Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
Elif Burcu Bali, Leyla A??k, Gül?in Akca, Meral Sarper, Mualla P?nar El?i, Ferit Avcu, Mecit Vural
1Health Services Vocational School, University of Gazi, Ankara, Turkey
2Department of Biology, Faculty of Science, University of Gazi, Ankara, Turkey
3Department of Medical Microbiology, Faculty of Dentistry, University of Gazi, Ankara, Turkey
4Cancer and Stem Cell Research Center, Gulhane Military Medical School, Ankara, Turkey
5Department of Hematology, Cancer and Stem Cell Research Center, Gulhane Military Medical School, Ankara, Turkey
Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
Elif Burcu Bali1*, Leyla A??k2, Gül?in Akca3, Meral Sarper4, Mualla P?nar El?i4, Ferit Avcu5, Mecit Vural2
1Health Services Vocational School, University of Gazi, Ankara, Turkey
2Department of Biology, Faculty of Science, University of Gazi, Ankara, Turkey
3Department of Medical Microbiology, Faculty of Dentistry, University of Gazi, Ankara, Turkey
4Cancer and Stem Cell Research Center, Gulhane Military Medical School, Ankara, Turkey
5Department of Hematology, Cancer and Stem Cell Research Center, Gulhane Military Medical School, Ankara, Turkey
PEER REVIEW
Peer reviewer
Dr. Ayten ?elebi Keskin, Department of Biology, Science and Art Faculty, K?r?kkale University, 71450, Yah?ihan, K?r?kkale.
Tel: 0 318 357 42 42/4092
E-mail: aytencelebi@yahoo.com
Comments
This is a well-organized study in which the authors investigated the antimicrobial activity of water, ethanol, methanol, n-hexan and EtAc extracts of T. turcica against A. actinomycetemcomitans and P. gingivalis as oral microaerophilic and anaerobic bacterial strains and evaluated the antioxidant potential and cytotoxic effects against human cancer (DU145, PC-3, K-562, HL60) and HGF cells. It has very good scope to further continue this work with more advanced tools to prove such plant potentials.
Details on Page 512
Objective:To investigate the in vitro antimicrobial potential of Thermopsis turcica Kit Tan, Vural & Kü?ük?dük against periodontopathogenic bacteria, its antioxidant activity and cytotoxic effect on various cancer cell lines.
Thermopsis turcica, Antimicrobial activity, Periodontopathogenic bacteria, Antioxidant effect, Phenolic content, Cytotoxic effect, Human gingival fibroblast, Acute promyelocytic leukemia
1. Introduction
Turkey has significant diversity of plant and rich flora. Its floral diversity is resulted from locating in intersection of three phytogeographic region (Mediterranean, Irano-Turanian and Euro-Siberian), being a bridge betweenflora of Southern Europe and Southwest Asia, being an origin and diversification center of numerous plant genus and possessing high endemism rate of plant species, probably concerning about ecological and phytogeographic diversity[1]. Medicinal plants play an important role in amelioration of some diseseaes such as infectious disease in Turkey[2,3]. Their parts used as a drog (flower, leave, seed, root, bark,ect.) ameliorate disease due to their effective compounds[4,5]. Their usage has increased on account of their low adverse effects and healthful features all over the world[6].
Plants of the genusThermopsis(Fabaceae) includes poisonous and harmful species with low feeding value. However, this genus has been used as a source of traditional oriental medicines and considered to be medicinal plants all over the world[7-9]. It belongs to Fabaceae family, including 25 species.Thermopsis turcica(T. turcica) (Kit Tan, Vural & Kü?ük?dük) is an unique endemic species of this genus in Turkey. It spreads narrowly between Eber Lake and Ak?ehir Lake in inner part of West Anatolia of Turkey[10,11]. It is known as Eber Sar?s? or Piyan[9]. Although it is categorized as critically endangered by the Red Data Book[12], the investigation of its biological activities might increase importance ofT. turcicaconservation in nature.
Thermopsisspecies have been constantly investigated in several areas. Total flavonoid contents of someThermopsisspecies contain six flavonoid components: formononetin, chrysoeriol, apigenin, luteolin, thermopsoside and cynaroside.Thermopsisextracts could be considered potential hypolipidemic and antisclerotic agent[13-16].Thermopsis alternifloracontains alkaloids, flavanoids, vitamin C, macro and microelements and its air-dried aerial part is used as a medicinal raw for a cytisine preparation[17].
There are limited investigations aboutT. turcicain literature. Studies ofT. turcicaconcern with its antimicrobial activity, morphology and anatomy, mutagenic potential, determination of its propagation by using conventional andin vitrotechniques and also investigation of its free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status[8,9,18-20].
The aim of this study was to investigate the antimicrobial activity of water, ethanol, methanol,n-hexane and ethyl acetate (EtAc) extracts ofT. turcicaagainstAggregatibacter actinomycetemcomitans(A.actinomycetemcomitans) andPorphyromonas gingivalis(P. gingivalis) as oral microaerophilic and anaerobic bacterial strains and evaluate the antioxidant potential and cytotoxic effects against human cancer [androgen-insensitive prostate cancer cells (DU145), androgen-sensitive prostate cancer cells (PC-3), promyelocytic leukemia (K-562), acute leukemia (HL60)] and human gingival fibroblast (HGF) cells.
2. Materials and methods
2.1. Chemicals
Chloroform, Folin-Ciocalteu’s phenol reagent, ethanol, methanol andn-hexane were purchased from Merck (Darmstadt, Germany). 3-[4,5-Dimethylthiazole-2-yl]-2,5-diphenyltetrazoliumbromide (MTT), Tween 40, dimethylsulphoxide (DMSO), ethyl acetate (EtAc), ethylenediaminetetraacetic acid, β-carotene, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(2-pyridyl)-5,6-bis(4-phenylsulphonic acid)-1,2,4-triazine (ferrozine), gallic acid, 2,6-di-tert-butyl-4-methylphenol (BHT) and linoleic acid were purchased from Sigma-Aldrich GmbH (Steinheim, Germany). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum were purchased from Gibco BRL (Gaithersburg, MD, USA). All other chemicals were analytical grade and obtained from either Sigma or Merck.
