Surveillance of multidrug resistant suppurative infection causing bacteria in hospitalized patients in an Indian tertiary care hospital
Nabakishore Nayak, Rajesh K. Lenka, Rabindra N. Padhy
1Central Resarch Laboratory, IMS & Sum Hospital, Siksha 'O' Anusandhan Univrsity, K-8, Kalinga Nagar, Bhubaneswar 751003, Odisha, India
2Department of Microbiologr, IMS & Sum Hospital, Siksha 'O' Anusandhan Univrsity, K-8, Kalinga Nagar, Bhubaneswar 751003, Odisha, India
Surveillance of multidrug resistant suppurative infection causing bacteria in hospitalized patients in an Indian tertiary care hospital
Nabakishore Nayak1,2, Rajesh K. Lenka2, Rabindra N. Padhy1*
1Central Resarch Laboratory, IMS & Sum Hospital, Siksha 'O' Anusandhan Univrsity, K-8, Kalinga Nagar, Bhubaneswar 751003, Odisha, India
2Department of Microbiologr, IMS & Sum Hospital, Siksha 'O' Anusandhan Univrsity, K-8, Kalinga Nagar, Bhubaneswar 751003, Odisha, India
Objective: To examine antibiograms of a cohort of suppurative bacteria isolated from woundswabs from hospitalized patients of all economic groups of a typical Indian teaching hospital.
Methods: In surveillance, antibiotic resistance patterns of 10 species of suppurative bacteria isolated from wound-swabs over a period of 24 months were recorded. Those were subjected to antibiotic sensitivity test, using 16 prescribed antibiotics of 5 different groups (3 aminoglycosides, 4 beta-lactams, 3 cephalosporins, 4 fluoroquinolones, and 2 stand-alone) in each 6-month interval of the study period. Results: Of 1 156 samples collected, 819 samples yielded pathogenic bacteria, of which, Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Enterococcus faecalis (E. faecalis), Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), Enterobacter aerogenes (E. aerogenes), Proteus mirabilis (P. mirabilis) and Proteus vulgaris (P. vulgaris) were isolated in the order of predominance. Isolated bacterial strains were floridly multidrug resistant. Strains of E. faecalis and S. aureus were found resistant to vancomycin, one of the newly introduced antibiotics. Conclusions: Of these S. aureus, particularly the methicillin resistant strain predominates, followed by strains of S. pyogenes and P. aeruginosa that were in the higher proportions of multidrug resistance.
ARTICLE INFO
Article history:
Received 8 August 2013
Received in revised form 15 September 2013
Accepted 24 September 2013
Available online 20 June 2014
Wound-swabs
Suppurative infections
Hospitalized patients
Pathogenic bacteria
Multidrug resistance
1. Introduction
Infections at burn and surgical wounds with Grampositive (GP) and Gram-negative (GN) aerobic bacteria have become a constant source of consternation to clinicians and surgeons. In the last few decades, management of wounds and associated co-morbidities has been creating increasing levels of failures in their control, because of the emergence of multidrug resistant (MDR) bacteria, escalating to other body parts as blood stream infections (BSIs). Moreover, the decaying part of a burn-wound picks up opportunistic infections including fungi even, widening the opportunity for super-infection by several bacteria, both from community/hospital settings originally unrelated to skin; eventually, this leads to bacteremia in several vital organs idiosyncratic to type and the extent of drug resistance of the infecting bacterium[1]. Bacteria,Staphylococcus aureus(S. aureus),Enterococcus faecalis(E. faecalis) andStreptococcus pyogenes(S. pyogenes), as GPs, andPseudomonas aeruginosa(P. aeruginosa),Klebsiellasp.,Escherichia coli(E. coli),Proteussp.,Haemophilussp. andMoraxellasp., among GN aerobic bacteria, as well asBacteroidessp. andFusobacteriumsp., as anaerobic ones, are found mainly causing suppurations at wound sites, which lead to cystitis, otitis, boils, mastitis, phlebitis, meningitis, pneumonia, osteomyelitis, endocarditis urinary tract infection (UTI), and a few more[2]. Among opportunistic GNs,P. aeruginosaespecially is the ill-famed notorious pathogen linked to doggedly intractable burn injuries[3], promoting urinary tract infections (UTIs) even, leading to renal failure or/and endocarditis leading to heart failure, due to suppurative bacteria with portal entry as BSI[4]. Indeed, extrication fromthe pandemonium of burn injury is a staggering victory, and its management looms large to the clinician due to multiple infections by MDR bacteria. Specifically, infected surgical wounds cause suppurative skin reactions, bacterial fluid lesions and subcutaneous nodules leading to metastasis, when not properly addressed. An unattended/ unaddressed wound-site is the most vulnerable point of entry of the marauding pathogen that may cause bacteremia latter on, because of its multidrug resistance. Indeed, certain GN bacteria (P. aeruginosa,Acinetobacter baumannii(A. baumannii), andK. pneumoniae) have emerged with versatile strains, resistance to all antibiotics of the present time and such strains are informally known as pandrug resistant (PDR) bacteria[5,6].
S. aureus, once known as a harmless commensal of nasal nares and soft tissues of the body, has became resistant to oxacillin/methicillin (penicillin-derivatives), and is known as, methicillin resistantS. aureus(MRSA). In an earlier study, IMS and Sum Hospital, Bhubaneswar, had reported resistance of MRSA to 23 antibiotics used at the present time[7,8], confirming it as the ‘superbug in health domain’, in this zone too. Intrinsically, MRSA has become the most common suppurative pathogen worldwide today, due to the armamentarium of multiple resistances, often creating an intractable situation in intensive and critical care units[7-9]. From the available literature, it could be spelled out that,S. aureushas several factors of potential virulence: (1) surface proteins promoting colonization in host tissues; (2) invasion-promoting proteins causing spread in host tissues; (3) surface factors inhibiting phagocytic engulfment by host cells of immune system, due to bacterial capsule and proteinase-A production; (4) inherent biochemical properties such as, catalase production; (5) immunological disguises such as, proteinase-A and coagulase productions by bacteria; (6) production of membrane degrading toxins, haemotoxin and leukotoxin that modify properties of eukaryotic cell membrane; (7) exotoxin production that damages host tissues by provoking symptoms of a disease[10]. Thus, the majority of diseases caused byS. aureusor MRSA and its other recent clonal nexuses are multifactorial. Human staphylococcal infections are more frequent, but they usually remain at the local entry spot because of limited activity of the host defense system at the injury spot. A hair follicle, needle-stick injury or a surgical wound itself would be common spots of portal entry of a pathogen.Staphylococcal pneumonia (S. pneumonia)is the frequent complication of pneumonia causing swelling, accumulation of pus and additional necrosis of lung-tissue in a more serious situation. Moreover, the inflamed area due toS. aureusis often a fibrin clot of bacteria and dead leukocytes, as a pus-filled boil or an abscess[11]. More serious infections byS. aureusof skin are furuncles or impetigo, and the localized bone infection is osteomyelitis; but BSI ofS. aureusleads to septicemia and bacteremia attacking lungs, kidney, heart, skeletal muscles and meninges, at least in old and immunocompromised patients, eventually causing utmost morbidity and significant mortality[1]. Other suppurative GP bacteria areStreptococcusandEnterococcus, which have developed parallel resistance to many antibiotics of the time[12]. More often than not, multiple bacterial infections are commonplace at injury sites.
