Prevalence and trends of aminoglycoside resistance in Shigella worldwide, 1999-2010
Bing Gu葒, Xing Ke葒, Shiyang Pan, Yan Cao Ling Zhuang, Rongbin Yu,
Huimin Qianc, Genyan Liua,b, Mingqing Tonga,b
aDepartment of Laboratory Medicine, the First Affiliated Hospital, Nanjing Medical University, Nanjing, Jiangsu 210029, China; bNational Key Clinical, Department of Laboratory Medicine, Nanjing, Jiangsu 210029, China;
cDepartment of Acute Infectious Disease Prevention and Control, Jiangsu Provincial Center for Disease Prevention and Control, Nanjing, Jiangsu 210029, China;
dDepartment of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 210029, China.Received 12 November 2012, Revised 09 December 2012, Accepted 17 January 2013, Epub 28 February 2013
INTRODUCTION
Acute gastroenteritis and diarrheal diseases continue to be a health problem worldwide, especially in developing countries. They account for approximately 2.5 million deaths per year in children < 5 years of age[1,2]. Worldwide, the most common bacterial pathogens causing these diseases are: Salmonella spp, Shigella (S.) spp, Campylobacter spp, Escherichia coli O157:H7, Listeria monocytogenes, Vibrio cholerae, and Yersinia enterocolitica[3,4]. The common route of infection by these pathogens is the ingestion of contaminated food and drinks[5]. Infection by Shigella species is an important global public health problem[6]. Shigella infections, especially S. flexneri and S. sonnei infections, can lead to illness ranging from mild, self-limited diarrhea to severe dysentery with frequent passages of blood and mucus, high fever, cramps, tenesmus, and in rare cases, bacteremia. Complications of shigellosis are seen most frequently in children, the elderly, and the immunocompromised. Therefore, shigellosis is recognized by the World Health Organization (WHO) as major global public health concern[7,8].
Prompt treatment with effective antimicrobial agents shortens the duration of symptoms and carriage, and reduces the spread of infection. However, antimicrobial resistance has complicated the selection of empirical agents for the treatment of shigellosis, particularly in children. Shigella isolates often showed resistance to commonly used, inexpensive antimicrobials, including ampicillin, piperacillin, trimethoprim-sulfamethoxazole, thereby drastically reducing therapeutic possibilities. Thus, the use of sulfonamide or β-lactam antibiotics would not be appropriate for empirical treatment of shigellosis. Shigella strains have become progressively resistant to multiple antimicrobial agents, initially to sulfonamides[9,10], shortly after they became commercially available; resistance to tetracycline, chloramphenicol, and streptomycin was seen less than 10 years after each was introduced, with subsequent resistance to ampicillin, kanamycin, and trimethoprim-sulfamethoxazole[11,12]. In certain eastern Africa populations and in a study from China, aminoglycoside resistance of Shigella is a common finding[13,14].
The present study aimed to identify the worldwide prevalence and distribution of aminoglycosideresistant Shigella using meta-analysis based on data gathered from a systematic review of articles reported between January 1999 and July 2012. The relevant estimates were evaluated for new cases and previously treated cases, respectively, which could provide a clear profile for the status of aminoglycoside-resistant Shigella globally.
MATERIALS AND METHODS
Literature identification
We conducted a computerized search of MEDLINE (January 1999--July 2012) and EMBASE (January 1999--July 2012) to identify all reports on aminoglycosides resistance associated with Shigella infections. The following keywords were used in searches: "bacterial surveillance" or "antimicrobial resistance" or "bacterial resistance" and "Shigella"[15]. We also attempted to identify potentially relevant articles by checking the references of the germane articles and through personal communications with colleagues.
Inclusion and exclusion criteria
Two investigators (BG and XK) reviewed potentially appropriate studies independently, to determine whether they met predetermined eligibility criteria. Disagreements between the reviewers were resolved by consensus. Studies obtained from the literature search were checked by title and citation. If an article appeared relevant, the abstract was reviewed. Relevant abstracts were examined in full text. The inclusion and exclusion criteria were established by the investigators prior to review of the literature. The inclusion criteria were as follows: original article, short communication, correspondence or letter which provided sufficient original data, and all strains isolated from stool. Studies were excluded if they met the following conditions: (1) review or case report; (2) not 1999--2010 data; (3) not separated by country/region; (4) non-human bacterial source; (5) did not include study drugs; (6) did not include resistance results for study pathogens; (7) inclusion/exclusion criteria were not presented; (8) non-recommended regimens/dosing; (9) susceptibility results were not presented. Before we excluded the studies, authors of such studies were contacted in an effort to obtain missing data.