2.2. Plant material
T. turcicawas collected from Afyon: Sultanda??, Dere?ine, Kavakl? k?yü, K?prülü?z, 965 m, near Ak?ehir Lake in Turkey during the flowering period in May 2011, 38°29’56”N, 31°18’49” E, Mecit Vural 10392 & Osman Tugay. Plant material was deposited at the herbarium of Gazi.
2.3. Preparation of Thermopsis extracts
The collected plants were dried at room temperature for 4-7 d. The dried plants (30 g) were ground into powder and extracted three times with 450 mL of ethanol, methanol, EtAc or water at ambient temperature for 24 h. The extracts were filtrated and concentrated to dryness with rotary evaporator at 40 °C. The dried extracts were stored in the dark at 4 °C until used.
2.4. Antimicrobial activity
2.4.1. Bacterial strains
A total of two microbial strains were used in the study.A. actinomycetemcomitans(ATCC 29523) andP. gingivalis(ATCC 33277) which were taken from microbiology laboratory of Gazi University, Faculty of Dentistry were used in the study. The strains were kept in Gazi University, Faculty of Dentistry, Medical Microbiology laboratory and cryopreserved at -86 °C. For experiments bacteria were inoculated onto Colombia agar (Merck, Germany) plates supplemented with hemine (5μg/mL), menadione (1 μg/mL) and 5% horse blood. Incubation was performed at 37 °C, under anaerobic conditions, for 5-7 d in an automated anaerobic chamber (Electrotek, UK) with an atmosphere of 90% N2, 5% CO2and 5% H2. After harvesting the bacteria, bacterial suspensions were prepared in the sterilized test tubes containing Colombia broth supplemented with hemine (5 μg/mL), menadione (1 μg/mL) and the inoculum were adjusted according to the turbidity of 0.5 McFarland standard. The density of bacterial suspensions was determined by using an Elisa reader (BioTek, USA) spectrophotometrically.
2.4.2. Disc diffussion method
The growth media was prepared by using the Colombia broth (Merck, Germany) supplemented with hemine (5 μg/ mL), menadione (1 μg/mL) and 100 μL amounts of bacterial suspension were spreaded onto them. Standard susceptibility discs without any additive chemicals (Bioanalyse, Turkey) were prepared by embedding them into the plants extract solutions. Then, they were put onto the plates and incubated as mentioned before according to the guidelines of Clinical Laboratory Standards Institute. After incubation the inhibition zones of the discs of extracts were measured with and total mean numbers were calculated for extracts.
2.4.3. Agar well diffusion method
After the wells with a diameter of 5 mm were prepared on the chosen agar medium plates, 100 μL amounts of bacterial suspension were spreaded onto them. Then, 10 μL of plant extracts and their solvents were added into these wells separetely. Plates were incubated as mentioned before. After the incubation, the inhibition zones were measured and the total mean numbers were calculated for the extracts.
2.4.4. Determination of the minimum inhibitory concentration (MIC)
Firstly, 100 μL amounts of plant extracts were added in to the first wells of the U-bottom-polistiren microplates and serially diluted in the range of 100-0.39 mg/mL. Then, 100 μL of bacterial suspensions were put onto them except negative control well. Polistiren microplates were incubated for 48 h in anaerobic cabin and after the incubation time, wells which contained minimum concentration of the extracts where no visible growth can be seen were expressed as MIC,i.e. the minimum concentration which completely inhibited bacterial growth. All experiments were performed in duplicates.
2.4.5. Determination of the minimum bactericidal concentration (MBC)
After determining the MIC, 10 μL amounts of solutions were taken from all of the wells where no visible growth was seen and put onto the same agar media plates and incubated as mentioned before. Then, the minimum concentration in which no grown colonies can be seen on the agar plates were expressed as MBC. After the colonies were counted, they were defined as CFU/mL.
2.5. Antioxidant activity
2.5.1.DPPHradical scavenging assay
The free radical scavenging activity ofThermopsisextracts was determined by the DPPH method[21]. Briefly, 0.1 mmol/ L solution of DPPH in methanol was added on toThermopsisextracts solutions at different concentrations. The reaction mixture was incubated for 30 min at room temperature in the dark. After 30 min, the absorbance was measured at 517 nm against a blank by a spectrophotometer. The inhibition of radical scavenging activity in percent (I%) was calculated using the following equation:
I%=[(Acontrol-Asample)/Acontrol]×100
Where Acontrolis the absorbance value of the control reaction (containing all reagents except the test compound) and Asampleis the absorbance of the test compound. IC50value of the extract concentration was calculated from the graph plotting inhibition percentage against extract concentration. BHT was used as positive control and a methanolic solution of the radical DPPH was prepared daily and protected from light.
2.5.2. Beta-carotene bleaching assay
The antioxidant activity ofThermopsisextracts was determined according to β-carotene bleaching assay[22]. A stock solution of β-carotene-linoleic acid mixture was prepared as follows: 0.5 mg β-carotene was dissolved in 1 mL of chloroform (high performance liquid chromatography grade). About 25 μL linoleic acid and 200 mg Tween 40 were added into this mixture. Chloroform was completely evaporated using a vacuum evaporator. Then, 100 mL distilled water saturated with oxygen (100 mL/min for 30 min) was added with a vigorous shaking. A total of 2 500 μL of this reaction mixture were dispensed into test tubes and 350 μL portions of the extracts, prepared at 2 g/mL concentrations were added and the emulsion system was incubated for up to 48 h at room temperature. The same procedure was repeated with synthetic antioxidant, BHT, as positive control, and a blank. After this incubation period, absorbances of the mixtures were measured at 490 nm. Antioxidative capacities of the extracts were compared with those of BHT and blank.
