Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
Laboratory of Applied Microbiology in Food, Biomedical and Environment (LAMAABE), Aboubekr Belka?d University, PO Box 9, 3000 Tlemcen, Algeria
2Laboratory of Natural Products Chemistry, University of Corsica, UMR CNRS 6134, Campus Grimaldi, BP 52, 20250 Corte, France
Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
Fethi Benbela?d1, Abdelmouna?m Khadir1, Mohamed Amine Abdoune1, Mourad Bendahou1*, Alain Muselli2, Jean Costa2
1Laboratory of Applied Microbiology in Food, Biomedical and Environment (LAMAABE), Aboubekr Belka?d University, PO Box 119, 13000 Tlemcen, Algeria
2Laboratory of Natural Products Chemistry, University of Corsica, UMR CNRS 6134, Campus Grimaldi, BP 52, 20250 Corte, France
PEER REVIEW
Peer reviewer
Malinee Pongsavee, Ph.D., Associate Professor, Department of Medical Technology, Faculty of Allied Health Sciences, Thammasat University, Rangsit Campus Patumthani 12121, Thailand.
Tel: 662-9869213 ext.7252
Fax: 662-5165379
E-mail: malineep@tu.ac.th
Comments
This study evaluated some EOs in treatment of intractable oral infections, principally caused by biofilm of multidrug-resistant E. faecalis. The results of this study is useful for E. faecalis infection treatment. The high yield and strong antimicrobial activity of three Algerian medicinal plants EOs used in eradication of MDR pathogens from oral ecosystem may contribut to the medical treatment for oral intractable infections caused by E. faecalis.
Details on Page 471
Objective:To evaluate some essential oils in treatment of intractable oral infections, principally caused by biofilm of multidrug-resistant Enterococcus faecalis (E. faecalis), such as persistent endodontic infections in which their treatment exhibits a real challenge for dentists.
Bacterial infections, Biofilm, Enterococcus faecalis, Essential oils, Multidrug-resistance
1. Introduction
Enterococci are commensal Gram-positive bacteria that inhabit in oral cavity, gastrointestinal tract, and vagina of humans and animals[1]. These bacteria can cause a wide variety of diseases in humans, especially, nosocomial infections and they now rank among the leading causative pathogens in the world[2].Enterococcus faecalis(E. faecalis) is responsible for up to 90% of human enterococcal infections[3], its pathogenicity ranges from life threateningdiseases in compromised individuals to less severe conditions particularly due to many virulence factors[4]. Enterococci are multidrug-resistant (MDR) bacteria to most antimicrobial drugs used to treat human infections which exhibit a considerable therapeutic challenges[5].
In oral cavity,E. faecalisis not considered to be part of the normal oral microbiota[6].E. faecalisis mainly responsible for several oral pathologies, particularly, dental caries[7], dental abscess[8], periodontal infections[9], apical periodontitis[10], and persistent endodontic infections, also known as post-treatment endodontic diseases, in whichE. faecalisis the etiological causative agent and responsible for serious complications[11,12]. This can be explained by the fact that this bacterium possesses not only many virulence factors, but also an endogenous resistance to extreme ecological conditions and antimicrobials[8], allowingE. faecalisto tolerate harsh environmental conditions in some sites within oral cavity, especially in root canal[11].
The resistance of microorganisms to harsh conditions is due to biofilm formation[13], a complex of lifestyle that allowing bacteria displaying specific properties, including an increase in resistance to antibiotics and antiseptic chemicals[14]. In fact, formation of these sessile communities and their inherent resistance to biocides are the origin of many persistent and chronic bacterial infections[15]. In dental root canal, eradication ofE. faecaliswith chemomechanical preparations and using antiseptics is difficult[11]. Even the most used antiseptics in endodontic treatments, sodium hypochlorite and chlorhexidine showed low ability to eliminateE. faecalis[16].
The lack of strategies forE. faecalisbiofilm elimination requires trying other substances except antiseptics and antibiotics, such as secondary metabolites of plants, especially, essential oils (EOs) one of the most important bioactive substances in medicinal plants[17]. Possessing a good antimicrobial activity[18], EOs can replace treatments with antibiotics and disinfection using antiseptics. Furthermore, EOs have many interesting medicinal properties which can contribute to the treatment of intractable oral infections such as anti-inflammatory[19,20], anti-oxidant and stimulating the immune system response activities[21,22].
Treatment of oral infections by plant preparations, such as decoctions and infusions, is very popular among Arab peoples. Major reason of using those herbs extracts is their effectiveness and availability. In Algeria, many herbs especially from Lamiaceae family are widely used in treatment of oral diseases such as candidiasis, dental caries and periodontal diseases. Present principally in wild, species of thyme, lavender, oregano and rosemary are even applied by local population as antiseptics and for oral cavity aromatization because of their refreshing scent.
In the lack of studies that evaluate EOs as treatments of intractable oral infections, such as persistent endodontic infections, the aim of this study was to evaluate some Algerian EOs as natural antiseptics and antimicrobials against MDRE. faecalis, one of the principal oral pathogens, in both planktonic and biofilm state.
2. Materials and methods
2.1. Plant material
We have selected ten medicinal plants for this study which are presented in Table 1. The choice of plant species is based on their use by the local population against oral infections, such as periodontal infections and dental caries. All species have been harvested from the region ofTlemcen located in the northwestern Algeria, from Jun 2011 to May 2012, in the wild and others cultivated. Specimens of all species in this study were identified by Laboratory of Ecology and Management of Natural Ecosystems, University of Tlemcen. All voucher specimens were deposited in our laboratory.