In face of accumulation of a vast majority of literature on MDR bacteria, it has become a matter of compulsion to conduct a regional surveillance on this exasperating class of pathogens, causing morbidity and mortality from general and surgical wound sites mainly[13]. This study records a gamut of antibiograms using 16 antibiotics of 5 groups of the time, with three GP and seven GN bacteria isolated from woundswabs of hospitalized patients of a typical Indian tertiary care hospital, a systematic study never reported before. This study should strengthen the epidemiological database of this vast subtropical country and other workers for a comparison as well as, in setting the stringent control of infections in a hospital. It is anticipated that this work would also benefit the pharmacy-world for further strategies in the crusade of the control of MDR bacteria, as wound infections give way to BSI and many more comorbidities. The infection dynamics of each bacterium studied here is so vast in literature that they would fill a book or two, when attempts would be made to describe their consternation in clinical management. This work examines antibiograms of a cohort of suppurative bacteria isolated from wound-swabs during 24 months from wards and cabins of the hospital, which treats patients of all economic groups, from slum dwellers and rural rustics to elite mass.
2. Materials and methods
2.1. Isolation and identification of bacteria
Strains of three GP,E. faecalis,S. aureus,S. pyogenes, and seven GNA. baumannii,E. coli,E. aerogenes,K. pneumoniae,P. mirabilis,P. vulgaris, andP. aeruginosawere isolated from the in-house patients during the study period. The isolated GP and GN bacteria were identified basing upon their colony morphology and results obtained from standard biochemical test, as done previously[7,14,15]. Microbial Type Culture Collection (MTCC) strain of each GP or GN bacterium was used as the reference control in each biochemical test.
2.2. Antibiotic susceptibility test
All bacterial strains including the standard MTCC strains of each bacterium were subjected to antibiotic sensitivity tests by the Kirby-Bauer’s method/ disc diffusion method, using a 4 mm thick Mueller-Hinton (MH) agar (HiMedia, Mumbai) medium[16]. An aliquot of 0.1 mL of 0.5 McFarlandequivalents,approximately from an exponentially growing culture was spread on agar for the development of lawn of a strain of a bacterium at 37℃ in a BOD incubator (Remi CIM-12S). Further, on the lawn-agar of each plate, 8 high potency antibiotic discs (HiMedia) of 16 prescribed antibiotics of 5 different groups were placed, individually at equal distances from one another. Plates were incubated for 18 h at 37℃ and were examined for size-measurements of zones of inhibition around each disc, following the standard antibiotic susceptibility test chart of Clinical Laboratory Standard Institute (CLSI) guidelines[17].
3. Results
From hospitalized patients of wards and cabins of IMS and Sum Hospital, 1 156 wound-swabs samples yielded 819 strains of pathogenic bacteria belonging to 10 species (three GP and seven GN bacteria) during the span of 24 months (April 2011- March 2013). All strains (E. faecalis,S. aureus,S. pyogenes,A. baumannii,E. coli,E. aerogenes,K. pneumoniae,P. mirabilis,P. vulgarisandP. aeruginosa) were identified by standard biochemical tests and were maintained as axenic cultures in suitable media. In total, there were 52 strains ofE. faecalis, 251 strains ofS. aureus, 210 strains ofS. pyogenes, 48 strains ofA. baumannii, 39strains ofE. aerogenes, 62 strains ofE. coli, 53 strains ofK. pneumoniae, 24 strains ofP. mirabilis, 21 strains ofP. vulgarisand 59 strains ofP. aeruginosa. Thus,S. aureuswas the maximally isolated suppurative-infection-causing bacterium, followed byS. pyogenes,E. coli,P. aeruginosa,K. pneumoniae,E. faecalis,A. baumannii,E. aerogenes,P. mirabilisandP. vulgaris(Table 1). During the last 6-month period, minimum numbers of pathogens were isolated. GP bacteria as medium to large, smooth, entire, slightly raised, creamy yellow, green-coloured betahaemolytic colonies on blood agar, found positive to catalase and coagulase tests were confirmed asS. aureus. Similarly, characteristics the rest other GP and GN bacteria are presented (Tables 2 and 3).
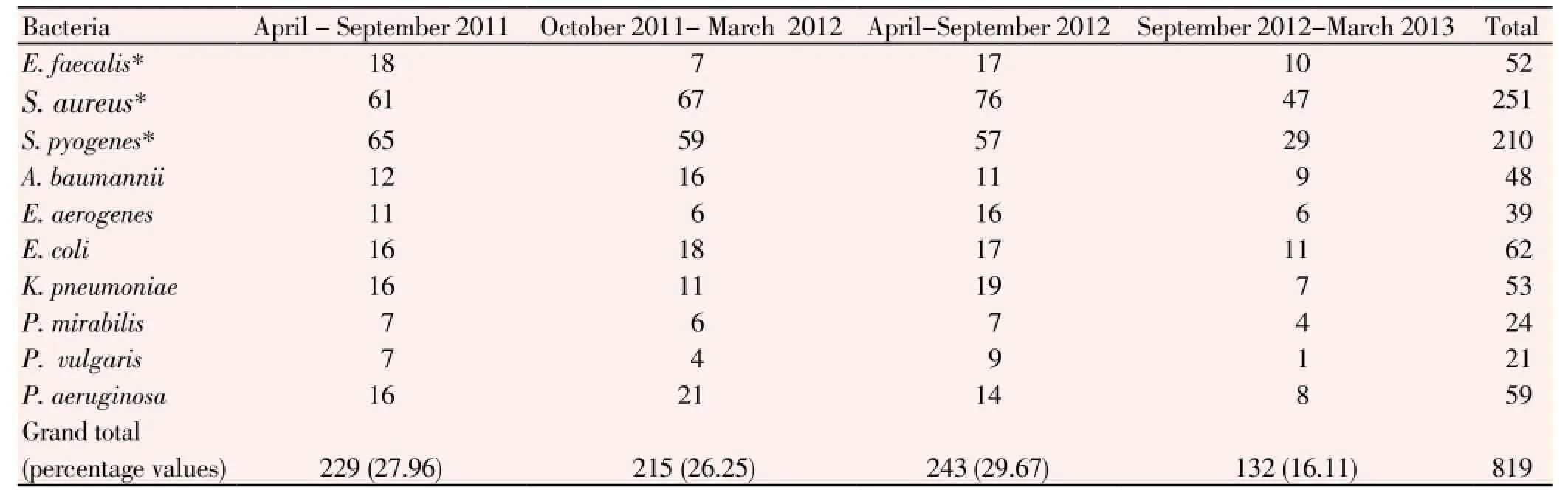
Table 1 Bacteria isolated from wound-swabs of the in-house wards patients.