2.5.3. Total phenolic contents of the extracts
Total phenolic contents ofThermopsisextracts weredetermined by using the Folin-Ciocalteu reagent according to the method of Singleton and Rossi using gallic acid as standard, with a slight modifications[23]. The extract solutions (100 μL) were mixed with 200 μL of 50% Folin-Ciocalteu reagent. The mixture was allowed to react for 3 min and 1 mL aqueous solution of 2% Na2CO3was added and shaked slightly. At the end of incubation for 1 h at room temperature, absorbance of each mixture was measured at 760 nm. The same procedure was also applied to the standard solutions of gallic acid. Total phenol contents were expressed μg gallic acid equivalents per mg of the extracts.
2.6. Cytotoxicity analysis
2.6.1. Cell culture and culture condition
The human cell lines including DU145, PC-3, K-562 and HL60 cells were obtained as a donation from GATA Research Center, and gingival fibroblast cells were obtained 20 years impacted tooth of healthy volunteers after inform concent and approval by the local ethics committee. All cancer cell lines and gingival fibroblast cells were cultured in RPMI-1640 and DMEM, respectively. Each medium was supplemented with 10% fetal bovine serum, 100 units/ mL penicilin and 100 μg/mL streptomycin. All cells were incubated at 37 °C with a humidified atmosphere of 95% air and 5% CO2.
2.6.2. Cytotoxic effect of the extracts against human cancer cell lines and normal gingival fibroblasts
Cytotoxic effects of the extracs were determined by MTT assay[24,25]. In this assay, cells were seeded into a 96-well plates (400 mm3cells/well) in 100 μL of medium and incubated at 37 °C with 5% CO2for 24 h. Then, the cells were treated with the extracts (ranging from 20 to 100 μg/mL) or without (vehicle control, 0.1% DMSO) extracts and incubated each cell lines for 24 h and 48 h. After incubation, 20 μL MTT solution was added into each well and incubated 4 h at 37 °C. The supernatant was removed and replaced with 100 μL of DMSO. The optical density of wells was measured with a microplate spectrophotometer reader (EIA Reader, ELX800, Biotek Instruments, Burlington, VT). The stock solution of the extracts was serially diluted with growth medium. The aqueous extract was dissolved in media. The same precedure was repeated for gingival fibroblast cells, but only the highest two concentrations (80 and 100 μg/mL) of the extracts were performed.
2.6.3. Statistical analysis
The extraction experiments were conducted in duplicate, and all the analyses were done in triplicate. The results were expressed as the mean±SD. One-way analysis of variance (ANOVA) was used in multiple group comparisons by using the SPSS 11.0 software package. Differences and values ofP<0.05,P<0.01 andP<0.001 were considered statistically significant.
3. Results
3.1. Antimicrobial activities of Thermopsis extracts on periodontopathogenic bacteria
In the present study, the average yields of ethanol, methanol, EtAc,n-hexane and water extracts ofT. turcicawere 9.14% (w/w); 18.76% (w/w); 9.70% (w/w); 0.50% (w/w) and 25.90% (w/w), respectively. Antimicrobial activity of the extracts and the antibiotics (standards) were carried out by agar well difussion method. Antimicrobial activity of the extracts was found at 25-100 mg/mL concentration range. The inhibition zones of the bacteria were in the range of (11.3±0.5) mm to (27.5±0.7) mm (Table 1). EtAc extract was found as the most effective extract in each concentration (25, 50 and 100 mg/mL) againstA. actinomycetemcomitansandP. gingivalis. Ethanol extract had an antimicrobial activity at 50 and 100 mg/mL concentrations against onlyP. gingivalis, whereas at the same concentrations, methanol had an antimicrobial effect on bothA. actinomycetemcomitansandP. gingivalis. Water andn-hexane extracts had no antimicrobial effect. Antimicrobial effect of the extracts increased according to concentrations.

Table 1 Antimicrobial activity of Thermopsis extracts on periodonto-pathogenic bacteria.
Ampicillin (Oxoid, 10 μg), clindamycin (Oxoid, 2 μg), tetracycline (Oxoid, 30 μg) antibiotic discs were used as standards for positive control. About 50% DMSO (solvent of the extracts) was used as a negative control by agar well diffusion method. The sensitivity of studied bacteria against ampicillin, clindamycin and tetracycline was given in Table 2.A. actinomycetemcomitansandP. gingivaliswere the most sensitive to ampicillin [(55.0±0.6) mm] and clindamycin [(74.6±0.5) mm], respectively. They were more resistance to tetracycline than ampicillin and clindamycin. Comparedwith the extracts, standard antibiotics were more effective on the bacteria. It was also detected MIC and MBC values of EtAc, methanol and ethanol extracts (Table 3).
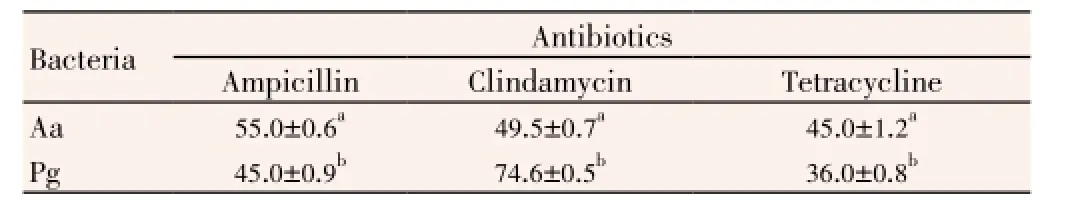
Table 2 Antimicrobial activity of standart antibiotics on periodontopathogenic bacteria.
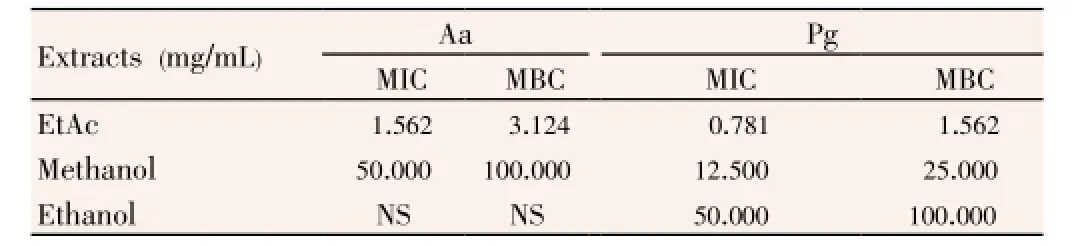
Table 3 MICs and MBCs of Thermopsis extracts against periodontopathogenic bacteria.