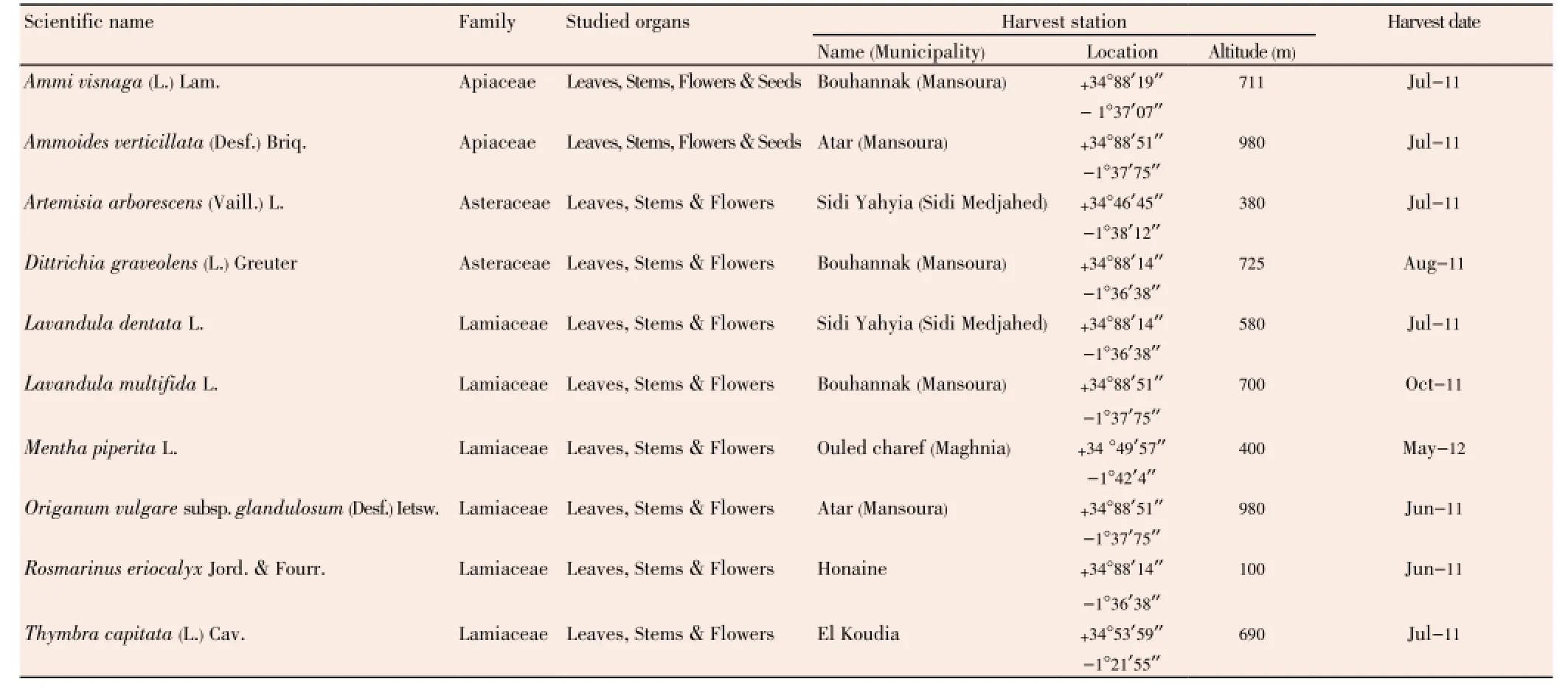
Table 1 Data on the studied plant material.
2.2. ObtainingEOs
For this purpose, we have used hydrodistillation with Clevenger-type apparatus of the fresh plant material, as recommended by Benbela?det al[23]. Recovered EOs were dried using magnesium sulfate and stored in smoked vials at 4 °C until analysis.
2.3.EOs analysis withGCandGC/MS
Gas chromatography (GC) analysis was performed using a Perkin Elmer Autosystem GC-type chromatograph, equipped with two flame ionization detectors, for the detection of volatile compounds, one injector/splitter, and two polar (Rtx-Wax, polyethylene glycol) and nonpolar (Rtx-1, polydimethylsiloxane) columns (60 m×0.22 mm inner diameter, film thickness 0.25 μm). The carrier gas was helium (1 mL/min) with a column head pressure of 25 psi. The injector temperature was 250 °C and that of the detector was 280 °C. The temperature was programmed to increase from 60 to 230 °C at the rate of 2 °C/min, and then maintained constant for 45 min at a level of 230 °C. The injection was done by split mode with a split ratio of 1/50. The amount of EO injected was 0.2 μL. Quantification was made by direct electronic integration of peak areas.
For the gas chromatography-mass spectrometry (GC/MS), analysis was performed using a Perkin Elmer Autosystem XL chromatograph, with an automatic injector and two polar (Rtx-Wax) and nonpolar (Rtx-1) columns (60 m×0.22 mm inner diameter, film thickness 0.25 μm), coupled with a Perkin Elmer TurboMass mass detector. The carrier gas was helium (1 mL/min) with a column head pressure of 25 psi. The injector temperature was 250 °C. The temperature was programmed to rise from 60 to 230 °C at the rate of 2 °C/min, and then kept constant for 35 min at a level of 230 °C. The injection was done by split mode with a split ratio of 1/80. The amount of EO injected was 0.2 μL. Detection was carried out by a quadrupole analyzer which consisted of an assembly of four parallel electrodes with cylindrical section. The source temperature was 150 °C. The device functioned in electron impact and fragmentation was performed at an electric field of 70 eV. The resulting mass spectra were acquired over the mass range of 35-350 Da.
To identify the constituents of the studied EOs, identification made by Kovats index was used, where the polar and nonpolar retention indices were calculated from the retention times of a series ofn-alkanes, and from databases of mass spectra, where the obtained mass spectra were compared with those of computerized libraries[24].
2.4. Microbial strains
Seven strains ofE. faecalishave been selected for this study; two of them are American Type Culture Collection strains with codes ATCC 29212 and ATCC 49452 (sensitive to antibiotics), while the rest are multidrug-resistant strains, selected from a collection of clinicalE. faecalisstrains obtained from patients with various oral infections, including, apical periodontitis, chronic periodontitis, aggressive periodontitis, and cervicofacial cellulitis which are summarized in Table 2. Strain samples were taken in the service of stomatology at University Health Center of Tlemcen from December 2011 to June 2012. At the first time, samples were enriched in Roth broth (Conda PronadisaTM, Spain) at 37 °C for 18 h. Thus, positive culture was inoculated in bile esculin agar (Fluka?, Switzerland) and incubated at 37 °C for 24 h, in order to isolate pure colonies. After purification, all isolated strains ofE. faecaliswere firstly identified by conventional microbiological methods, while the final identification confirmation was carried out by API Strep? gallery (BioMérieux?, France). Finally, allE. faecalisstrains were conserved in brain heart infusion broth (Conda PronadisaTM, Spain) with glycerol (Fluka?, France) (8:2,v/v) at -20 °C.
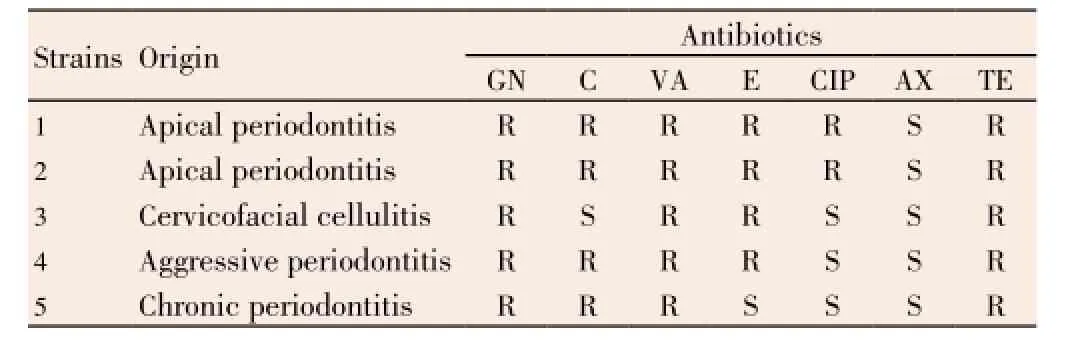
Table 2 Data on studied E. faecalis strains.