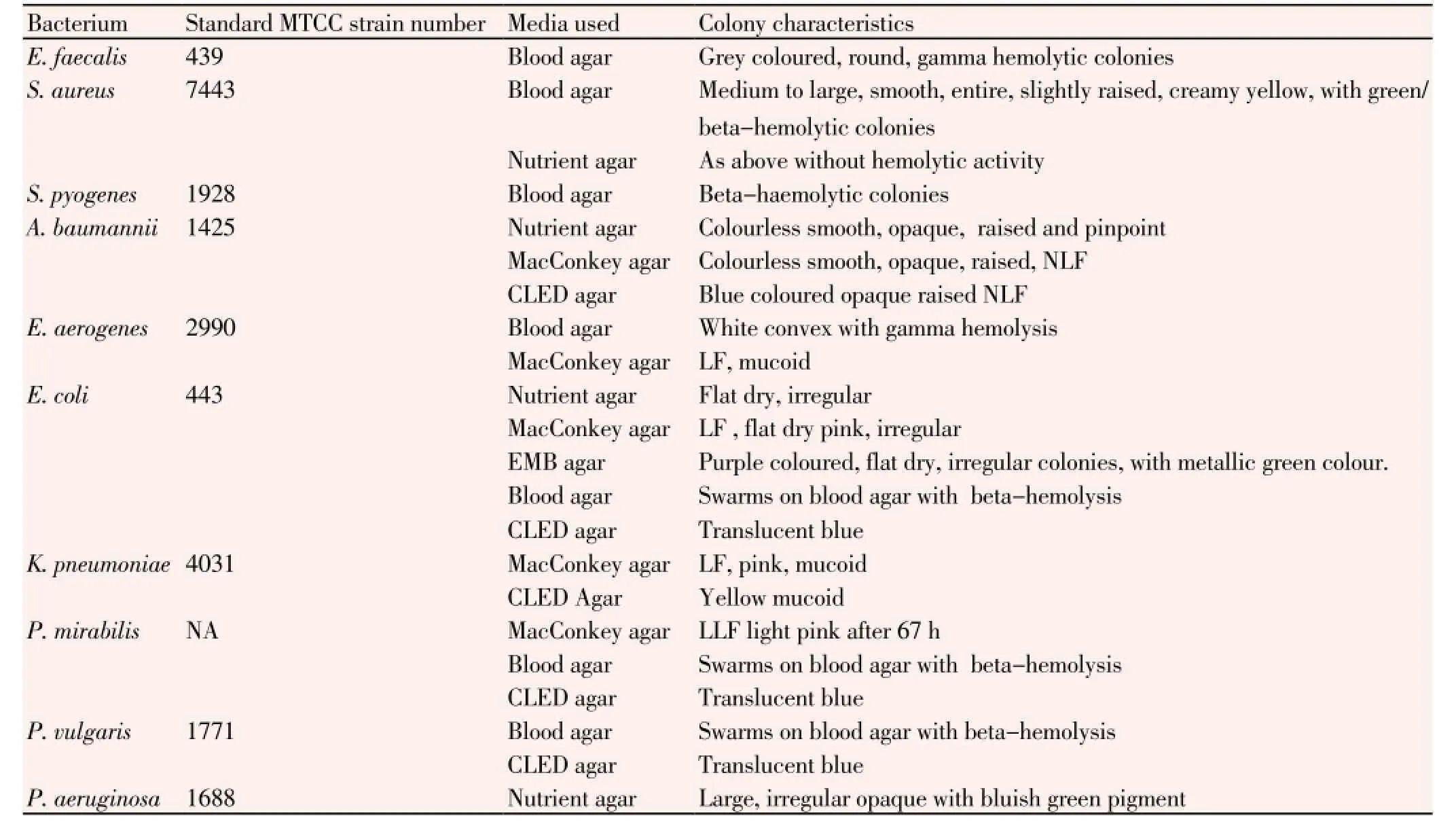
Table 2 Media used for isolation and maintenance pathogenic bacteria from urine samples and their colony characteristics.
All isolated bacterial strains were subjected to antibiotic sensitivity tests with all antibiotics used, in each 6-month period. Three aminoglycoside antibiotics (μg/disc), amikacin-30, gentamicin-10 and tobramycin-10 were moderately resistant to ten species of pathogens used, in ranges, 27% to 76% of 52 strains ofE. faecalis, 31% to 79% of 251 strains ofS. aureus, 51% to 76% of 210 strains ofS. pyogenes, 25% to 71% of 48 strains ofA. baumannii, 36% to 78% of 39 strains ofE. aerogenes, 61% to 81% of 62 strains ofE. coli, 66% to 79% of 53 strains ofK. pneumoniae, 66% to 89% of 24 strains ofP. mirabilis, 61% to 71% of 21 strains ofP. vulgaris, 66% to 86% of 59 strains ofP. aeruginosa. Among these three antibiotics, amikacin was the most resistant antibiotic to these pathogens (Table 4). Mean percent values of resistant strains of individual bacteria are discernible (Table 4).
Similarly, percentages of resistance patterns of 3 GP bacteria with four antibiotics of the beta-lactam group are detailed (Table 5); resistance patterns were in ranges: 52 to 74% of 52 strains ofE. faecalis, 61 to 82% of 251 strains ofS. aureus, and 47 to 76% of 210 strains ofS. pyogenes. Likewise, GN bacteria were tested for three beta-lactams only with resistance patterns as given: 47% to 77% of 48 strains ofA. baumannii, 52% to 81% of 39 strains ofE. aerogenes, 51% to 89% of 62 strains ofE. coli, 54% to 83% of 53 strains ofK. pneumoniae, 22% to 43% of 24 strains ofP. mirabilis, 34% to 48% of 21 strains ofP. vulgaris, 41% to 75% of 59 strains ofP. aeruginosa. For GN bacteria, antibiotics were resistant in the order: ampicillin >piperacillin/ tazobactam > amoxyclav. But with GP bacteria such an order would be: ampicillin > piperacillin/ tazobactam > oxacillin > amoxyclav (Table 5). Mean percentvalues of resistant strains of individual bacteria are evident in the Table 5.
