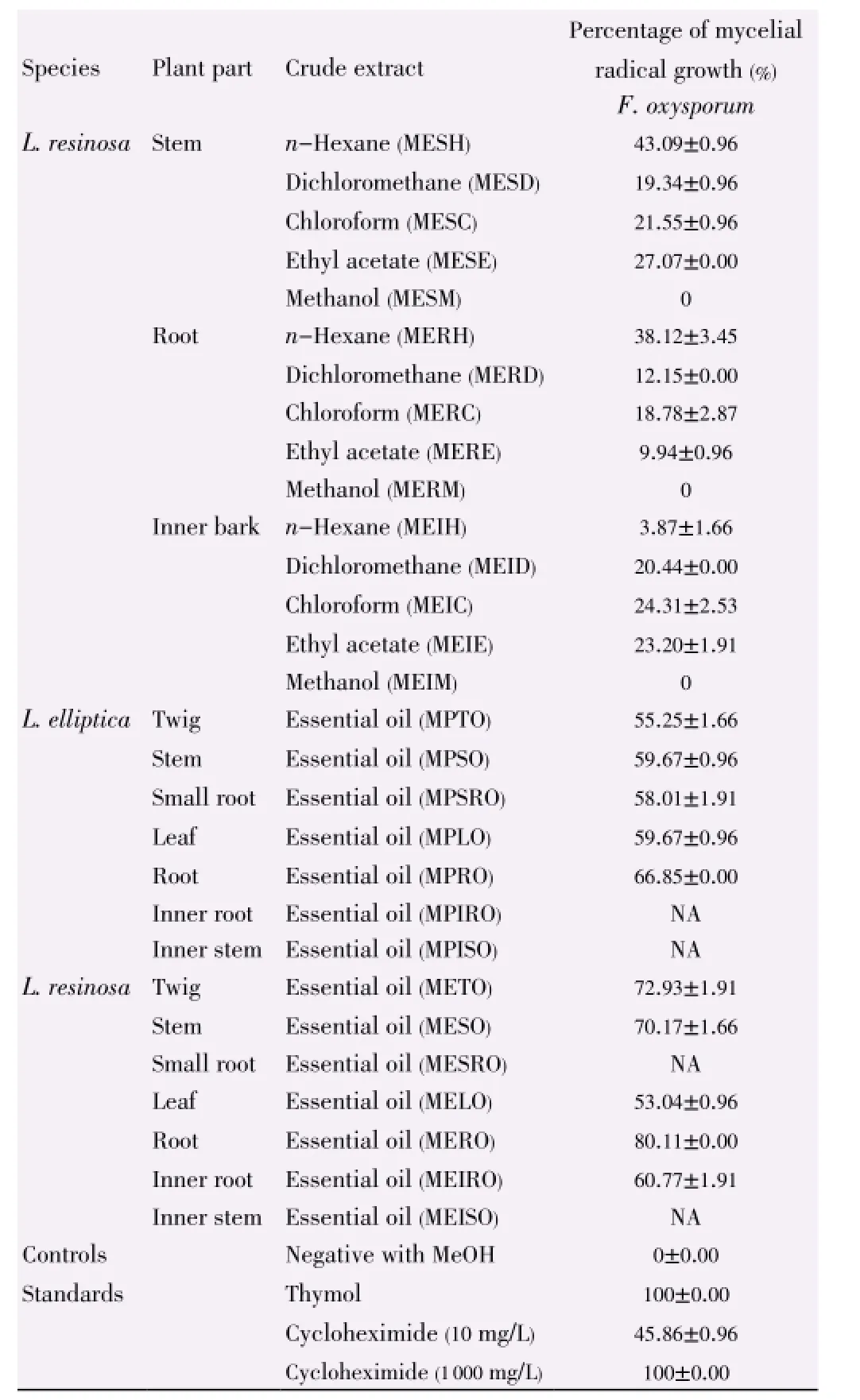
Table 7 Antifungal activity of L. elliptica and L. resinosa towards F. oxysporum.
Both extracts ofL. resinosaandL. ellipticashowed different level of antifungal activities against test strains as stated in Table 7. Unlike the crude extracts, essential oils from the root ofL. resinosaandL. ellipticahave the highest inhibition activities in this assay, which is (80.11±0.00)% and (66.85±0.00)% respectively.
4. Discussion
From the DPPH assay, the absorbance of samplereagent mixture varied inversely with the free radical scavenging and antioxidant activity. This is due to the fact that absorbance decreases when antioxidant donates proton to DPPH radical. Results showed that methanol extracts from root ofL. resinosaandL. ellipticashowed stronger scavenging activity than standard BHT with EC50value of 28.18 mg/L. This indicates that methanol extracts of root and stem from both species might containantioxidant agents which are useful as potential sources for natural antioxidants that are comparable to the synthetic antioxidant, BHT.