As a result of MIC and MBC, EtAc extract had the highest antimicrobial effect onA. actinomycetemcomitans(MIC: 1.562 mg/mL, MBC: 3.124 mg/mL) andP. gingivalis(MIC: 0.781 mg/ mL, MBC: 1.562 mg/mL). After EtAc, methanol extract had lower antimicrobial effect onA. actinomycetemcomitans(MIC: 50.000 mg/mL, MBC: 100.000 mg/mL) andP. gingivalis(MIC: 12.500 mg/mL, MBC: 25.000 mg/mL). The ethanol extract had the lowest antimicrobial activity against only P. gingivalis, which had 50.000 mg/mL MIC and 100.000 mg/ml MBC values. The inhibition zone ofThermopsisextracts with agar well diffusion method, showed a significant correlation with MIC and MBC values (P<0.001).
3.2. DPPH assay
Antioxidant activity of the extracts was tested by the DPPH radical scavenging. Polyphenols, such as BHT are known to be effective antioxidants[26]. In the present study, synthetic antioxidant BHT was used as a standard with which antioxidant activities of the extracts were compared. Free radical scavenging properties ofT. turcicaextracts in different concentrations were shown in Figure 1.n-Hexane and water extracts had poor radical scavenging activities. However, EtAc, methanol, ethanol extracts showed significant antioxidant activities. For each concentration (50-250 μg/mL), these extracts had radical scavenging effects (Figure 1). DPPH radical scavenging properties of the extracts are presented in Table 4. Lower IC50value demonstrates higher antioxidant activity. The EtAc extract [IC50=(30.0±0.3) μg/mL] exhibited higher DPPH scavenging activity than the methanol [IC50=(52.5±0.1) μg/mL] and the ethanol extracts [IC50=(71.3±0.6) μg/mL]. Also, DPPH scavenging activities of the EtAc, methanol and ethanol extracts were lower than the standard BHT [IC50=(27.5±0.2) μg/mL]. But antioxidant activity of the EtAc extract was similar to that of BHT. DPPH radical scavenging activity of test samples was in the order BHT>EtAc>methanol>ethanol.

Table 4 Antioxidant activities of T. turcica extracts.
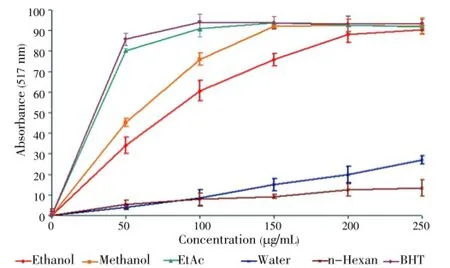
Figure 1. DPPH radical scavenging activity of Thermopsis extracts. Results are mean±SD of triplicate measurements.
3.3. Beta-carotene bleaching assay
Beta-carotene bleaching assay is based on the mechanism of occurence free radical growing out of the hydroperoxides formed from lineloic acid. In this assay, β-carotene underwent rapid discoloration in the absence of antioxidant. The lineloic acid was a free radical which attacked the highly unsaturated β-carotene molecules. Since this oxidation brought about breaking of double bands of β-carotene, the compounds lost its characteristic orange colour, which could be measured spectrophotometrically. The presence of various extracts blocked the streching of β-carotene bleaching by neutralising the free radicals occured in the system[27]. The relative antioxidative activities (RAAs) of the extracts were evaluated from the equation, RAA=Asample/ABHT, where ABHTis the absorbance of the control (BHT) and Asampleis the absorbance of the extracts. The calculated RAAs of the extracts are shown in Table 4. The inhibition values of linoleic acid oxidation were evaluatedas (74.35±0.30)%, (69.57±0.50)%, (60.81±1.20)%, (33.72±2.50)% and (16.78±0.90)% for EtAc, methanol, ethanol,n-hexane and water extracts, respectively. BHT had the highest inhibition value [(100.00±0.60)%]. Among the extracts, EtAc extract had the most effective in this method like in the other antioxidant activity methods. A relationship between the DPPH scavenging ability and β-carotene bleaching extent was found for EtAc, methanol and ethanol extracts. In both assays, the ordering of the antioxidant activities for the extracts solved in different solvents and BHT was the same (BHT>EtAc>methanol>ethanol) and the EtAc extract exhibited higher antioxidative capacity than the other extracts. Like DPPH assay, in this method,n-hexane and water extracts had weak antioxidant activity.
3.4. Total phenolic content
The amount of total phenolics inThermopsisextracts were detected spectrometrically according to the Folin-Ciocalteu method and calculated as gallic acid equivalents. The standard curve equation is,y(absorbance)=0.008 5x(μg gallic acid)-0.020 9,R2=0.999 9. The absorbance value was inserted in equation above and the total amount of phenolic compound was calculated by the same equation. In Table 4, the total phenolic contents of EtAc, methanol, ethanol, water andn-hexane were (162.5±1.2) μg/mg, (80.84±2.20) μg/mg, (44.96±0.90) μg/mg, (21.04±0.70) μg/mg and (9.63±1.20) μg/mg, respectively. As a standard, the total phenolic content of BHT was (277.24±10.06) μg/mg. The results demonstrated that after BHT, EtAc, methanol and ethanol extract contained high amount of phenolic compounds, respectively. Although, then-hexane and water extracts had lower phenolic content. As a result of antioxidant activity assays, the EtAc, methanol and ethanol extract were found to be effective antioxidants in differentin vitroassays including DPPH radical scavenging and β-carotene bleaching. The EtAc extract included the highest phenolic content and also possessed the highest DPPH scavenging and β-carotene-bleaching effect.