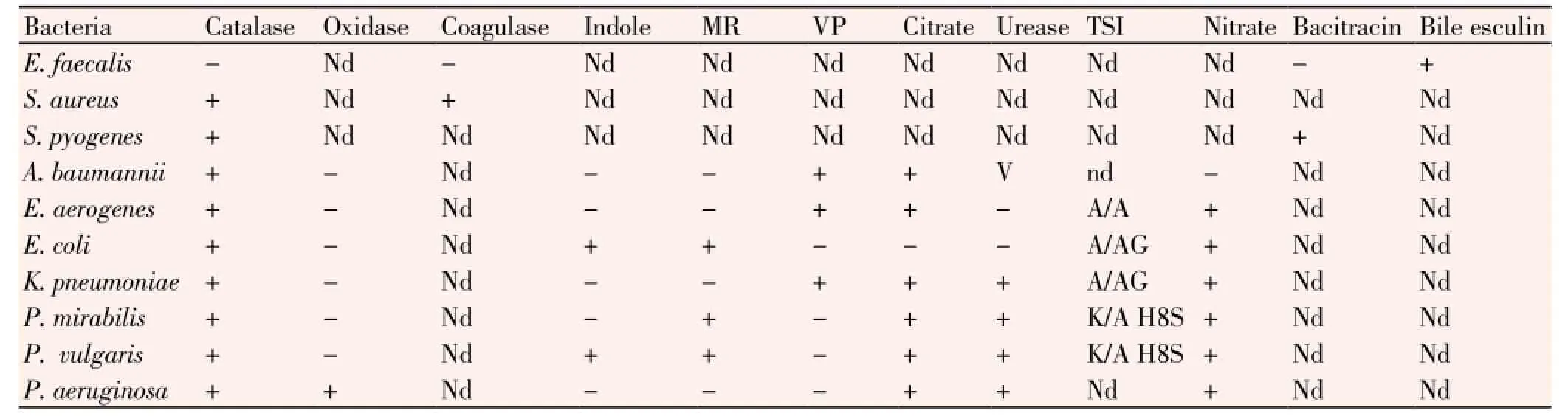
Table 3 Biochemical identifications of isolated Gram-positive and Gram-negative bacteria.
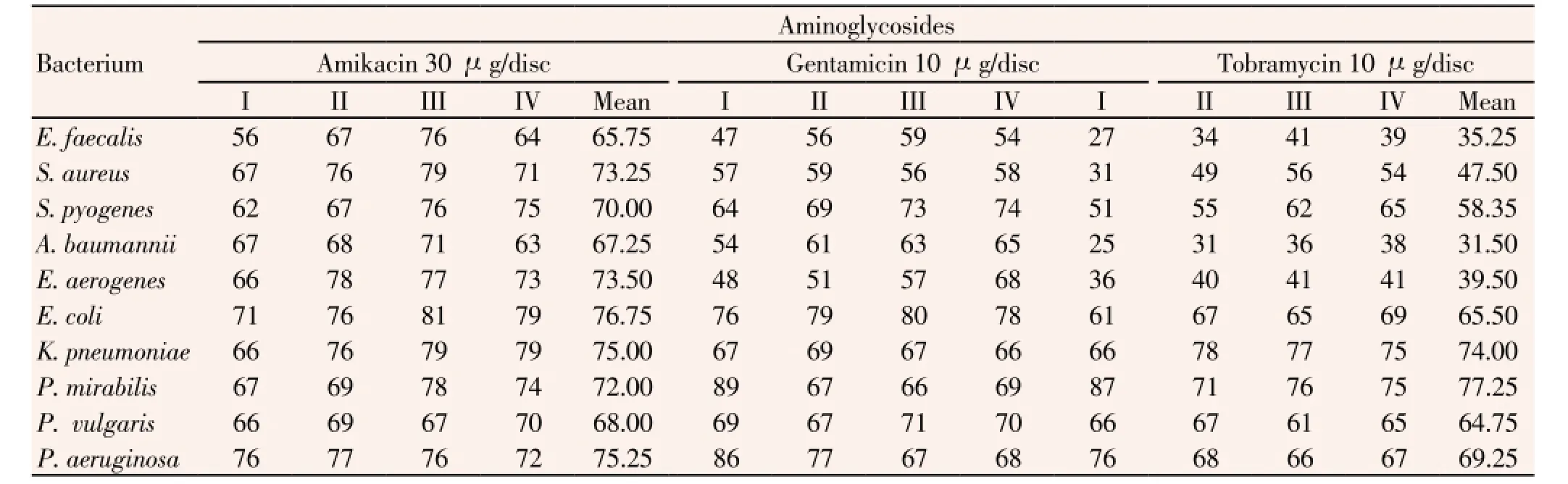
Table 4 Percentage of resistance of all clinically isolated bacteria to three antibiotics of aminoglycoside group.
Further, resistance-percent values of suppurativeinfection-causing bacteria to the cephalosporin group (cefepime, ceftazidime and ceftriaxone), in three 6-month phases were in ranges, 57% to 76% of 52 strains ofE. faecalis, 51% to 76% of 251 strains ofS. aureus, 51% to 85% of 210 strains ofS. pyogenes, 45% to 64% of 48 strains ofA. baumannii, 52% to 72% of 39 strains ofE. aerogenes, 75% to 88% of 62 strains ofE. coli, 54% to 74% of 53 strains ofK. pneumoniae, 32% to 51% of 24 strains ofP. mirabilis, 36% to 46% of 21 strains ofP. vulgaris, 49% to 77% of 59 strains ofP. aeruginosa(Table 6). All these three antibiotics were almost equally resistant to the isolated suppurative pathogens, confirming the consistence in the production of ESBL by majority of isolates.
Similarly, resistance-percent values of suppurative infection causing bacteria to antibiotics of the fluoroquinolone group (gatifloxacin, levofloxacin, ciprofloxacin and ofloxacin) in three 6-month phases were in ranges, 51% to 76% of 52 strains ofE. faecalis, 68% to 89% of 251 strains ofS. aureus, 44% to 65% of 210 strains ofS. pyogenes, 45% to 77% of 48 strains ofA. baumannii, 47% to 81% of 39 strains ofE. aerogenes, 60% to 87% of 62 strains ofE. coli, 58% to 81% of 53 strains ofK. pneumoniae, 25% to 49% of 24 strains ofP. mirabilis, 25% to 45% of 21 strains ofP. vulgaris, 54% to 81% of 59 strains ofP. aeruginosa(Table 7). These antibiotics were resistant to all these pathogens in the order: ofloxacin > gatifloxacin > norfloxacin >levofloxacin; the later one was newly introduced.
Lastly, detailed percentage values of GP bacteria were: 26% to 32% of 52 strains ofE. faecalis, 36% to 39% of 251 strains ofS. aureusand 34% to 47% of 210 strains ofS. pyogenesfor linezolid. The resistance patterns of 2 GP bacteria to the stand-alone antibiotic, vancomycin were in ranges was in ranges, 15% to 19% of 52 strains ofE. faecalis, and 11% to 17% of 251 strains ofS. aureus(Table 8). The emergence of vancomycin resistant mutants is a matter of clinical concern. Of 819 bacterial isolates in this study,S. aureus,S. pyogenes,P. aeruginosa,E. faecalis,K. pneumoniae,A. baumannii,E. aerogenesandProteussp. were found in the order of predominance to antibiotic resistance. This study recorded that all of these pathogens isolated from clinical samples were resistant to all antibiotics individually used.