2.5. Antibiogram
For selection ofE. faecalismultidrug-resistant strains from clinical collection, we have performed an antibiogram according to Clinical and Laboratory Standards Institute (CLSI) recommendations[25]. Antibiogram was determined with the following antimicrobial agent-containing disks: amoxicillin (25 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), erythromycin (15 μg), gentamicin (15 μg), tetracycline (30 μg) and vancomycin (30 μg) (Oxoid?, England).
2.6. Antimicrobial assay
2.6.1. Inocula preparationPreviously identified and conserved strains were taken andinoculated in Mueller-Hinton broth (Fluka?, India). After incubation at 37 °C for 24 h, suspensions were taken and shaken well using the vortex then diluted for standardizing. Inocula were set to 0.5 McFarland or an optical density from 0.08 to 0.13 at 625 nm wavelength, which corresponds to 108CFU/mL[25].
2.6.2. Antimicrobial activity ofEOs against planktonic E. faecalis strains
2.6.2.1. Disk diffusion method
We have used Kirby-Bauer’s agar disk diffusion modified method[26], where antimicrobial activity of EOs againstE. faecalisstrains was evaluated in plates with Mueller-Hinton agar (Fluka?, India) pre-inoculated by swabbing of standardized microbial suspension at 108CFU/mL. Whatman No. 3 paper disks impregnated with 10 μL of EO, were placed on the surface of agar, each disk has a 6 mm diameter. After incubation at 37 °C for 24 h, the results were read by measuring the diameter of inhibition zones in millimeters (mm) by vernier scale. All tests were performed in triplicate.
2.6.2.2.MICdetermination
The minimum inhibitory concentrations (MICs) of EOs were determined by modified broth micro-dilution method from Wiegandet al[27]. Briefly, concentrations of each EO were prepared by 1/2 dilution series in Mueller-Hinton broth with 1% of Tween 80, starting from 40.00% to 0.08%. After that, 96-well microplate was filled by distributing 90 μL of 5× 105CFU/mL inoculum (prepared by 1/200 dilution of 108CFU/ mL inoculum) with 10 μL of each concentration. The final concentration of EOs in wells was ranging from 4% to 0.008%, and the final concentration of Tween 80 was 0.1% in each well. After 24 h incubation at 37 °C, the MIC was defined as the lowest concentration of EO inhibiting visible growth. In addition, two wells of every range of microplate were filled with inoculum and inoculum with 0.1% of Tween 80, and served as positive controls for each strain. All tests were performed in triplicate.
2.6.3. Antimicrobial activity ofEOs against E. faecalis strains in biofilm
2.6.3.1. Biofilm inhibitory concentration
The biofilm inhibitory concentrations (BICs) of studied EOs againstE. faecalisstrains in biofilm case were determined as described by Nostroet al.[28] with modification. Firstly, 96-well microplate was filled by distributing 100 μL of inoculum at 108CFU/mL in each well. After 24 h of incubation at 37 °C, medium was gently removed and all wells were washed three times with sterile phosphate buffered saline. In the same moment, ten concentrations of each EO were prepared by 1/2 dilution series in Mueller-Hinton broth with 3.33% of Tween 80, starting from 40.00% to 0.08%. After removing of planktonic cells from microplate, all wells were filled with 70 μL of sterile Mueller-Hinton broth with 30 μL of each concentration, the final concentrations of EO in wells were ranging from 12% to 0.02%, and the final concentration of Tween 80 was about 1% in each well. Biofilm inhibitory concentration was determined after 24 h incubation at 37 °C, as the lowest concentration with no culture in well, visually determined and confirmed by no increase in optical density compared with the initial reading. Two ranges of wells for each microplate were filled with sterile Mueller-Hinton broth and serve as positive controls. All tests were performed in triplicate.
2.6.3.2. Biofilm eradication concentration
Biofilm eradication concentration (BEC) is the lowest concentration of EO that kills all viable cells ofE. faecalispresent and protected in biofilm. BEC was defined also as described by Nostroet al.[28]with modification. Protocol was continued in the same 96-well microplate used in determination of BIC, in the same day where the result of BIC was read. Supernatant fluid was gently removed and the wells were well washed three times with sterile phosphate buffered saline and one time with 20% sterile ethanol solution (Scharlau?, Spain) (in order to eliminate remaining traces of EOs). Then, microplate wells were filled with sterile tryptic soy agar (Conda PronadiaTM, Spain) and incubated for 72 h at 37 °C. BEC was determined as the lowest concentration with no culture visually determined and confirmed by no increase in optical density compared with the initial reading. Positive control was performed to verify that 20% of ethanol has no effect on strains. All tests were performed in triplicate.
2.7. Statistical analysis
Statistical analyses used in this study have been carried out using Microsoft? Excel. Where, comparison between antimicrobial activity against reference and clinical strains was performed by Studentt-test at 95% level (P<0.05) in both planktonic and biofilm state.
3. Results
3.1. Chemical composition of studiedEOs
Quantitative and qualitative analytical results of the studied EOs by GC and GC/MS are shown in Table 3. In which we notice variability in composition of EOs between studied plants. EOs of species which belong to Lamiaceae and Apiaceae family are mainly rich in oxygenated monoterpenes, especially alcohols, such as thymol, carvacrol, and linalool with a high percentage.Thymbra
capitata(T. capitata) andLavandula multifida(L. multifida) are constituted principally by carvacrol (58.68% and 57.75%, respectively), beta-bisabolene (22.91%) is the second major component inL. multifidaEOs. Thymol followed by gamma terpinene and para-cymene are the main constituents in EO ofOriganum glandulosum(O. glandulosum) (41.62%, 27.03%, and 17.07%, respectively), and thymol followed by para-cymene and limonene in EO ofAmmoides verticillata(A. verticillata) (50.13%, 15.58%, and 15.02%, respectively). Linalool and linalyl acetate (51.59% and 21.12%, respectively) are the major constituents ofMentha piperita(M. piperita). While the rest of studied EOs are constituted principally by non-alcoholic terpenes, such as beta-thujone (47.58%) inArtemisia arborescens(A. arborescens), bornyl acetate (56.16%) inDittrichia graveolens(D. graveolens), 1,8-cineole (36.72%) inLavandula dentata(L. dentata), and camphor (35.50%) inRosmarinus eriocalyx(R. eriocalyx).