Validity assessment
Studies were assessed for quality and only high quality studies were included for analysis. Characteristics of high quality studies were: prospective cohort, retrospective consecutive cohort; provided basic data including study period and area, total tested numbers and resistant numbers; susceptibility test was performed in accordance with guidelines established by the Clinical and Laboratory Standard Institute (CLSI)[16]; reported at least one of three antimicrobials (gentamicin, kanamycin and amikacin) with quality control; individuals included in studies had no infections other than bacillary dysentery. Only one representative case for each outbreak was included, unless the isolates had different antibiotic susceptibility patterns. When study strains overlapped, we included strains from the more recent and larger study in the analysis. If the strains from the smaller study provided data that was not reported in the larger study, results were included for that specific variable.
Data extraction and statistical analysis
Data extraction was performed by two reviewers (BG and XK) using a standardized extraction form. When there was disagreement, the relevant paper was reviewed and differences were resolved by consensus. Microsoft Excel (version 12.0) software was used for data entry and analysis. In our review, considering the possibility of significant heterogeneity between studies which were tested with the Q test (P < 0.10 was considered indicative of statistically significant heterogeneity), random effect models or fix effect models were chosen by P value for meta-analysis. Freeman-Tukey arcsin transform to stabilize variances, and after the meta-analysis, investigators can transform the summary estimate and the CI boundaries back to proportions using sin function. Specific conversion details were previously described[17]. Data manipulation and statistical analyses were undertaken using the Statistical Software Package (STATA) 11.0 (STATA Corporation, College Station, TX, USA).
RESULTS
Studies and endpoints
We reviewed 3,176 publications from MEDLINE and EMBASE reported from 1999 to 2012. Candidate articles are shown in Fig. 1. After exclusion based on title and abstract evaluation, 580 articles were retrieved for detailed, full-text evaluation. As shown in Fig. 1, among the included articles, 46 studies were reviews or case reports. Ninety-one articles did not use data that was within the 12-year study period. Findings in 18 articles were not separated by country/region. Human or resistance results for study pathogens were not presented in 28 and 69 studies, respectively. Detailed results of drug susceptibility testing (DST) with respect to study drugs were not provided in 181 studies. Recommend regimens, recommended dosing or data on minimal inhibitory concentrations (MIC) was not included in 9, 42, and 28 studies, respectively. Finally, 68 studies, addressing the prevalence of aminoglycoside-resistant Shigella in new cases or in previously treated cases, were identified.
Status of aminoglycoside-resistant Shigella
Table 1 shows the meta-analysis of the global status of Shigella aminoglycoside resistance in new cases or in previously treated cases worldwide. The summarized prevalence of gentamicin, kanamycin and amikacin resistance was found to be 3.95% (95%CI: 3.59%-4.22%) (n/N= 937/14,059), 6.88% (6.36%-7.43%) (n/N=1,106/8,647) and 1.29% (0.97%-1.68%) (n/N=432/8,614), respectively. Importantly, evident heterogeneity was observed (P < 0.001). In the stratified analyses, the prevalence of any drug resistance was observed to vary by geographic areas, study years and subtypes. Lower rates were observed for studies from Europe-America and the period of 1999 to 2004, while the rates from Asia-Africa and using the subtypes of S. flexneri were higher. The end time for enrollment of the cases (after 2008) did not significantly change the results.