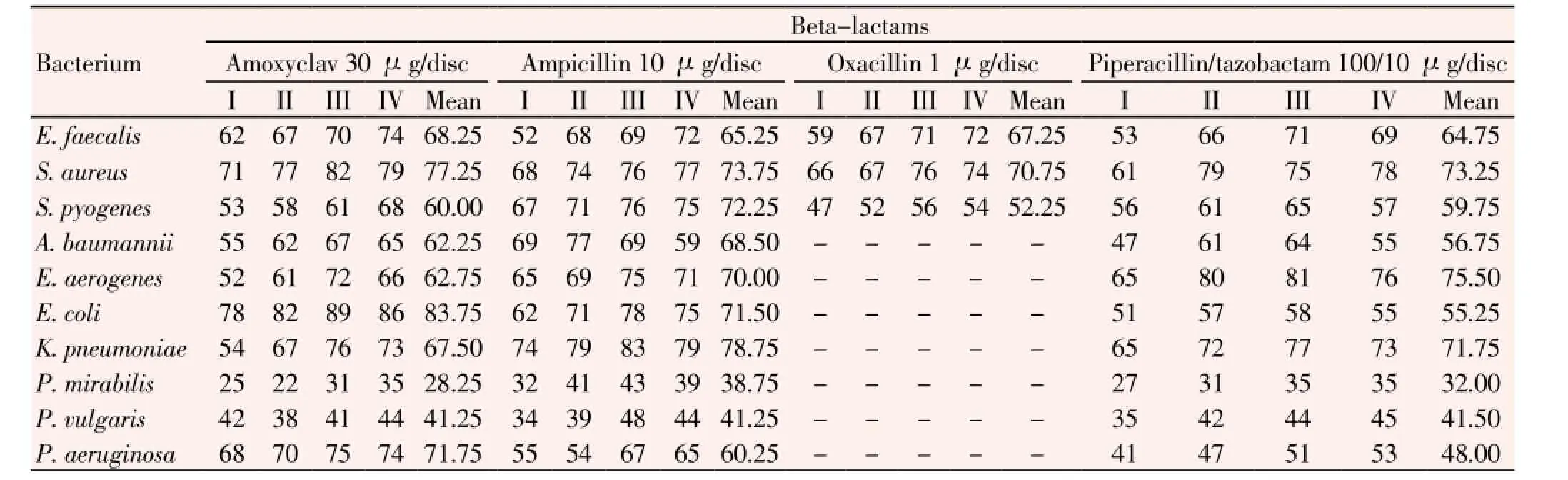
Table 5 Percentage of resistance of all clinically isolated bacteria to four antibiotics of β-lactam group.
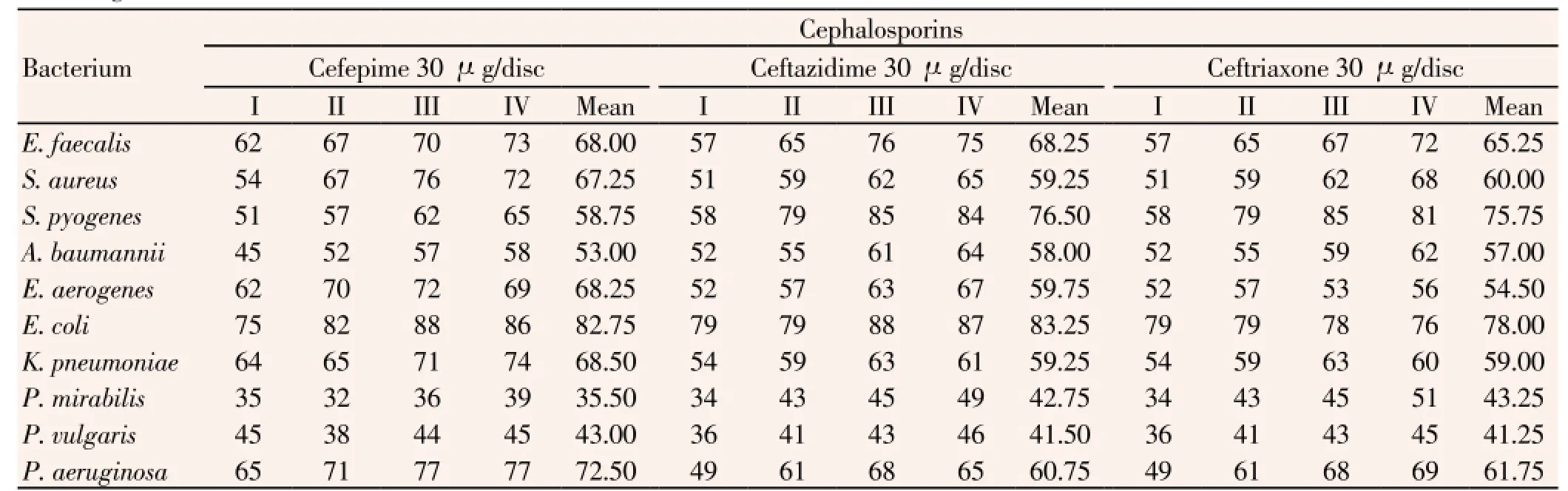
Table 6 Percentage of resistance of all clinical isolated bacteria to three antibiotics of cephalosporin group.
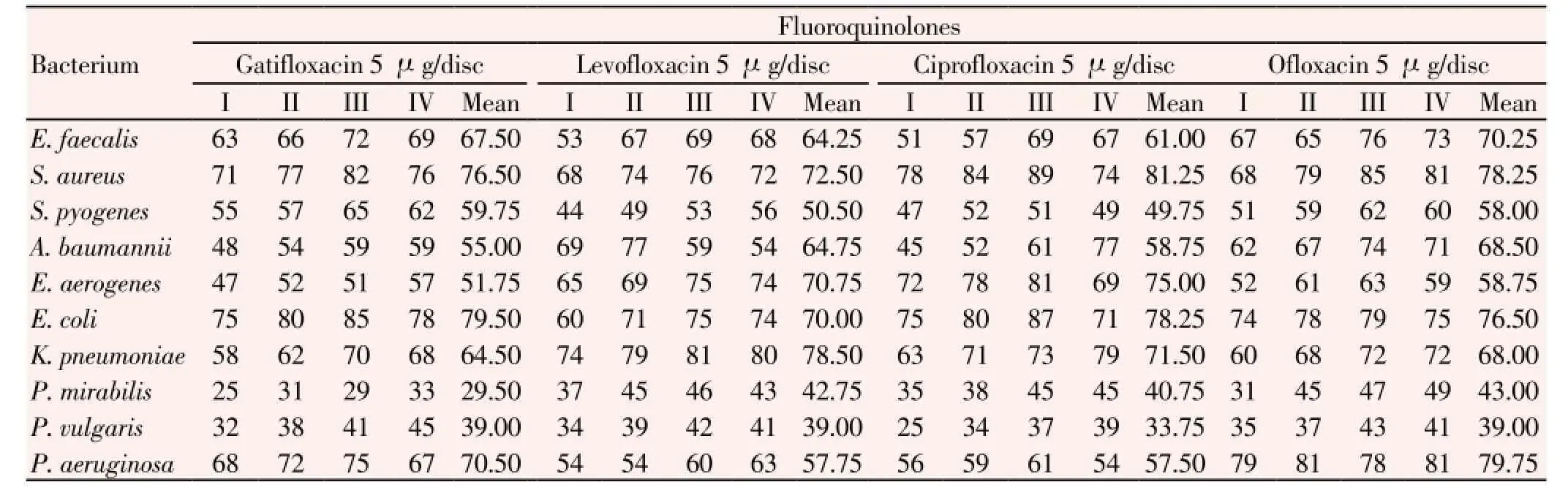
Table 7 Percentage of resistance of all clinical isolated bacteria to four antibiotics of fluoroquinolone group.