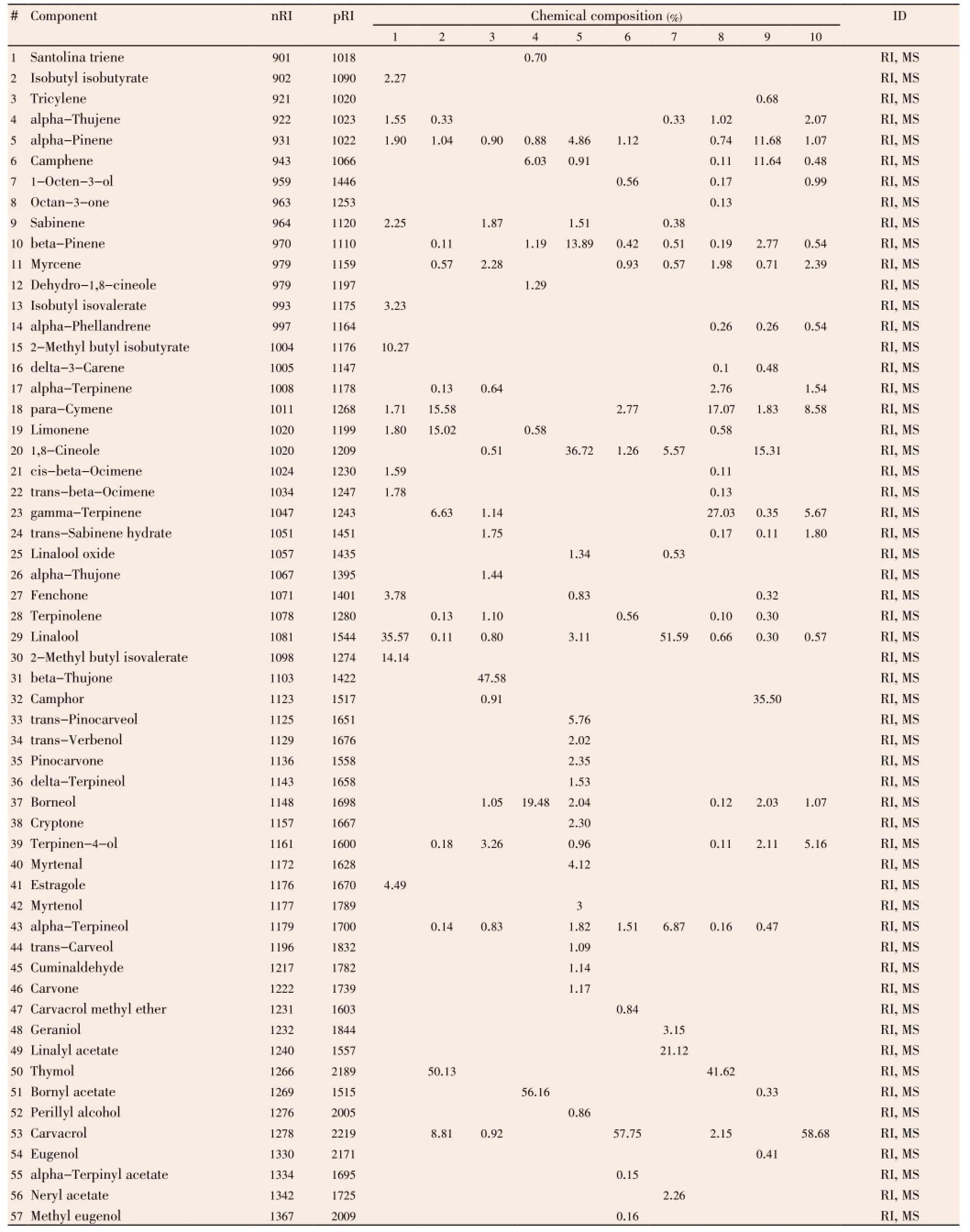
Table 3 Chemical composition of studied EOs.
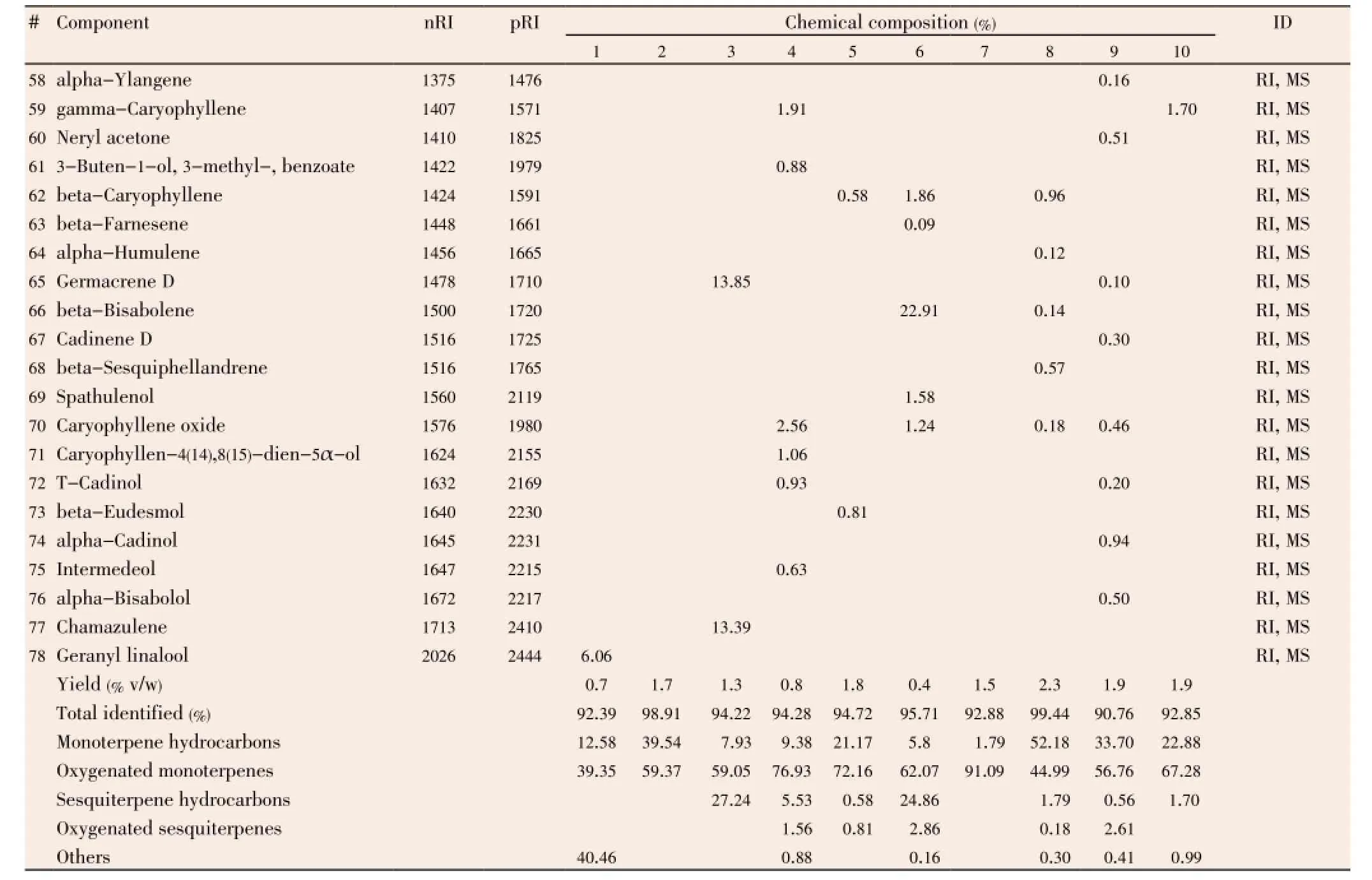
Table 3, continued Chemical composition of studied EOs.