3.5. Cytotoxic effects of Thermopsis extracts on human cancer and normal cell lines
The cytotoxic effects ofThermopsisextracts were analysed on DU145, PC-3, K-562, HL60 human cancer cell lines and also HGF cell lines. The cytotoxic effects of them were evidenced by MTT assay. Sincen-hexane extract had low antioxidant effect and phenolic content, it wasn’t studied for cytotoxic experiment. The cytotoxicity of the extracts on HL60 cells for 24 and 48 h was shown in Figures 2 and 3.

Figure 2. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on HL60 cells for 24 h.Each value represents the mean±SD of six wells.*P<0.05,**P<0.01,***P<0.001 versus control.

Figure 3. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on HL60 cells for 48 h.Each value represents the mean±SD of six wells.*P<0.05,**P<0.01,***P<0.001 versus control.
Although water extracts had no cytotoxic effect for 24 and 48 h, EtAc and ethanol extract had a powerful cytotoxic effect on HL60 cells. IC50values of the EtAc extract was (70.0±0.9) μg/mL and (50.0±3.6) μg/mL for 24 and 48 h, respectively. On the other hand, the ethanol extract had a cytotoxic effect [IC50=(80.0±1.2) μg/mL] for 48 h. It was also found that methanol extract had low cytotoxic effect within 24 h. For 48 h, there was no toxicity on HL60 cells. Figures 4 and 5 are shown cytotoxic effect of the extracts on K562 cells for 24 and 48 h. In Figure 4, it was demonstrated that only ethanol extract had cytotoxic effect at 80 and 100 μg/mL concentrations (P<0.001) within 24 h. The other extracts were no toxic on K562.For 48 h, it was observed that both EtAc and ethanol extracts were toxic on K562 at 80 and 100 μg/mL concentrations (P<0.001) (Figure 5). Methanol and water extracts had no cytotoxic effect on K562 cells. The cytotoxic activity of EtAc and ethanol extracts on HL60 cells were higher than K562 cells.

Figure 4. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on K562 cells for 24 h.Each value represents the mean±SD of six wells.*P<0.05,**P<0.01,***P<0.001 versus control.

Figure 5. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on K562 cells for 48 h.Each value represents the mean±SD of six wells.*P<0.05,**P<0.01,***P<0.001 versus control.
The extracts were also tested on DU145 and PC-3 cell lines at the same concentrations and time. However, the extracts exerted no cytotoxic effects on prostate cancer cell lines (data was not shown). The results indicated that the cytotoxicity of EtAc and ethanol extracts was influenced by the concentration (20, 40, 60, 80 and 100 μg/mL) and time (24 and 48 h). Unlike water, ethanol and methanol extract, EtAc exhibited remarkable cytotoxic effect on HL60 cells. Two highest concentrations of the extracts were also tested on HGF as a control for 24 and 48 h. Our findings showed that there was no toxic effect on HGF cells at 80 and 100 μg/mL concentrations for 24 and 48 h (Figures 6 and 7). Among all of the cancer cells, only HL60 cells was the most susceptible to EtAc extract.
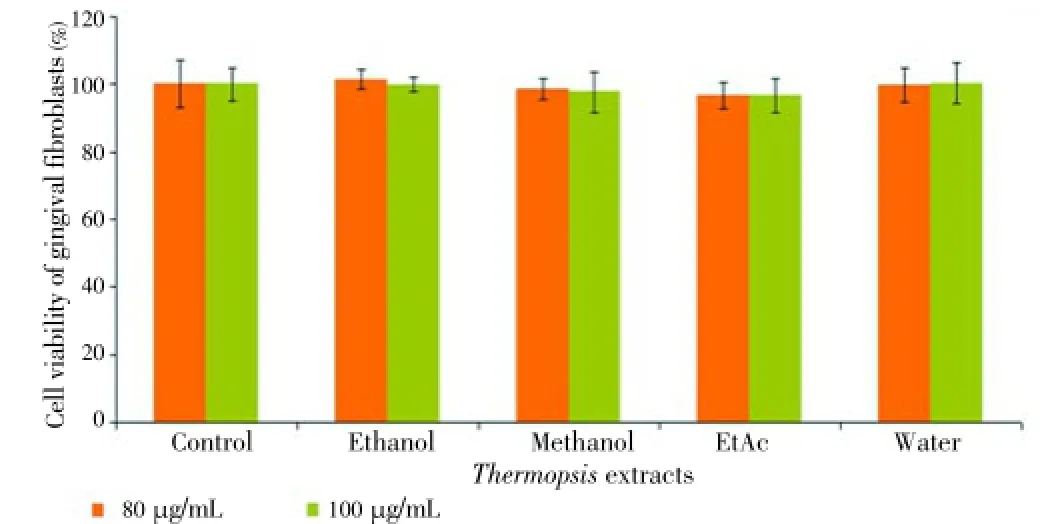
Figure 6. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on HGF cells for 24 h.

Figure 7. Cytotoxic effect of Thermopsis extracts at concentrations (20, 40, 60, 80 and 100 μg/mL) on HGF cells for 48 h.
4. Discussion
Several advances in understanding and potentially treating human diseases can take root from investigations of the toxic principles derived from poisonous plants[28]. Although some studies have been practiced toT. turcica, there are a few study about this poisonous plant. It was reported that leaf, rhizome stem and callus extracts ofT. turcicahave strong antimicrobial compounds, including alkaloids as main metabolites, against human pathogenic microorganisms (Candida albicans, Pseudomonas aeruginosaATCC 27853,Staphylococcus aureusATCC 25923,Escherichia coliATCC 25922, methicillin resistantStaphylococcus aureus,Salmonella enteriditis, Salmonellasp.,Klebsiella pneumoniae,Proteus vulgaris) at 100 μg/mL concentration by disc diffusion method[19]. In our study, though no antimicrobial activity was detected by disc diffusion method, antimicrobial activity of methanol, ethanol and EtAc extracts of this plant was detected by using agar well diffusion method. Among them, EtAc extract had more powerful antimicrobial effect than ethanol and methanol extracts againstA. actinomycetemcomitansandP. gingivalis. These bacteria are useful indicators of increased risk of gingival attachment loss and bring about active diseases such as chronic periodontitis, atherosclerosis, and atherosclerotic cardiovascular disease[29-31]. According to antimicrobial results, ethanol, methanol and EtAc extracts ofT. turcicahad different antimicrobial effects against these pathogens, and these effects may result from their phytochemical possessions including also phenolic compounds.