The most common drug resistance was observed for kanamycin. Among kanamycin resistance, the highest drug resistance rate by geographic areas was found in Asia with a prevalence of 16.78% (7.58%-28.71%). Similarly, the most common resistance was observed for 2005-2007 and S. flexneri with a summarized combined prevalence of 12.05% (11.18%-14.21%) and 9.25% (7.69%-10.96%), respectively.Studies considered for primary analysis
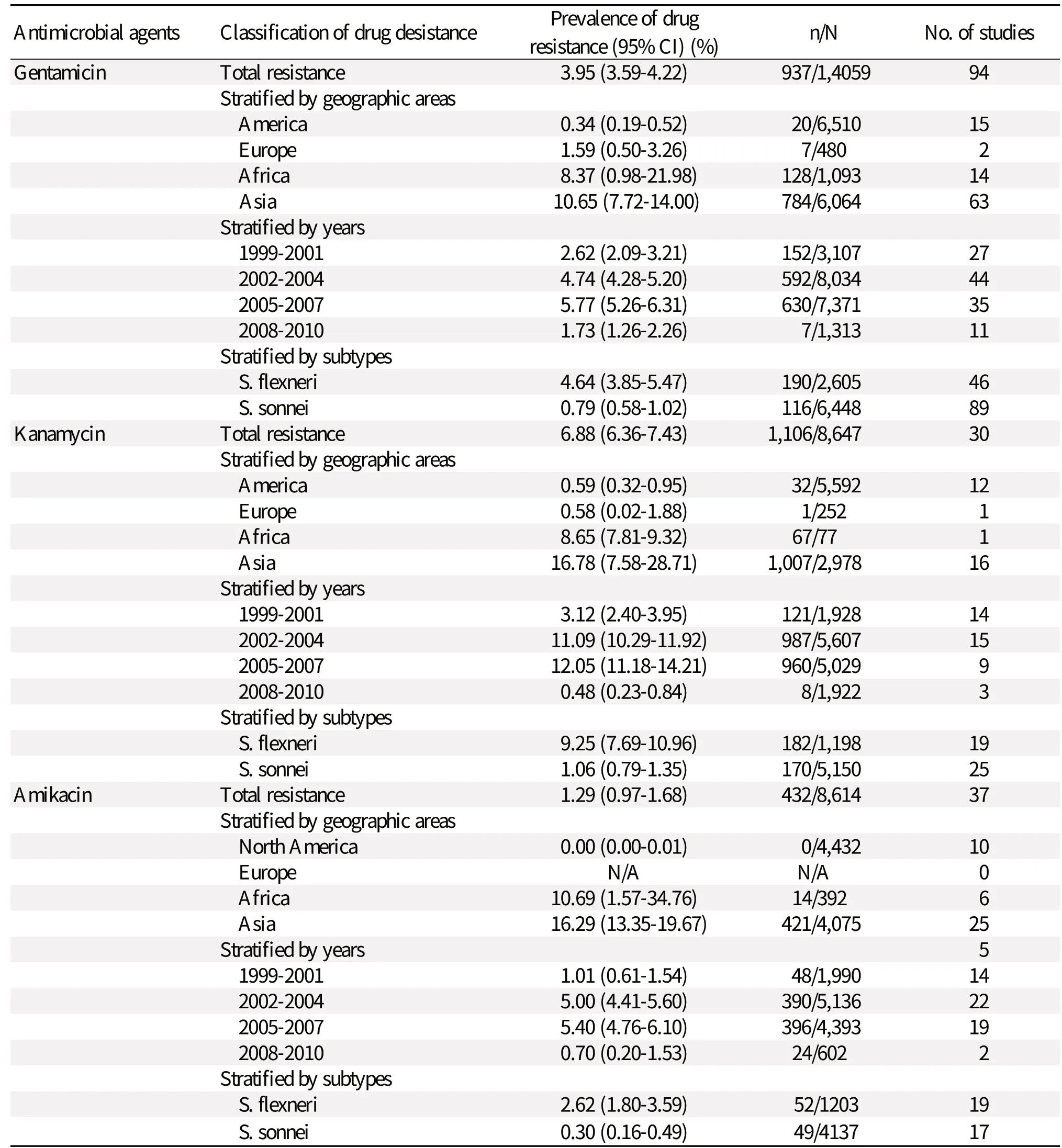
Table 1 Status of aminoglycoside-resistant Shigella from 1999-2010
A total of 68 reports were included in the metaanalysis. As specified a priori in our analysis plan, meta-analyses were performed for outcomes in which there were one or more observations of pediatric or adult group resistance that could be aggregated in the three drugs. Analyses were conducted across the geographic areas, study years and different subtypes for selected primary endpoints, including the resistance of Shigella to gentamicin, kanamycin and amikacin; S. flexneri to gentamicin, kanamycin and amikacin; S. sonnei to gentamicin, kanamycin and amikacin (Supplementary Table 1 available online).