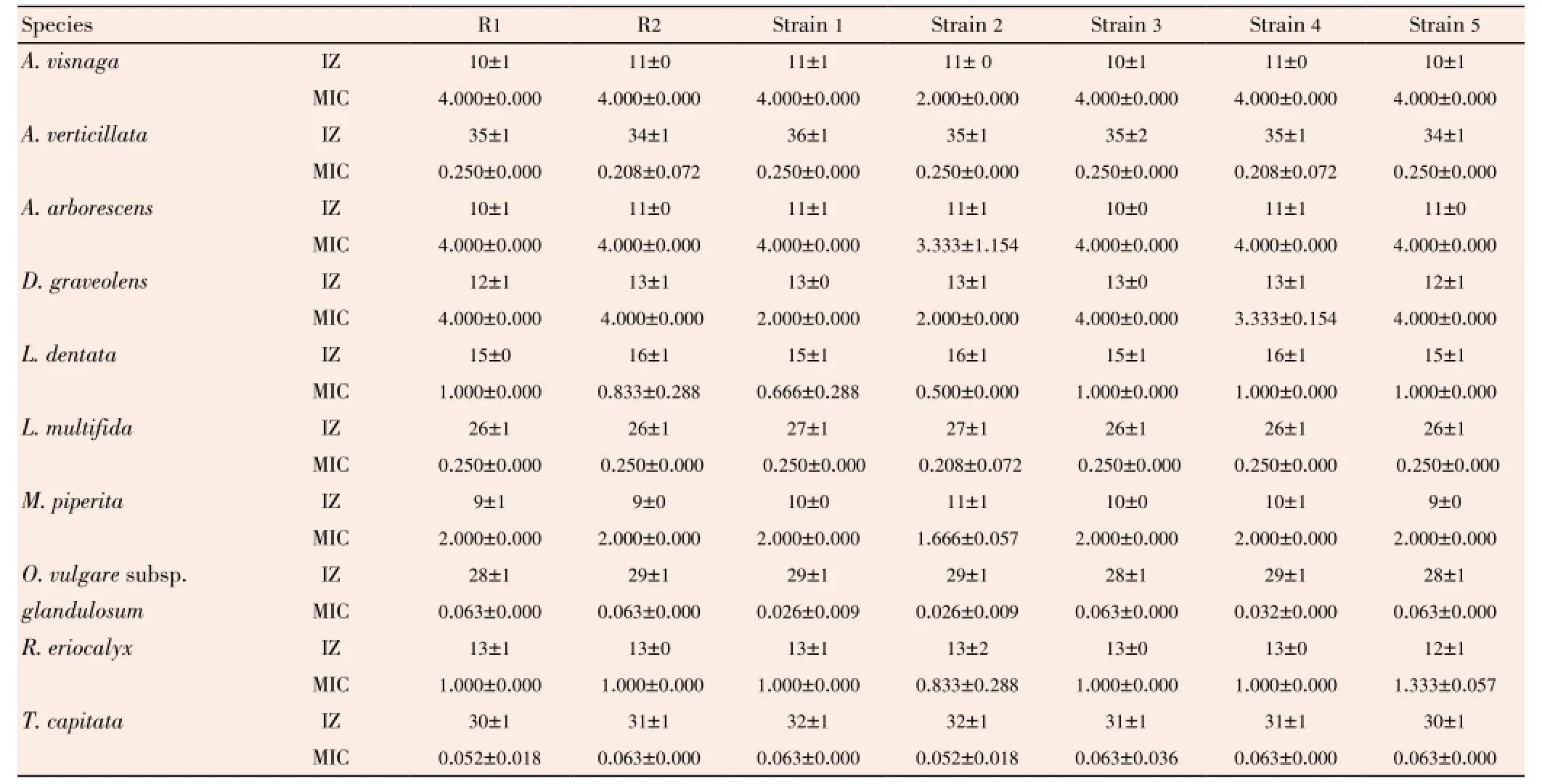
Table 4 Inhibition effect of studied EOs against planktonic E. faecalis strains. Expressed by the diameter inhibition zones (IZ in mm) and minimum inhibitory concentration (MIC in % v/v) methods.
3.2. Antimicrobial activity ofEOs againstMDR E. faecalis
During sampling at the Service of Dentistry at University Health Center of Tlemcen, we have remarked thatE. faecaliswas responsible for several oral complications, especially, apical periodontitis. Antibiogram results ofE. faecalisstrains have shown that almost all of these bacteria are multidrug-resistant to majority of antibiotics, especially vancomycin and gentamicin, only amoxicillin was effective on all theseE. faecalisstrains.
Antimicrobial activity evaluation of studied EOs againstE. faecalisstrains was very interesting, whether in planktonic or biofilm state. In planktonic, both methods, agar disk diffusion and MIC determination (Table 4), have shown that EOs ofT. capitataandO. glandulosumwere the most active, with inhibition zone larger than 27 mm and MIC less than 0.07% whether for susceptible or resistant strains. At less degree,A. verticillata,L. multifida,L. dentata, andM. piperitahave a good antimicrobial activity on all strains, while the rest of EOs have shown a moderate activity.
In biofilm state (Table 5), both EOs ofT. capitataandO. glandulosumwere also the effective oils, which can inhibit biofilm growth of allE. faecalisstrains at 0.75%, and can eradicate all viable microbial cells protected in biofilm at 1.50% only. Other EOs have shown an interesting antimicrobial activity againstE. faecalisstrains in biofilm, notablyA. verticillataandL. multifidawith their BECs range between 1.50% and 3.00%.