Phenolic compounds (e.g. phenolic acids, flavonoids, quinones, coumarins, lignans, stilbenes, tannins) possess a wide range of pharmacological properties such as antiinflammatory, antiviral, antimicrobial, antioxidant, anticancer, cardioprotective and vasodilatory effects[32-35]. The flavonoids are almost ubiquitous in plants and they are powerful chain-breaking antioxidants acting as metal chelators and free radical scavengers[36]. Nitrogen compounds (alkaloids), vitamins, terpenoids (including carotenoids), and some other endogenous metabolites in plants also play important roles in antioxidant activity[32]. Aksoyet al. reported that acetone and methanol extracts ofT. turcicahad radical scavenging effects at all concentrations (50, 100, 200 μg/mL) and they found that the radical scavenging effect of BHT was higher than acetone and methanol extract like in our antioxidant results[20]. While acetone extract was higher radical scavenging effect than methanol extract in their results. In contrast, in our study, EtAc extract exhibited stronger radical scavenging effectthan methanol and ethanol extract.
Several antioxidant compounds have different polarities, therefore different solvents are frequently used for the isolation of antioxidants. Antioxidant activity of the extracts are drastically dependent on the type of solvents[37]. According to our antioxidant results, the extract which was solved in EtAc had the most powerful antioxidant activity in DPPH free radical scavenging and β-carotene-bleaching methods. Besides, it included the highest phenolic compounds. There was a significant correlation between the content of phenolic compounds, the DPPH free radical scavenging and β-carotene-bleaching effect[20,33,38-40].
Vattemet al.reported that both total phenolics and antioxidant capacity correlated well with the increase in antimicrobial activity of their extracts against patogen bacteria. Their results indicated that a partial hydrophobic phytochemical extract with a good antioxidant capacity could have a high antimicrobial activity[33]. Our results showed that the extract of EtAc which was an hydrophobic organic solvent had the most powerful antimicrobial and also antioxidant capacity. Our antimicrobial results were also in agreement with the literatures that exhibited a good correlation between antimicrobial and antioxidant
activity[41,42].
In cytotoxic assay, ethanol and EtAc extracts exhibited cytotoxic effects on HL60 and K-562 cells. EtAc extract had more powerful cytotoxic effect than ethanol extract on HL60 cells indicated that the compounds in EtAc extract included toxic phytochemicals. It was also the unique extract that possessed the highest cytotoxic effect against HL60 cells. There are some reports which contributes to the relationship of cytotoxicity with antioxidant activity[32,40,42,43]. Therefore, antioxidant potential of the EtAc extract may contribute to its cytotoxic activity. But it was unexpected that methanol extract, which had more powerful antioxidant effect than ethanol extract, didn’t exhibit any cytotoxic effect on all of the cancer cell lines (K-562, HL60, DU145 and PC-3).
For K-562 cells, ethanol extract was more effective than EtAc extract. However, both of the extracts exhibited weak cytotoxic activities within 24 and 48 h. On the other hand, water extract had also no cytotoxic effect on all of the cancer cell lines indicated that the compounds present in these extracts are non-toxic. All of the extracts had also non-toxic chemicals against HGF cells.
The genusThermopsisare rich in flavonoids and alkaloids, such as N-formycytisine, thermopsine, N-methylcytisine, anagyrine, cytosine, and anagyrine[44,45]. ?eneret al.reported that different tissues ofT. turcicacontained high anagyrine with some lupine alkaloids, coumarins, steroids, flavonoids and cardioactive glycosides[46]. These phenolic compounds and alkaloids could be responsible for the observed cytotoxic activity of the EtAc and ethanol extract.
T. turcicais an endemic species under the threat of extinction. Therefore, we think that it is important to investigate its biological activity. The cytotoxic activity ofThermopsisextracts have not been reported up to now. Although additional research is needed to investigate the cytotoxicity ofT. turcica, thesein vitrostudy shows that its EtAc extract could be significant source of antioxidants and also might include anticancer agents.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by Ministry of Development of Turkish Republic for foundation of Moleculer Biology Research Center (Grant No. 1998K121480). The authors also wish to thank to Associate Prof. Dr. Osman Tugay, Department of Biology, Faculty of Science and Art, Sel?uk University, for collaboration of the collection and identification of the plant material.
Comments
Background
Medicinal plants have played a key role in the worldwide maintenance of health. That’s why it appears useful to obtain a scientific basis for the possible use of herbal drugs in the treatment of diseases such as cancer, infectious diseases and those associated with oxidative damage. Natural products of higher plants are an important source of therapeutic agents; therefore, many research groups are currently screening the different biological activities of the plants.
Research frontiers
The study investigated the antimicrobial activity of water, ethanol, methanol,n-hexan and EtAc extracts ofT. turcicaagainst periodonto-pathogenic bacterial strains and evaluated the antioxidant potential and cytotoxic effects against human cancer and HGF cells.
Related reports
It was reported that leaf, rhizome stem and callus extracts ofT. turcicahave strong antimicrobial compounds, including alkaloids as main metabolites, against human pathogenic microorganisms. Vattemet al.(2004) reported that both total phenolics and antioxidant capacity correlated well with the increase in antimicrobial activity of their extracts against patogen bacteria. The antimicrobial results of this study were also in agreement with the literatures that exhibited a good correlation between antimicrobial and antioxidant activity.
Innovations and breakthroughs
The cytotoxic activity ofThermopsisextracts have not been reported up to now. This study suggests that ethanol and EtAc extracts ofT. turcicaexhibited cytotoxic effects on HL60 and K-562 cells. EtAc extract had more powerful cytotoxic effect than ethanol extract on HL60 cells indicated that the compounds in EtAc extract included toxic phytochemicals.