Alln-hexane and most dichloromethane extracts of both species possessed weak antioxidative properties with EC50values higher than 1 000 mg/L. The activity increases as the concentration of the samples increases but is varied inversely to the EC50values. Polarity and characteristics of solvent used in extraction influence the antioxidant activity due to the variation in chemical composition, compounds solubility and content of the extracts obtained. In addition, scavenging behavior is affected by concentrations, types of extracts, plant parts used and species tested.
Generally, Gram positive bacteria have higher susceptibility than Gram negative bacteria due to the lack of lipopolysaccharides and protein cell wall structures. However, in this study, Gram negative bacteria tested showed greater susceptibility in the plant extracts antibacterial assay. Gram positiveP. aeruginosashowed the most notable results (> 95% of the plant extracts) towards bacteria through the inhibition as shown in Table 3 due to its restrictive external membrane barrier[25]. This might be due to the difference in concentrations of extracts, plant types[26] or permeability barrier by cell wall[27]. This is supported by the studies carried out on major Australian native plant by Palombo and Semple (2001) which indicated significant results towards Gram negative bacteria instead of Gram positive bacteria[28].
Test strains showed higher sensitivity towards hexane, dichloromethane and chloroform extract than methanol extract. There might be some active compounds present in these plants that are less polar and readily dissolved in these solvents. Of all the plant parts, extracts from stem of both species showed the most significant activity towards test strains. Negative results do not mean that the bioactive compound is absent or not bioactive. This phenomenon can be explained by the insufficient amount or concentration of active phytocompounds present in that particular plant extracts[4,29]. Thus, these compounds can be further accumulated and studied. Besides, the plant extracts might be active towards other bacterial strains rather than those tested[29].
Essential oils from both species showed greater activities as compared with crude extracts in antifungal assay. This matched with the studies carried out on essential oils ofLitseaspp. such asLitsea kostermansii[30] andLitsea akoensis[31] demonstrating excellent antibacterial or antifungal activities. Mycelial radial growth assay for both species showed that hexane extracts showed the strongest inhibitory activities whilst methanol extracts from every part of the plants have weak or no antifungal activities towards the test strainF. oxysporum. Noteworthy, it was the essential oil from root and stem extracts from both plants exhibiting greater antifungal activities.
The overall results suggest that the less polar compounds in both species have the potency to be attributed as novel antimicrobial products while extracts with higher polarity may be an alternative in substituting the current synthetic antioxidant which will be harmful to health in long term.
Further studies such as identification and accumulation of phytocompounds and antioxidative agents can be performed to observe their mechanism of activities.
In conclusion, the methanol extracts of root and stem of bothL. ellipticaandL. resinosashowed significant scavenging activity in comparison with the standard BHT. The extracts can be the potential sources for natural antioxidants that are comparable to the synthetic antioxidant.L. resinosashowed strong antibacterial activities compared toLitsea elipticaand less polar extracts such as hexane and dichloromethane extracts showed significant activities as compared to methanol extracts which is polar. Essential oil of both species showed great potential in inhibiting fungusF. oxysporum.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was funded by Universiti Malaysia Sarawak (UNIMAS) research grant E14052-F07-49-792/2011(2), and scholarship to the postgraduate by Universiti Teknologi MARA (UiTM).
Comments
Background
Microorganisms are responsible for many diseases and there is a need for discovery of products from natural sources with anti-microbial properties. In addition, antioxidants from natural sources could be a substitute to current synthetic antioxidants which may have undesirable side effects.
Research frontiers
The present research shows different solvents extracts of different parts ofL. ellipticaandL. resinosain antioxidant activity, anti-bacterial activities and anti-fungal activity of essential oil of both species.
Related reports
The toxicity effects ofL. ellipticawere studied (Siti Nor Ainet al., 2011)[17]. Large amount of essential oil constituents ofL. resinosahas been studied but not their activities[18].
Innovations and breakthroughs
Authors have demonstrated extracts of root and stem of bothL. ellipticaandL. resinosawhich showed significant scavenging activity in modified DPPH radical scavenging antioxidant assay.
Applications
L. ellipticawas shown to be not toxic (Siti Nor Ainet al.,2011)[17] and the present study supported the use of this plant extract as an antioxidant.
Peer review
Results of this study are important contributions to the body of knowledge. It has been shown that the methanol extracts of root and stem of bothL. ellipticaandL. resinosashowed significant scavenging activity andL. resinosashowed strong antibacterial activities compared toL. elipticaand less polar extracts such as hexane and dichloromethane extracts showed significant activities as compared to methanol extracts which is polar. Essential oil of both species also showed great potential in inhibiting fungusF. oxysporum.