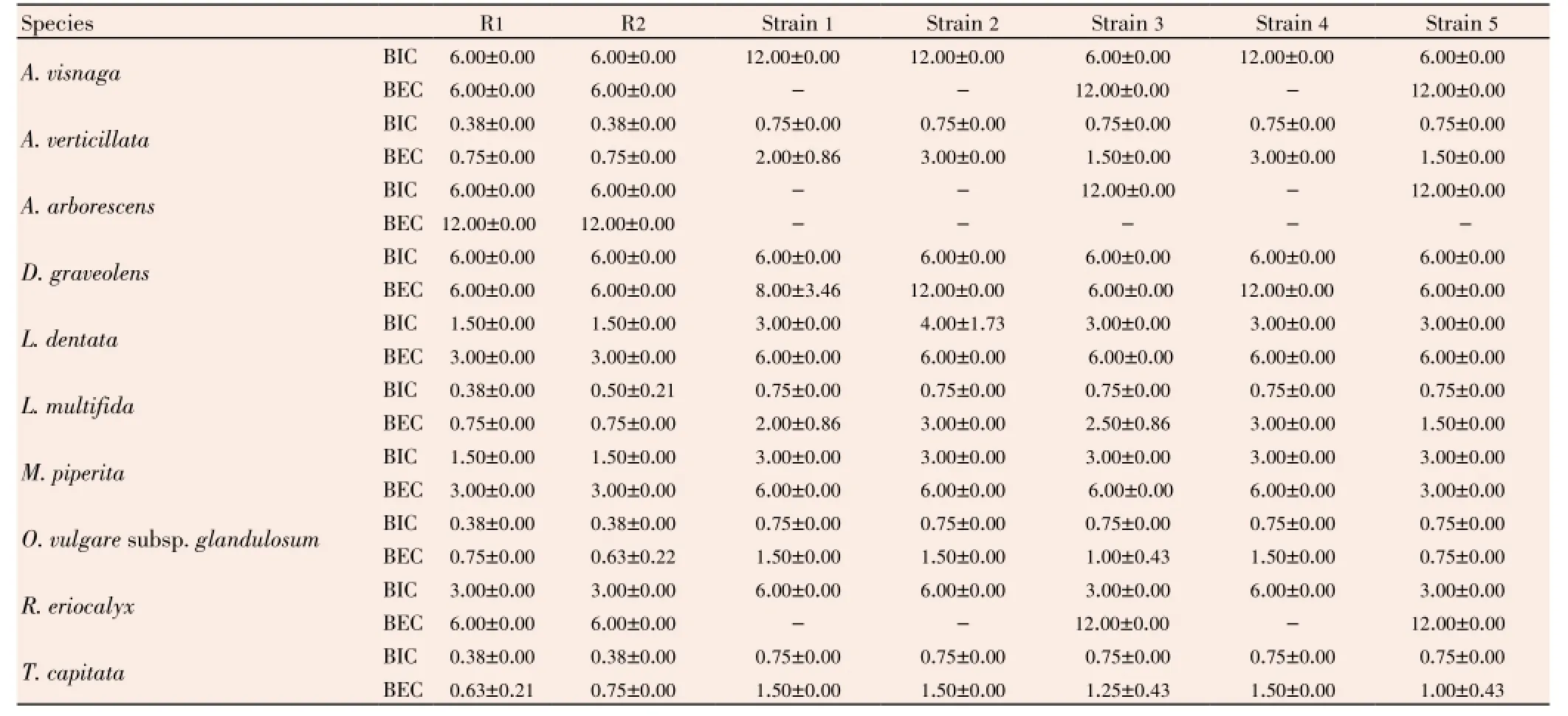
Table 5 Inhibition effect of studied EOs against strains of E. faecalis in biofilm. Expressed by biofilm inhibitory concentration (BIC in %v/v) and biofilm eradication concentration (BEC in %v/v) methods.
4. Discussion
In the present study, we have evaluated some Algerian EOs against oral MDRE. faecalis. The choice of this species is based on many factors. Firstly, because of its multiple virulence factors[29], the presence of this Gram-positive bacterium in the oral cavity has, in fact, a relation with several dental diseases, especially apical periodontitis[10]. In a research released by Salahet al.[30] allE. faecalisisolates in their study were recovered only from patients with dental diseases, especially necrotic pulps, while noE. faecalisstrains were found in healthy patients. Secondly,E. faecalisis a nosocomial bacterium which can resist against many antibiotics[31], chemo-mechanical preparations, andantiseptics, which represent a real challenge for dentists in some intractable infections, as seen in post-treatment endodontic diseases[11]. Thirdly,E. faecalishas an ability to form biofilm which seems to be a key factor in many bacterial infections and resistance of microorganisms to disinfections[13,32,33]. So, all these factors makeE. faecalisas one of the major pathogens in the oral cavity, with a high risk of treatment failure because of its resistance to both antibiotics and antiseptics. Therefore, elimination of this harmful bacterium from oral ecosystem needs other solutions.
As alternative to antibiotics and antiseptics, we have tested some EOs against oral multidrug-resistantE. faecalis, especially in biofilm state, which is the real existence state of this harmful bacterium in the oral cavity, and the reason for its ability to survive under severe conditions causing pathogenesis during chronic infections. Antimicrobial activity evaluation of studied EOs againstE. faecalisstrains was very interesting, whether in planktonic or biofilm state. When we compare the ability to totalE. faecaliseradication of studied EOs with well-used endodontic antiseptics, we find these EOs are competitive, especially in biofilm state. In study realized by Senaet al.[34], it was found that chlorhexidine at 2% and NaOCl at 5.25% can eradicateE. faecalis, on condition when supplemented with mechanical agitation. While, some authors indicate that chlorhexidine and NaOCl show a low ability to eliminateE. faecalis[16]. For example, Arias-Molizet al.[35] found that chlorhexidine at 4% did not eradicateE. faecalisbiofilm. So, we conclude that studied EOs are good alternative antiseptic which can be used instead of chlorhexidine or NaOCl, especially againstE. faecalisbiofilm.
Comparison of the antimicrobial effect of studied EOs, using Studentt-test at 95% confidence level (P<0.05), between sensitive and resistant strains have shown no difference in activity at planktonic state. While in biofilm state, Studentt-test at 95% confidence level (P<0.05) show a significant difference between strains, where studied EOs were more effective against sensitive strains ofE. faecalisthan clinical ones. This could explain that biofilm formation was prevalent among isolates with MDR phenotype[36], as well as the slow metabolic rate of microorganisms in biofilms[37], and the extracellular matrix of the biofilm impede the effectiveness of many antimicrobials, which deters these agents to gain the protected cell inside[38]. We concluded that MDR strains ofE. faecaliswere less sensitive to EOs mainly due to their high ability to biofilm formation. But even if there is a mild resistance of biofilm to EOs, these antimicrobial agents remained effective, especially those ofT. capitataandO. glandulosum.