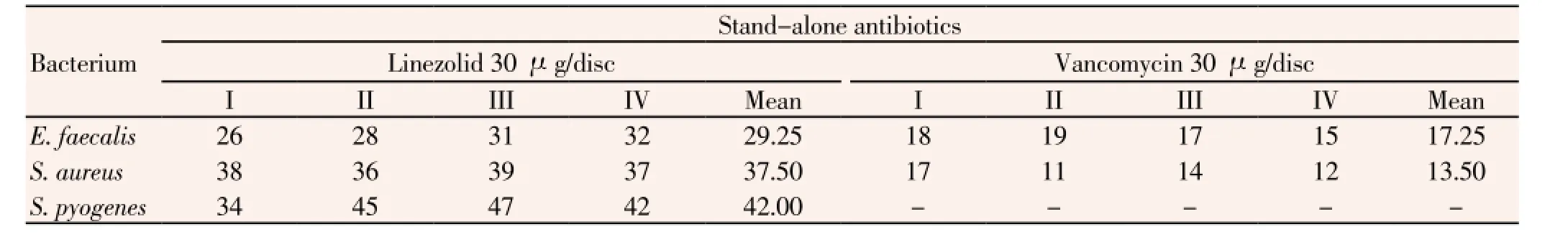
Table 8 Percentage of resistance of all clinical isolated Gram-positive bacteria to two stand-alone antibiotics.
Figure 1. Colonies of S. pyogenes on blood agar.
4. Discussion
Surgical wound-sites are generally taken care of suitably, as those wounds are planned, but burn injuries create beleaguered conditions in hospitals. Burn wounds, occurring impromptu, promote multiple infections due to the damage of the skin, the physical barrier, and cause immune suppression, independently[18]. Wounds being the invasive site for staphylococci, streptococci and enterococci among GPs, and GNs including pugnacious/PDR strains ofAcinetobacter,Klebsiella, Pseudomonasand a few more genera, are a priory, the prominent reckless nosocomial pathogens. Moreover, the ominousA. baumanniiin wounds is the most rife or notorious peripatetic, etiological agent in hospital acquired infections[19]; it is a PDR pathogen[5,14,20], affording much difficulty for its control.
For the control of wounds, topical antibacterial agents are used in addition to systemic ones. The popular topical antimicrobial, Acticoat with the silver sulfadiazine influences the respiratory chain at the cytochrome level, by disrupting the microbial electron transport system mediated by the silver ion[21]. In fact, because of the slow release of silver ions from Acticoat, a stronger and longer antimicrobial effect had been recorded, compared to the other classical silver containing topical agents. Thus, it was found as the more effective agent against aerobic, anaerobic GPs and GNs, fungi, and viruses on wound sites[21]. Another broad spectrum topical antibiotic obtained fromPseudomonas fluorescens, mupirocin had been recorded strongly inhibiting protein and RNA synthesis in pathogens[22]. Moreover,A. baumanniiin burn injury infections causes delay in the wound healing process, probably due to its ability to cause systemic circulation; sepsis mostly occurs ending with high mortality rates with patients who were inadequately treated[20,23]. Acticoat, however, was independently reported to control MDRA. baumannii,in vivoby other workers[18,24]. Octanidiene dihyrocholride is another topical antibacterial agent used in the control of active and chronic wounds[23,25]without any disadvantage of the other chemical, silver sulfadiazine, as the later has a lower escher-penetration rate, needing frequent applications, whereas octenidine dihydrochloride based formulations are effective against GPs, GNs and fungi[26]. Quintessentially, Acticoat had appreciable control actions over octanidiene dihyrocholride or chlorhexidine acetate onA. baumannii[23]. Sofra-tulle (framycetin sulphate bp 1%), framyzyne are sulfamylone creams, also are used as topical applications for burn injuries withPseudomonasinfections. Indeed, aggressive uses of antibiotics lead to conditions for the growth of fungi in burns;Candida albicansis the most common associated fungal pathogen[27]. Powdered sulfonamides, as well as, its cream-based formulations had been a popular topical medicine for burn wounds, with a limited absorption of sulfonamides at injured sites. Further, in children, agranulocytes are induced with another control agent, sulfapyridine, when applied to burn injuries. Therefore, sulfonamide creams are rarely used for burn injuries. Nevertheless, sulfadyazine are less readily absorbed in the body in comparison to sulfonamides[28].
GP bacteria particularly survive the thermals of burning, unless an immediate topical treatment is done around 48 hours; staphylococci come up from the sweat glands and hair follicles colonizing at the burn site[29]. In a typical study, from surgical wounds analyzed with 343 patients,S. aureuswas prevalent at 28.2%,P. aeruginosaat 25.32%,E. coliat 7.8%,S. epidermidisat 7.1% andE. faecalisat 5.6%[30].Proteussp. particularly was reported to have been isolated from an Indian tertiary care hospital; all the isolated strains of pathogens were resistant to the entire antibiotics group used, among which,S. aureusin GPs,A. baumanniiandP. aeruginosaamong GNs had the maximum resistance values[31]. In a study from Netherlands, it was reported that a cohort of 11 patients and nurses of the surgical ward of a hospital were infected withS. pyogeneswith a particular serotype of low virulence; another group of patients from the same hospital had infection with another serotype ofS. pyogenescausing heavy delay in wound healing[32]. This study clearly demonstrated the outbreak ofS. pyogenesinfections in hospital settings.