[1] Tanaka H, Yatsuhashi S, Yasuda T, Sato M, Sakai E, Xiao C, et al. A new amide from the leaves and twigs of Litsea auriculata. J Nat Med 2009; 63: 331-334.
[2] Hosamath PV. Evaluation of antimicrobial activity of Litsea glutinosa. Int J Pharm Appl 2011; 2(1): 105-114.
[3] Kofi A, Stephen G, Francis A. Antibacterial and radical scavenging activity of fatty acids from Paullinia pinnata L. L Pharmacogn Mag 2009; 5(19): 119-123.
[4] Delahaya C, Rainford L, Nicholson A, Mitchell S, Lindo J, Ahmad M. Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. J Med Biol Sci 2009; 3(1): 1-7.
[5] Feng T, Zhang RT, Tan QG, Zhang XY, Liu YP, Cai XH, et al. Two new isoquinoline alkaloids from Litsea cubeba. Z Naturforsch 2009; 64: 871-874.
[6] Cheng MJ, Wang TA, Lee SJ, Chen IS. A new butanolide and a new secobutanolide from Litsea lii var. nunkao-tahangensis. Nat Prod Ros 2010; 24(7): 647-656.
[7] Wang YS, Huang R, Lu H, Li FY, Yang JH. A new 2’-oxygenated flavone glycoside from Litsea glutinosa (Lour.) C. B. Rob. Biosci Biotechnol Biochem 2010; 74(3): 652-654.
[8] Pan JY, Zhang S, Jun W, Li QX, Xiao ZH. Litseaglutinan A and lignans from Litsea glutinosa. Helv Chim Acta 2010; 93(5): 951-957.
[9] Agrawal N, Choudhary AS, Sharma MC, Dobhal MP. Chemical constituents of plants from the genus Litsea. Chem Biodivers 2011; 8: 223-243.
[10] Chowdhury JU, Bhuiyan NI, Nandi NC. Aromatic plants of Bangladesh: Essential oils of leaves and fruits of Litsea glutinoosa (Lour.) C.B, Robinson. Bangladesh J Bot 2008; 37(1): 81-83.
[11] Wang H, Liu Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem Biodivers 2010; 7: 229-235.
[12] Chang SY, Cheng MJ, Kuo YH, Lee SJ, Chang HS, Chen IS. Secondary metabolites from the stem bark of Litsea akoensis and their cytotoxic activity. Helv Chim Acta 2008; 91(6): 1156-1165.
[13] Hosseinzadeh M, Mohamad J, Khalilzadeh MA, Zardoost MR, Haak J, Rajabi M. Isolation and characterization of bioactive compounds from the bark of Litsea costalis. J Photochem Photobiol B 2013; 128: 85-91.
[14] Jia XJ, Dong LH, Yang Y, Yuan S, Zhang ZW, Yuan M. Preliminary structural characterization and antioxidant activities of polysaccharides extracted from Hawk tea (Litsea coreana var. lanuginosa). Carbohydr Polym 2013; 95: 195-199.
[15] Devi P, Meera R. Study of antioxdant, anti-inflammatory and wound healing activity of extracts of Litsea glutinosa. J Pharm Sci Res 2010; 2(2): 155-163.
[16] Guzmán-Gutiérrez SL, Navarrete A. Pharmacological exploration of the sedative mechanism of hesperidin indentified as the active principle of Citrus sinensis flowers. Planta Med 2009; 75: 295-301.
[17] Silva KA, Klein Junior LC, Cruz SM, Cáceres A, Quint?o NL, Monache FD, et al. Anti-inflammatory and anti-hyperalgesic evaluation of the condiment laurel (Litsea guatemalensis Mez.) and its chemical composition. Food Chem 2012; 132(4): 1980-1986. [18] Masran SN, Salji MR, Othman H, Budin SB, Taib IS. Acute toxicity (oral) information of Litsea elliptica Blume essential oil. Int Conference Biosci Biochem Bioinf 2011; 5: 399-403.
[19] Ahmad FB, Jantan IB, Bakar BA, Ahmad AS. A comparative study of the composition of the leaf oils of three Litsea species from Borneo. J Essent Oil Res 2005; 17(3): 323-326.
[20] Tyagi AK, Malik A. Antimicrobial potential and chemical composition of Eucalyptusglobulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem 2011; 126: 228-235.
[21] Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, et al. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res 2002; 519: 103-119.