A huge number of studies that evaluate the antimicrobial activity of many EOs were published in recent years, from which some authors concluded that antimicrobial activity of EOs was strongly correlated with the content of terpenoid phenols such as carvacrol, eugenol and thymol and some other oxygenated monoterpenes such as nerol, linalool, α-terpineol, fenchol and terpinen-4-ol[18,39,40]. In this study, we have found the same remark, where chemical analyses of studied EOs showed that among the most active EOs against MDRE. faecalisstrains,O. glandulosum,T. capitata,L. multifida, andA. verticillataEOs are constituted principally by terpenoid phenols, 41.62% thymol, 58.68% carvacrol, 57.75% carvacrol, and 50.13% thymol, respectively. WhileM. piperitawas constituted principally by oxygenated monoterpenes, 51.59% linalool. In the other hand, synergetic effect between all major compounds of EOs was reported in some studies, where EO was more antimicrobially active than its major compound that was responsible for activity. For example, in study realized by Veraset al.[41] they found that EO ofLippia sidoideswas more effective againstStaphylococcus aureusthan thymol alone, its major compound. In addition, Mulyaningsihet al.[42] found a good synergetic effect between compounds ofEucalyptus globulusEO against multidrugresistant bacteria,Staphylococcus aureusandEnterococcus faecalis. As well as, multiple combinations between terpenes were effective against microorganisms[43], the use of the entire EO as antiseptic in mouthwash seems to be better than use of those terpenes alone, in both antimicrobial activity and natural treatment.
In summary, the findings of this study indicate that EOs extracted from aromatic plants can be used in treatment of oral intractable infections caused byE. faecalis, especially persistent endodontic infections. Because of their high yield and strong antimicrobial activity in biofilm state, the activity of three Algerian medicinal plantsO. glandulosum,T. capitataandA. verticillataas solution used in eradication of MDR pathogens from oral ecosystem seems to be very important in both medical and economical point of view.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The Authors profusely thank Dr. Boumediene Medjahdi, Department of Agronomy and Forestry, Aboubekr Belka?d University of Tlemcen, for botanical assistance. This work was financially supported by the Ministry of Higher Education and Scientific Research of People’s Democratic Republic of Algeria (Grant No. B05/N125).
Comments
Background
Enterococci are the commensal Gram-positive bacteria that inhabit in oral cavity, gastrointestinal tract and vagina of humans and animals. Enterococci are multidrug-resistant bacteria to most antimicrobial drugs used to treat human infections and they exhibit a considerable therapeutic challenges.E. faecalisis responsible for several oral pathologies, particularly, dental caries, dental abscess, periodontal infectionsetc. The secondary metabolites of plants, especially, EOs are one of the most important bioactive substances in medicinal plants. Possessing a good antimicrobial activity, EOs can replace treatments with antibiotics and disinfection using antiseptics.
Research frontiers
Ten medicinal plants were studied in this research. The choice of plant species was based on their use by the local population against oral infections, such as periodontal infections and dental caries. Seven strains ofE. faecalishave been selected for this study; two of them were American Type Culture Collection strains with codes ATCC 29212 and ATCC 49452 (sensitive to antibiotics). While the rest were multidrug-resistant strains, selected from a collection of clinicalE. faecalisstrains obtained from patients with various oral infections, including apical periodontitis, chronic periodontitis, aggressive periodontitis, and cervicofacial cellulitis. Antimicrobial activity of EOs against planktonicE. faecalisstrains assay and antimicrobial activity of EOs againstE. faecalisstrains in biofilm assay were done.
Related reports
E. faecalisis the etiological causative agent where it’s responsible for serious complications. It is the fact that this bacterium possesses not only many virulence factors, but also an endogenous resistance to extreme ecological conditions and antimicrobials, allowing E.faecalis to tolerate harsh environmental conditions in some sites within oral cavity, especially in root canal. EOs have many interesting medicinal properties which can contribute to the treatment of intractable oral infections such as anti-inflammatory, anti-oxidant and stimulating the immune system response activities.
Innovations and breakthroughs
EOs extracted from aromatic plants in this study can be used in treatment of oral intractable infections caused byE. faecalis, especially persistent endodontic infections.
Applications
EOs are good alternative antiseptics which can be used instead of chlorhexidine or NaOCl, especially against MDRE. faecalisbiofilm. It might be applied to use in other virulence bacterial strains.
Peer review
This study evaluated some EOs in treatment of intractable oral infections, principally caused by biofilm of multidrugresistantE. faecalis. The results of this study is useful forE. faecalisinfection treatment. The high yield and strong antimicrobial activity of three Algerian medicinal plants EOs used in eradication of MDR pathogens from oral ecosystem may contribut to the medical treatment for oral intractable infections caused byE. faecalis.
[1] Wang QQ, Zhang CF, Chu CH, Zhu XF. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int J Oral Sci 2012; 4(1): 19-23.
[2] Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000; 21(8): 510-515.
[3] Teixeira LM, Merquior VLC. Enterococcus. In: De Filippis I, Mckee ML, editors. Molecular typing in bacterial infections. New York: Humana Press; 2013, p. 17-26.
[4] Kayaoglu G, ?rstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med 2004; 15(5): 308-320.
[5] Arias CA, Contreras GA, Murray BE. Management of multi-drug resistant enterococcal infections. Clin Microbiol Infect 2010; 16(6): 555-562.
[6] Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43(11): 5721-5732.
[7] Kouidhi B, Zmantar T, Mahdouani K, Hentati H, Bakhrouf A. Antibiotic resistance and adhesion properties of oral Enterococci associated to dental caries. BMC Microbiol 2011; 11(1): 155.
[8] R??as IN, Siqueira JF Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod 2004; 30(5): 315-320.
[9] Souto R, Colombo AP. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch Oral Biol 2008; 53(2): 155-160.
[10] Takahashi Y, Yoshida A, Nagayoshi M, Kitamura C, Nishihara T, Awano S, et al. Enumeration of viable Enterococcus faecalis, a predominant apical periodontitis pathogen, using propidium monoazide and quantitative real-time polymerase chain reaction. Microbiol Immunol 2011; 55(12): 889-892.
[11] Portenier I, Waltimo TMT, Haapasalo M. Enterococcus faecalisthe root canal survivor and ‘star’ in post-treatment disease. Endod Topics 2003; 6(1): 135-159.
[12] Zhu X, Wang Q, Zhang C, Cheung GSP, Shen Y. Prevalence,phenotype, and genotype of Enterococcus faecalis isolated from saliva and root canals in patients with persistent apical periodontitis. J Endod 2010; 36(12): 1950-1955.