Applications
The results of the present study provided evidence for the antioxidant, anticancer and antimicrobial activities of the studied plant extracts, and bring supportive data for future investigations that will lead to their use in cancer, antioxidant and antimicrobial therapy.
Peer review
This is a well-organized study in which the authors investigated the antimicrobial activity of water, ethanol, methanol,n-hexan and EtAc extracts ofT. turcicaagainstA. actinomycetemcomitansandP. gingivalisas oral microaerophilic and anaerobic bacterial strains and evaluated the antioxidant potential and cytotoxic effects against human cancer (DU145, PC-3, K-562, HL60) and HGF cells. It has very good scope to further continue this work with more advanced tools to prove such plant potentials.
[1] Dagci EK, ?zmirli M, Di?rak M. [A research of the antimicrobial activities of some tree species grown in Kahramanmaras city]. KSU J Sci Eng 2002; 5(1): 38-46. Turkish.
[2] Kültür S. Medicinal plants used in K?rklareli province (Turkey). J Ethnopharmacol 2007; 111(2): 341-364.
[3] Dogan NM, Cansaran A, Acar G, ?ztekin M. Antimicrobial activity of extracts of some plants from Amasya (Turkey). Adv Bio Res 2010; 1(1) : 87-91.
[4] Baytop T. [Therapy with medicinal plants in Turkey (past and present)]. Istanbul: Istanbul University Publications; 1999, p. 92. Turkish.
[5] Dulger B, Gonuz A. Antimicrobial activity of some endemic Verbascum, Salvia, and Stachys species. Pharm Biol 2004; 42(4-5): 301-304.
[6] Ba?gel S, Erdemo?lu SB. Determination of mineral and trace elements in some medicinal herbs and their infusions consumed in Turkey. Sci Total Environ 2006; 359(1-3): 82-89.
[7] Saito K, Takamatsu S, Murakoshi I, Ohmiya S, Otomasu H. Isolation of a new alkaloid (-)-0-acetylbaptifoline and the absolute stereochemical relationships of lupine alkaloids in Thermopsis chinensis. J Nat Prod 1989; 52(5): 1032-1035.
[8] ?zdemir C, Dural H, Ertu?rul K, Kü?ük?dük M, Baran P, ?anda MA. Morphology and anatomy of endemic Thermopsis turcica Kit Tan, Vural & Kü?ük?dük. Bangladesh J Bot 2008; 37(2): 105-114.
[9] Liman R, Eren Y, Akyil D, Konuk M. Determination of mutagenic potencies of aqueous extracts of Thermopsis turcica by Ames test. Turk J Biol 2012; 36: 85-92.
[10] Tan K, Vural M, Kü?ük?dük M. An unusual new Thermopsis from Turkey. Notes Roy Bot Gard Edinburgh 1983; 40(3): 515-518.
[11] Davis PH, Mill RR, Kit T. Flora of Turkey and the East Aegean Islands. Edinburg: Edinburg University Press; 1988, p. 111-112.
[12] Ekim T, Koyuncu M, Vural M, Duman H, Ayta? Z, Adigüzel N. Red data book of Turkish plants (pteridophyta and spermatophyta). Ankara: TDKA and Van Centennial University Press; 2000.
[13] Faizieva SK, Khushbaktova ZA, Syrov VN, Yuldashev MP, Batirov éK, Sagdullaev SS. The total flavonoids from Thermopsis alterniflora, Th. dolichocarpa, Vexibia alopecuroides, and Rhaponticum carthamoides and their hypolipidemic activity. Chem Nat Compd 1999; 35(2): 155-158.
[14] Khushbaktova ZA, Faizieva SK, Syrov VN, Yuldashev MP, Batirov éK, Mamatkhanov AU. Isolation, chemical analysis, and study of the hypolipidemic activity of the total flavonoid extract from Thermopsis altherniaflora. Pharm Chem J 2001; 35(3): 155-158.
[15] Kotenko LD, Mamatkhanov AU, Turakhozhaev MT, Yuldashev MP. Quantitative analysis of flavonoids in the total preparation of Thermopsis alterniflora. Chem Nat Compd 2001; 37(2): 137-139.
[16] Satimov GB, Mamatkhanov AU, Kotenko LD, Turakhodzhaev MT, Madrakhimov N, Faizieva S, et al. A total flavonoid extract from Thermopsis altherniflora: development of the production technology, creation of a medicinal preparation. Pharm Chem J 2003; 37(4): 203-206.
[17] Asilbekova DT. Lipids of Thermopsis alterniflora bean seeds and shells. Chem Nat Compd 2004; 40(6): 532-534.
[18] Cenkci S, Temel M, Kargio?lu M, Dayan S. Propagation of endangered Thermopsis turcica Kit Tan, Vural & Kü?ük?dük using conventional and in vitro techniques. Turk J Biol 2009; 33:327-333.
[19] Korcan SE, Cigerci IH, Dilek M, Kargioglu M, Cenkci S, Konuk M. Antimicrobial activity of an endemic species, Thermopsis turcica, Turkey. Kuwait J Sci Eng 2009; 36: 101-112.
[20] Aksoy L, Kolay E, A??l?nü Y, Aslan Z, Karg?o?lu M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J Biol Sci 2013; 20(3): 235-239.
[21] Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-1200.
[22] Miller HE. A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc 1971; 48(2): 91.
[23] Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 1965; 16(3): 144-158.
[24] Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 1990; 131(2): 165-172.
[25] Ural AU, Avcu F, Candir M, Guden M, Ozcan MA. In vitro synergistic cytoreductive effects of zoledronic acid and radiation on breast cancer cells. Breast Cancer Res 2006; 8(4): R52.
[26] Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 1998; 46(10): 4113-4117.
[27] Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001; 73(3): 285-290.
[28] Welch KD, Panter KE, Gardner DR, Stegelmeier BL. The Good and the bad of poisonous plants: an introduction to the USDAARS Poisonous Plant Research Laboratory. J Med Toxicol 2012; 8: 153-159.