[22] Lin CT, Chu FH, Tseng YH, Tsai JB, Chang ST, Wang SY. Bioactivity investigation of Lauraceae trees grown in Taiwan. Pharm Biol 2007; 45(8): 638-644.
[23] Wang SY, Kuo YH, Chang HN, Kang PL, Tsay HS, Lin KF, et al. Profiling and characterization antioxidant activities in Anoectochilus formosanus hayata. J Agric Food Chem 2002; 50: 1859-1865.
[24] Tan M, Zhou L, Huang Y, Hao X, Wang J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globules Labil. Nat Prod Res 2008; 22(7): 569-575.
[25] Mann CM, Cox SD, Markham JL. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett Appl Microbiol 2000; 30: 294-297.
[26] Cock IE. Antibacterial activity of selected Australian native plant extracts. Internet J Microbiol 2008; 4(2): 1-8.
[27] Wei LS, Musa N, Sengm CT, Wee W, Shazili NA. Antimicrobial properties of tropical plants against pathogenic bacteria isolated from aquatic organisms. Afr J Biotechnol 2008; 7(13): 2275-2278.
[28] Palombo EA, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. J Ethnopharmacol 2001; 77: 151-157.
[29] Parekh J, Chanda SV. Antibacterial activity of aqueous and alcoholic extracts of 34 Indian medicinal plants against some Staphylococcus species. Turk J Biol 2008; 32: 63-71.
[30] Ho CL, Wang EI, Hsu KP, Lee PY, Su YC. Composition and antimicrobial activities of the leaf essential oil of Litsea kostermansii from Taiwan. Nat Prod Commun 2009; 4(8): 1123-1126.
[31] Ho CL, Lin CY, Wang EI, Su YC. Composition, antioxidant and antimicrobial activities of leaf and twig essential oils of Litsea akoensis from Taiwan. Nat Prod Commun 2011; 6(6): 901-904.
10.12980/APJTB.4.2014C1129
*Corresponding author: Wong Mui Hung, Faculty of Applied Sciences, Universiti Teknologi MARA Sarawak; Samarahan Campus, Jalan Meranek, 94300 Kota Samarahan, Sarawak, Malaysia.
Tel: (+6082) 677439
Fax: (+6082) 677300
E-mail: Wongmh@sarawak.uitm.edu.my, wongmuihung@gmail.com
Foundation Project: Funded by Universiti Malaysia Sarawak (UNIMAS) research grant E14052-F07-49-792/2011(2) and scholarship to the postgraduate by Universiti Teknologi MARA (UiTM).
Article history:
Received 13 Feb 2014
Received in revised form 23 Feb, 2nd revised form 3 Mar, 3rd revised form 13 Mar 2014
Accepted 12 Apr 2014
Available online 28 May 2014
Methods:In vitro method -2,2-diphenyl-1-picrylhydrazyl radical scavenging assay was conducted for antioxidant activity determination while antimicrobial assay consisted of agar well diffusion assay and mycelial radial growth assay.
Results:Methanol extracts of root and stem of L. elliptica and L. resinosa exhibited the highest antioxidant activity with EC50of 23.99, 41.69, 11.22 and 35.48 mg/L respectively. All methanol extracts of L. resinosa as well as root extracts from L. elliptica showed significant scavenging activity. Hexane extract from stem of L. resinosa presented the largest inhibition zone in Gramnegative bacteria Pseudomonas aeruginosa and Escherichia coli while chloroform extract from inner bark of L. resinosa showed major inhibition towards Gram-positive bacteria Bacillus subtilis. Essential oils from the root of both species showed significant antifungal activities which are 80.11% and 66.85% respectively.
Conclusions:Overall, methanol extracts from root and stem of both species showed antioxidant activity comparable to standard butylated hydroxytoluene. Extracts from L. resinosa demonstrated stronger antimicrobial properties compared to that from L. elliptica.
 Asian Pacific Journal of Tropical Biomedicine2014年5期
Asian Pacific Journal of Tropical Biomedicine2014年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ethnobotanical survey of folklore plants used in treatment of snakebite in Paschim Medinipur district, West Bengal
- Pharmacognostic studies of stem, roots and leaves of Malva parviflora L.
- Rapid detection of coliforms in drinking water of Arak city using multiplex PCR method in comparison with the standard method of culture (Most Probably Number)
- Salvia fruticosa reduces intrinsic cellular and H2O2-induced DNA oxidation in HEK 293 cells; assessment using flow cytometry
- Antisickling activity of butyl stearate isolated from Ocimum basilicum (Lamiaceae)
- Tamarind seed coat extract restores reactive oxygen species through attenuation of glutathione level and antioxidant enzyme expression in human skin fibroblasts in response to oxidative stress