[13] George S, Kishen A, Song P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod 2005; 31(12): 867-872.
[14] H?iby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010; 35(4): 322-332.
[15] Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284(5418): 1318-1322.
[16] Estrela C, Silva JA, De Alencar AH, Leles CR, Decurcio DA. Efficacy of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: a systematic review. J Appl Oral Sci 2008; 16(6): 364-368.
[17] Kalemba D, Matla M, Sm?tek A. Antimicrobial activities of essential oils. In: Patra AK, editor. Dietary phytochemicals and microbes. Germany: Springer; 2012, p. 157-183.
[18] Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 2007; 21(4): 308-323.
[19] Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules 2010; 15(12): 9252-9287.
[20] Mendes SS, Bomfim RR, Jesus HC, Alves PB, Blank AF, Estevam CS, et al. Evaluation of the analgesic and antiinflammatory effects of the essential oil of Lippia gracilis leaves. J Ethnopharmacol 2010; 129(3): 391-397.
[21] ?nan ?, ?zcan MM, Al Juhaimi FY. Antioxidant effect of mint, laurel and myrtle leaves essential oils on pomegranate kernel, poppy, grape and linseed oils. J Clean Prod 2012; 27: 151-154.
[22] Alexander M. Aromatherapy & immunity: how the use of essential oils aid immune potentiality Part 2 mood-immune correlations, stress and susceptibility to illness and how essential oil odorants raise this threshold. Int J Aromather 2001; 11(3): 152-156.
[23] Benbela?d F, Abdoune MA, Khadir A, Bendahou M. Drying effect on yield and antimicrobial activity of essential oils. Int J Med Aromat Plants 2013; 3(1): 93-101.
[24] Adams PR. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Illinois: Allured Publishing Corporation; 2007, p. 802.
[25] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests; approved standard-ninth edition. Pennsylvania, USA: CLSI; 2006.
[26] Klancnik A, Piskernik S, Jersek B, Mozina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods 2010; 81(2): 121-126.
[27] Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3(2): 163-175.
[28] Nostro A, Sudano Roccaro A, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 2007; 56: 519-523.
[29] Zoletti GO, Pereira EM, Schuenck RP, Teixeira LM, Siqueira JF Jr, dos Santos KR. Characterization of virulence factors and clonal diversity of Enterococcus faecalis isolates from treated dental root canals. Res Microbiol 2011; 162(2): 151-158.
[30] Salah R, Dar-Odeh N, Abu Hammad O, Shehabi AA. Prevalence of putative virulence factors and antimicrobial susceptibility of Enterococcus faecalis isolates from patients with dental diseases. BMC Oral Health 2008; doi: 10.1186/1472-6831-8-17.
[31] Nallapareddy SR, Singh KV, Sillanp?? J, Garsin DA, H??k M, Erlandsen SL, et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006; 116(10): 2799-2807.
[32] Crémet L, Corvec S, Batard E, Auger M, Lopez I, Pagniez F, et al. Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diagn Microbiol Infect Dis 2013; 75(3): 252-255.
[33] H?iby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen P?, et al. The clinical impact of bacterial biofilms. Int J Oral Sci 2011; 3(2): 55-65.
[34] Sena NT, Gomes BP, Vianna ME, Berber VB, Zaia AA, Ferraz CC, et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J 2006; 39(11): 878-885.
[35] Arias-Moliz MT, Ferrer-Luque CM, González-Rodriguez MP, Valderrama MJ, Baca P. Eradication of Enterococcus faecalis biofilms by cetrimide and chlorhexidine. J Endod 2010; 36(1): 87-90.
[36] Sanchez CJ Jr, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis 2013; doi: 10.1186/1471-2334-13-47.
[37] Chávez De Paz LE, Hamilton IR, Svens?ter G. Oral bacteria in biofilms exhibit slow reactivation from nutrient deprivation. Microbiology 2008; 154(7): 1927-1938.
[38] Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003; 2(2): 114-122.
[39] Guarda A, Rubilar JF, Miltz J, Galotto MJ. The antimicrobial activity of microencapsulated thymol and carvacrol. Int J Food Microbiol 2011; 146(2): 144-150.
[40] Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C 2007; 62(7-8): 507-513.
[41] Veras HN, Rodrigues FF, Colares AV, Menezes IR, Coutinho HD, Botelho MA, et al. Synergistic antibiotic activity of volatile compounds from the essential oil of Lippia sidoides and thymol. Fitoterapia 2012; 83(3): 508-512.
[42] Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010; 17(13): 1061-1066.
[43] Gallucci MN, Oliva M, Casero C, Dambolena J, Luna A, Zygadlo J, et al. Antimicrobial combined action of terpenes against the foodborne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Fragr J 2009; 24(6): 348-354.
10.12980/APJTB.4.2014C1203
*Corresponding author: Dr. Mourad Bendahou, Department of Biology, Faculty of Sciences of Nature, Life, Earth and Universe, Aboubekr Belka?d University, P.O. Box 119, 13000 Tlemcen, Algeria.
Tel: +21343211572
Fax: +21343286308
E-mail: bendahou63@yahoo.fr
Foundation Project: Supported by the Ministry of Higher Education and Scientific Research of People’s Democratic Republic of Algeria (Grant No. B05/N125).
Article history:
Received 13 Feb 2014
Received in revised form 20 Feb, 2nd revised form 25 Feb, 3rd revised form 3 Mar 2014
Accepted 12 Apr 2014
Available online 28 Jun 2014
Methods:Ten chemically analyzed essential oils by gas chromatography-mass spectrometry were evaluated for antimicrobial activity against sensitive and resistant clinical strains of E. faecalis in both planktonic and biofilm state using two methods, disk diffusion and broth microdilution.
Results:Studied essential oils showed a good antimicrobial activity and high ability in E. faecalis biofilm eradication, whether for sensitive or multidrug-resistant strains, especially those of Origanum glandulosum and Thymbra capitata with interesting minimum inhibitory concentration, biofilm inhibitory concentration, and biofilm eradication concentration values which doesn’t exceed 0.063%, 0.75%, and 1.5%, respectively.
Conclusions:Findings of this study indicate that essential oils extracted from aromatic plants can be used in treatment of intractable oral infections, especially caused by biofilm of multidrugresistant E. faecalis.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A retrospective evaluation of the quality of malaria case management at twelve health facilities in four districts in Zambia
- Study of the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions
- Pharmacognostical study and establishment of quality parameters of aerial parts of Costus speciosus-a well known tropical folklore medicine
- Hepatocurative potential of Vitex doniana root bark, stem bark and leaves extracts against CCl4-induced liver damage in rats
- In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit
- Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