[29] Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 1995; 63(10): 3878-3885.
[30] Ready D, D’Aiuto F, Spratt DA, Suvan J, Tonetti MS, Wilson M. Disease severity associated with presence in subgingival plaque of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Tannerella forsythia, singly or in combination, as detected by nested multiplex PCR. J Clin Microbiol 2008; 46(10): 3380-3383.
[31] Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosiswith an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol 2010; 59(2): 143-151.
[32] Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004; 74(17): 2157-2184.
[33] Vattem DA, Lin YT, Labbe RG, Shetty K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Process Biochem 2004; 39(12): 1939-1946.
[34] Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 2006; 99(1): 191-203.
[35] Kilani S, Ben Sghaier M, Limem I, Bouhlel I, Boubaker J, Bhouri W, et al. In vitro evaluation of antibacterial, antioxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus. Bioresour Technol 2008; 99(18): 9004-9008.
[36] Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000; 52(4): 673-751.
[37] Gong Y, Liu X, He WH, Xu HG, Yuan F, Gao YX. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012; 83(3): 481-489.
[38] Barros L, Ferreira MJ, Queirós B, Ferreira ICFR, Baptista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portugese wild edible mushrooms and their antioxidant activities. Food Chem 2007; 103(2): 413-419.
[39] Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWTFood Sci Technol 2008; 41(6): 1067-1072.
[40] Allahghadri T, Rasooli I, Owlia P, Nadooshan MJ, Ghazanfari T, Taghizadeh M, et al. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J Food Sci 2010; 75(2): H54-61.
[41] Pérez J, Rubia TD, Moreno J, Martínez J. Phenolic content and antibacterial activity of olive oil waste waters. Environ Toxicol Chem 1992; 11(4): 489-495.
[42] Sulaiman GM, Hussien NN, Marzoog TR, Awad HA. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am J Biochem Biotechnol 2013; 9(1): 41-46.
[43] Hou J, Sun T, Hu J, Chen S, Cai X, Zou G. Chemical composition, cytotoxic and antioxidant activity of the leaf essential oil of Photinia serrulata. Food Chem 2007; 103(2): 355-358.
[44] Dement WA. Biological implication of flavonoid chemistry in Baptisia and Thermopsis. Biochem Syst Ecol 1975; 3(2): 91-94.
[45] Saito K, Takamatsu S, Ohmiya S, Otomasu H, Yasuda M, Kano Y, et al. Lupin alkaloids from the seeds of Thermopsis lupinoides. Phytochemistry 1988; 27(11): 3715-3716.
[46] ?ener B, Koyuncu M, Ergun F, editors. Proceedings of the 9th symposium on Plant Drugs Eski?ehir (Turkey); 1992 May 16-19; Eskisehir, Turkey.
10.12980/APJTB.4.2014APJTB-2013-0010
*Corresponding author: Dr. Elif Burcu Bali, Assistant Professor, Health Services Vocational School, University of Gazi, Ankara, Turkey.
Tel: +90 (312) 484 56 35-175
Fax: +90 (312) 484 36 49
E-mail: e.burcubali@gmail.com, burcubali@gazi.edu.tr
Foundation Project: Supported by Ministry of Development of Turkish Republic for foundation of Moleculer Biology Research Center (Grant No. 1998K121480).
Article history:
Received 25 May 2014
Received in revised form 2 Jun, 2nd revised form 7 Jun, 3rd revised form 14 Jun 2014
Accepted 26 Jun 2014
Available online 28 July 2014
Methods:In vitro antimicrobial activities of ethanol, methanol, ethyl acetate (EtAc), n-hexane and water extracts of Thermopsis turcica herb against periodontopathogenic bacteria, Aggregatibacter actinomycetemcomitans ATCC 29523 and Porphyromonas gingivalis ATCC 33277 were tested by agar well diffusion, minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). Antioxidant properties of the extracts were evaluated by 1,1-diphenyl-2-picryl-hydrazyl radical scavenging activity and β-carotene bleaching methods. Amounts of phenolic contents of the extracts were also analysed by using the Folin-Ciocalteu reagent. Additionally, cytotoxic activity of the extracts on androgen-insensitive prostate cancer, androgen-sensitive prostate cancer, chronic myelogenous leukemia and acute promyelocytic leukemia human cancer cell lines were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Human gingival fibroblast cells were used as a control.
Results:Our data showed that EtAc extract had the highest antimicrobial effect on Aggregatibacter actinomycetemcomitans (MIC: 1.562 mg/mL, MBC: 3.124 mg/mL) and Porphyromonas gingivalis (MIC: 0.781 mg/ mL, MBC: 1.562 mg/mL). In antioxidant assays, EtAc extract exhibited also the highest radical scavenging activity [IC50=(30.0±0.3) μg/mL] and the highest inhibition [(74.35±0.30)%] against lineloic acide oxidation. The amount of phenolic content of it was also the highest [(162.5±1.2) μg/mg gallic acid]. In cytotoxic assay, only ethanol [IC50=(80.00±1.21) μg/mL] and EtAc extract [IC50=(70.0±0.9) μg/mL] were toxic on acute promyelocytic leukemia cells at 20-100 μg/mL (P<0.05). However, no toxic effect was observed on human gingival fibroblast cells.
Conclusions:According to our findings, owing to its antioxidant and cytotoxic potential, EtAc extract might include anticancer agents for acute promyelocytic leukemia.
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Reticulo-cutaneous fistula due to the ingestion of a long metallic rod in a cow
- Clinical, radiological and molecular diagnosis correlation in serum samples from patients with osteoarticular tuberculosis
- Anti-diabetic effects of Caulerpa lentillifera: stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes
- Down-regulated expression of NPM1 in IMS-M2 cell line by (-)-epigallocatechin-3-gallate
- Knowledge, attitude, and practices related to cutaneous leishmaniasis in an endemic focus of cutaneous leishmaniasis, Southern Iran
- An initial study of insect succession on decomposing rabbit carrions in Harare, Zimbabwe