Apart from surgical wounds, post-operative peritonitis is an important determinant in toxic shock syndrome in ICU patients. In a study from Paris, it was found that GP bacteria were prevalent in 40% of cases andE. faeciumwas the leading organism (19%), followed by streptococci (11%),S. aureus(3%), coagulase negative staphylococci (8%). Similarly, among infections from GNs, Enterobacteriaceae members contribute 37% of the total infection;E. coliwere 18%, and the rest other bacteria were as follows:Enterobacter8%,Klebsiella5%,Morganella3%,Proteus2%,Citrobacter2%;Pseudomonas6% andA. baumannii1%. The prevalence of the total anaerobic bacteria causing post-operative peritonitis in this study was 13% with the predominance ofBacteroidessp. Among all these bacteria, the prevalence of MDR Enterobacteriaceae was 5% and that ofP. aeruginosawas 2%[33]. An Indian study, however, recorded increasing incidences of MDRKlebsiellasp., isolated from 72% infected hospitalized patients, out of which, 3.44% isolates were capable of the production ofKlebsiella pneumoniaecarbapenemase enzyme, of which 26 strains from hospital and 8 strains were from community isolates[34]. From Greece, MDR GN bacteria were reported from ICU surgical patients; it was reported that hospitalization for more than 5 days increased the susceptibility towards MDR infections[13]. Sternal wound infections after cardiac surgery were caused by MRSA and GNs in 22.4%, within two years of study with 1895 patients in France[35]. It is consensus that a patient admitted to a hospital, more often with resource-limited settings, for a specific problem or even due to problems of old age, picks up one or more MDR infectious bacteria. Risk-factors of post-discharge invasive infection from MRSA in hospitalized patients can be imagined, as monitored with MRSA in the USA through BSI for its high rates of antibiotic resistance, for example[36].
It is believed that antibiotics are symbol and substance of clinical management today, and it is hard to think of an aspect of contemporary life that does not depend on antibiotics. Taking to surprise, nosocomial spread of MDR bacteria has become so commonplace that clinicians of both developing and developed countries get often emotionally grueled during treatment to delirious hospitalized patients of any ailment, with whom an infection on hospitalization with an MDR bacterium could be a terminal illness. As known, several factors contribute to the problem of the emergence of MDR bacteria: (1) Device-and-fomite-associated nosocomial infections are frequent in hospital settings everywhere and always[37], with begrimed settings. (2) The drug resistance character has ramifications: bacteria may be resistance to representative, frequently used antibiotics of several classes; paradigmatically, the production of extended spectrum β-lactamase has rendered resistance to antibiotics of penicillin/cephalosporin group[38]; carbapenemase production affords resistance in GN bacteria to carbapenems (imipenem, meropenem and ertapenem) in use. (3) Mutation rates in bacteria are faster because of simple, plastic genomes — one mutant cell in 106 to 108 bacterial cells in the presence of an antibiotic-stress is known to be drug resistant[39]. (4) To avoid host toxicity, antibiotics doses are mostly fixed at some lower concentration that is often below the mutant preventive concentration [40], giving ways to the development of mutants. (5) Genetic recombinationmechanisms (bacterial transformation and conjugation) are operative in natural systems, such as hospital sewages, facilitating the creation of a pool of drug resistant characters in camaraderie MDR strains[41]. (6) Absence of a stringent antibiotic policy triggers the emergence of resistant bacteria as both clinicians and patients use antibiotics belonging to higher generations without often being warranted. (7) Patients, particularly, often do not complete the course of prescribed dose of an antibiotic, because of the blithesome effect of the control of infection from the start of the course. Thus, bacterial resistance to antibiotics is a consistently complex and dynamic affair, involving major genetic and biochemical mechanisms, bacterial transformations, interchange of integrons, hypermutabilty, plasmids mediated improvements in resistance factors, ending with drug efflux mechanism and gaining of characters to synthesize indigenous and exogenous antibiotic degrading enzymes.
This study demonstrated that most of these pathogens isolated from clinical samples were MDR and they are potential enough to destroy the clinical totem pole of a hospital and to precipitate devastating episodes in the community. As analyzed, suppurative infections are one of the major problems of health, as MDR bacteria could attack several organs such as lungs, heart and kidneys, through BSI. Compared to infection studies on suppurative pathogens from other hospitals globally, the surveillance data of this typical hospital clearly gives high values of wound infections among which, the MRSA infection predominates. Burn injuries are needed to be attended more stringently in this peninsula too. Steps to prevent invasive infections of most of these pathogens, particularly MRSA in communities must be started in the hospital with proper/ prior post-discharge settings.
Conflicts of interests
The authors declare that they have no conflict of interests.
Acknowledgements
This work is a part of PhD thesis of N Nayak in Biotechnology of S‘O’A University and he is too supported by the university as JRF. Part of the work was supported by project no. 21/ (0859)/11/EMR-II, CSIR, New Delhi to RN Padhy. IMS and Sum Hospital provided extended facilities.
[1] Pop-Vicas A, Tacconelli E, Gravenstein S, Lu B, D’Agata EM. Influx of multidrug-resistant, gram-negative bacteria in the hospital setting and the role of elderly patients with bacterial blood stream infection. Infect Control Hosp Epidemiol 2009; 30: 325-331.
[2] Nia KM, Sepehri G, Khatmi H, Shakibaie MR. Isolation and antimicrobial susceptibility of bacteria from chronic suppurative otitis media patients in Kerman, Iran. Iran Red Crescent Med J 2011; 13: 891-894.
[3] Bielecki P, Pucha?ka J, Wos-Oxley ML, Loessner H, Glik J, et al. In vivo expression profiling of Pseudomonas aeruginosa infections reveals niche-specific and strain-independent transcriptional programs. PLoS ONE 2011; 6(9): e24235.
[4] Dryden MS. Complicated skin and soft tissue infections. J Antimicrob Chemother 2010; 65: 35-44
[5] Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis 2005; 5: 24.
[6] Kujath P, Kujath C. Complicated skin, skin structure and soft tissue infections - are we threatened by multi-resistant pathogens? Eur J Med Res 2010; 15: 544-553.
[7] Dubey D, Padhy RN. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethno-medicinal plants used by an aborigine of India. Asian Pacif J Trop Dis 2012; 2: 273-281.
[8] Dubey D, Rath S, Sahu MC, Pattnaik L, Debata NK, Padhy RN. Surveillance of infection status of drug resistant Staphylococcus aureus in an Indian teaching hospital. Asian Pacif J Trop Dis 2012; 3: 133-142.
[9] Sisirak M, Zvizdic A, Hukic M. MRSA as a cause of nosocomial wound infections. Bosn J Basic Med Sci 2010; 10: 32-37.
[10] Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008; 46: S350-S359.
[11] Zeller JL, Burke AE, Glass RM. JAMA patient page. MRSA infections. JAMA 2007; 298: 18-26.
[12] Sood S, Malhotra M, Das BK, Kapil A. Enterococci were originally classified as enteric Gram-positive cocci and later included in the genus Streptococcus. Indian J Med Res 2008; 128: 111-121.
[13] Alexiou VG, Michalopoulos A, Makris GC, Peppas G, Samonis G, Falagas ME. Multidrug resistant Gram-negative bacterial infection in surgical patients hospitalized in the ICU: A cohort study. Eur J Clin Microbiol Infect Dis 2012; 31:557-566.
[14] Rath S, Dubey D, Sahu MC, Debata NK, Padhy RN. Surveillance of multidrug resistance of 6 uropathogens in a teaching hospital and in vitro control by 25 ethnomedicinal plants used by an aborigine of India. Asian Pacif J Trop Biomed 2012; 2: S818-S829.
[15] Rath S, Padhy RN. Monitoring in vitro antibacterial efficacy of Terminalia alata Heyne ex. Roth, against multidrug resistant enteropathogenic bacteria. J Act Med 2013; 3: 93-102
[16] Sahu MC, Rath S, Dubey D, Debata NK, Padhy RN. Multidrug resistance of Pseudomonas aeruginosa as known from surveillanceof nosocomial and community infections in an Indian teaching hospital. J Pub Health 2012; 20: 413-423.
[17] CLSI-Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing: twenty-first informational supplement. Document M 200-S21; Wayne, 2011.
[18] Glasser JS, Guymon CH, Mende K, Wolf SE, Hospenthal DR, Murray CK. Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns 2010; 36: 1172-1184.
[19] Dallo SF, Weitao T. Insights into Acinetobacter war wound infections, biofilms and control. Adv Skin Wound Care 2010; 23: 169-174.
[20] Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. Nitric oxide releasing nanoparticles are therapeutic for Acinetobacter baumannii wound infections. Virulence 2010; 1: 62-67.
[21] Ulkur E, Okul O, Karagoz H, Yeniz E, Celikoz B. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigras), and fusidic acid 2% (Fucidin) for topical antibacterial effect in methicillin-resistant staphylococci-contaminated, fullskin thickness rat burn wounds. Burns 2005; 31: 874-877.
[22] Acikel C, Oncul O, Ulkur E, Bayram I, Celikoz B, Cavuslu S. Comparison of silver sulfadiazine 1%, mupirocine 2% and fusidic acid 2% for topical antibacterial effect in methicillin resistant staphylococci-infected full-skin thickness rat burn wounds. J Burn Care Rehabil 2003; 24: 37-41.
[23] Uygur F, Oncul O, Evinc R, Diktas H, Acar A, Ulkur E. Effects of three different topical antibacterial dressings on Acinetobacter baumannii contaminated full-thickness burns in rats. Burns 2009; 35: 270-273.
[24] Davis SC, Pisanni F, Montero RB. Effects of commonly used topical antimicrobial agents on Acinetobacter baumannii: an in vitro study. Mil Med 2008; 173: 74-78.
[25] Wolcott RD, Rhoads DD, Bennett ME, Wolcott BM, Gogokhia L, Costerton JW, et al. Chronic wounds and the medical bio-film paradigm. J Wound Care 2010; 19: 45-46, 48-50, 52-53
[26] Rigopoulos D, Rallis E, Gregoriou S, Larios G, Belyayeva Y, Gkouvi K, et al. Treatment of Pseudomonas nails infections with 0.1% octenidine dihydrochloride solution. Dermatology 2009; 218: 67-68.
[27] Isibor JO, Oseni A, Eyaufe A, Osagie R, Turay A. Incidence of aerobic bacteria and Candida albicans in post-operative wound infections. Afr J Microbiol Res 2008; 2: 288-291.
[28] International consensus. Appropriate use of silver dressings in wounds. An expert working group consensus[Online]. Available from: http://www.woundsinternational.com. [Accessed January 15, 2013]
[29] Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns 2004; 30: 357-361.
[30] Giacometti A, Cirioni O, Schimizzi AM, Del Prete MS, Barchiesi S, D’Errico MM, et al. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol 2000; 38: 918-922
[31] Ravisekhar G, Bernu D, Vishnu B, Hartla S, Arti K, Ammini AC, Rama C. Clinical microbiological study of diabetic foot ulcers in an Indian tertiary-care hospital. Diabetic Care 2006; 29: 1727-1732.
[32] Davies BI, Hirsch J, Werink TJ, Toenbreker H, Bainczijk F, van Leeuwen WJ. A Streptococcus pyogenes outbreak caused by an unusual serotype of low virulence: the value of typing techniques in outbreak investigations. J Infect 1999; 38: 185-190.
[33] Augustin P, Kermarrec N, Muller-Serieys C, Lasocki S, Chosidow D, Marmuse JP, et al. Risk factors for multidrug resistant bacteria and optimization of empirical antibiotic therapy in postoperative peritonitis. Crit Care 2010; 14: R20.
[34] Jadhav S, Misra RN, Gandham N, Ujagare M, Ghosh P, Kalpana A, et al. Increasing incidence of multidrug resistance Klebsiella pneumoniae infections in hospital and community settings. Int J Microbiol Res 2012; 4: 253-257.
[35] Maillet JM, Oghina G, Le Besnerais P, Thierry S, Bouquet G, Mesnildrey P, et al. Preoperative carriage and postoperative samespecies sternal wound infection after cardiac surgery. Interact Cardiovasc Thorac Surg 2011; 13: 381-385.
[36] Duffy J, Dumyati G, Bulens S, Namburi S, Gellert A, Fridkin SK, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013; doi.org/10.1016/j.ajic.2012.10.020.
[37] Crnich CJ, Drinka P. Medical device - associated infections in the long-term care setting. Infect Dis Clin North Am 2012; 26: 143-164.
[38] Shahcheraghi FM, Rahbar SM, Zahraei VS, Shooraj F. Transmission of Vibrio cholera O1 serotype inaba in a rural area of qazvin, Iran Associated with Drinking Water. Asian J Epidemiol 2009; 2: 66-71.
[39] Gillespie SH, Basu S, Dickens AL, O’Sullivan DM, McHugh TD. Effect of sub-inhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J Antimicrob Chemother 2005; 56: 344-348.
[40] Ferrari R, Magnani M, Souza RB, Tognim MCB, Oliveira TCRM. Mutant prevention concentration (MPC) of ciprofloxacin against Salmonella enterica of epidemic and poultry origin. Curr Microbiol 2011; 62: 628-632.
[41] Perron GG, Lee AEG, Wang Y, Huang WE, Barraclough TG. Bacterial recombination promotes the evolution of multi-drugresistance in functionally diverse populations. Proc Biol Sci 2012; 279: 1477-1484.
ment heading
10.1016/S2221-6189(14)60033-0
*Corresponding author: Prof. Dr. R. N. Padhy, CSIR Scientist, Central Research Laboratory, IMS & Sum Hospital, Siksha ‘O’ Anusandhan University, K-8, Kalinga Nagar, Bhubaneswar 751003, Odisha, India.
Tel: +919437134982
E-mail: rnpadhy54@yahoo.com
Part of the work was supported by project no. 21/(0859)/11/EMR-II, CSIR, New Delhi to RN Padhy.
 Journal of Acute Disease2014年2期
Journal of Acute Disease2014年2期
- Journal of Acute Disease的其它文章
- Acute anaphylactic reaction to expired chlorpheniramine injection
- An insidious presentation of thrombotic thrombocytopenic purpura: A case report and brief literature review
- Acute anaphylaxis after red fire ant bite
- Acute reaction after administration of nimotuzumab: a concern
- Acute natural disaster relief by role of military medicine
- HIV/AIDS related deaths from three district hospitals of West Bengal: An observation