Resistance patterns in European-American and Asian-African countries
Gentamicin
Table 2 shows the resistance rates of total Shigella isolates in European-American and Asian-African countries. A lower prevalence of gentamicin resistance was found in European-American countries at 0.68% (0.39%-1.05%). After analyzing the study data on years, we observed a minimal change in the resistance prevalence of gentamicin, from 0.25% (0.04%-0.64%) to 0.84% (0.08%-2.40%) in Europea-American countries, in contrast to data in Asian-African countries, which fluctuated from 6.05% (1.18%-14.28%) to 20.83% (12.67%-30.40%). It is worth noting that the resistance prevalence of gentamicin increased annually in Asian-African countries, while the resistance prevalence decreased year by year in European-American countries.
Kanamycin
The kanamycin resistance calculations of Shigella isolates among different areas are shown in Table 2. The prevalence of gentamicin resistance in Asian-African countries increased sharply from 14.00% (3.97%-28.85%) in 2002-2004, to 20.96% (3.37%-48.11%) in 1999-2001 and to 32.40% (17.87%-48.91%) in 2005-2007. Data for Asian-African regions from 2008-2010 were not found. The changes in kanamycin resistance in European-American countries were minimal; in fact, the resistance prevalence decreased annually.
Amikacin
Table 2 compares the amikacin resistance of Shigella isolates between European-American and Asian-African countries. In European-American regions, a lower amikacin resistance was also found during the 12-year study period. In fact, amikacin resistance decreased from 0.28% (0.00-1.08) to 0.05% (0.04-0.40). The highest resistance of Shigella isolates to amikacin was only 0.28% (0.00%-1.08%). We observed that the prevalence of amikacin resistance remarkably increased from 6.39% (1.40%-14.63%) to 48.06% (34.57%-61.65%) in Asian-African countries.
Comparison between S. flexneri and S. sonnei
Comparison of the data from Europe-America or Asia-Africa revealed that S. flexneri resistance to gentamicin [0.65% (0.30%-1.14%); 9.66% (5.51%-14.81%)] was greater than the resistance calculated for S. sonnei [0.39% (0.21%-0.61%); 13.66% (7.72%-20.96%)] (Tables 3, 4, and 5). Similarly, S. flexneri resistance to kanamycin [1.59% (0.88%-2.51%); 31.28% (13.97%-51.86%)] was greater than the resistance calculated for S. sonnei [0.30% (0.15%-0.51%); 18.10% (4.89%-37.20%)]. S. flexneri resistance to amikacin [0.37% (0.05%-0.96%); 7.72% (3.37%-13.66%)] was greater than the resistance calculated for S. sonnei [0.07% (0.01%-0.18%); 6.91% (1.75%-15.09%)]. Tables 3 to 5 also show that gentamicin and kanamycin were observed for the most common drug resistance system with a summarized prevalence of 33.95% (3.75%-75.11%) and 64.16% (57.04%-70.99%) in Asia-Africa, respectively. In European-American or Asian-African regions, amikacin resistance incidence of S. flexneri was not very high at 0.37% (0.05%-0.96%) and 7.72% (3.37%-13.66%), respectively. A difference was found in European-American countries where the prevalence of S. sonnei resistance was greater in the period of 2008-2010, giving a 7.18-fold increase in gentamicin-resistance and 4.41-fold increase in kanamycin-resistance from 1999 to 2010. In Asian-African regions, resistance data about S. flexneri or S. sonnei during 2008-2010 were not found.Comparison between children and adults
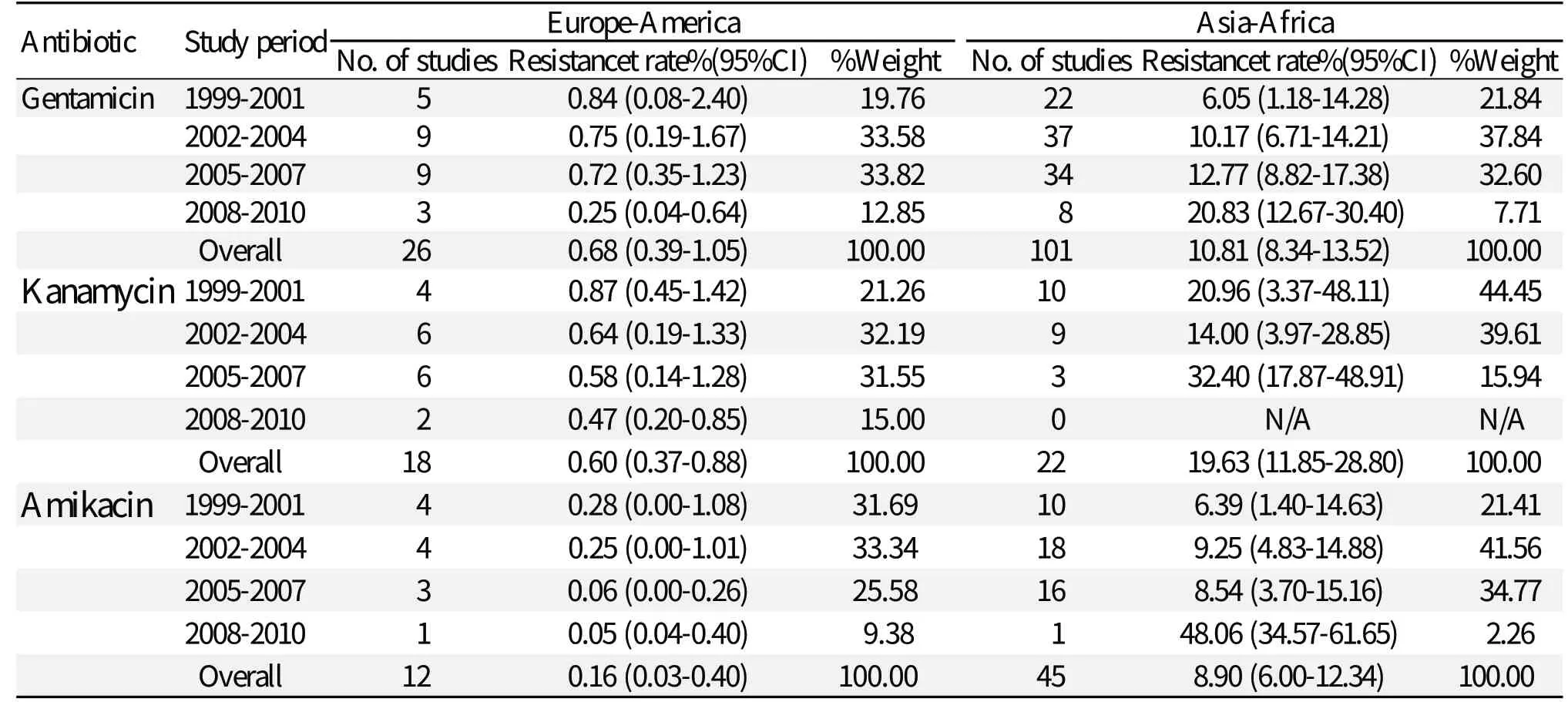
Table 2 Resistance to gentamicin, kanamycin and amikacin in Shigella spp. collected during 1999-2010
Table 6 shows the clear relationship between the resistance rate and the age of patients with diarrhea. Strains explicitly isolated from children were naturally classified into the pediatric group, whereas the remaining strains were viewed as isolates from adults. For Shigella, an additional analysis was conducted for a pediatric group population (34 studies).
In this pediatric group, the resistance of Shigella to gentamicin was higher than that among the adult group population [5.93% (3.97%-8.23%) and 18.34% (9.81%-28.76%)]. Kanamycin resistance in the pediatric group was significantly higher than that in the adult group, which showed 70.72% (33.95%-96.25%) versus 5.40% (1.87%-10.62%) for kanamycin. Similarly, greater resistance to amikacin was shown in the pediatric group than in the adults group [8.43% (3.26%-15.71%) vs 2.23% (0.81%-4.35%)].
DISCUSSION
In China, Shigella spp. is the most frequently isolated gastrointestinal pathogen and accounts for up to 1.7 million episodes of bacillary dysentery annually, with up to 200,000 patients admitted to hospitals[18]. Any of four subtypes of Shigella (S. dysenteriae, S. flexneri, S. boydii, and S. sonnei) can cause shigel
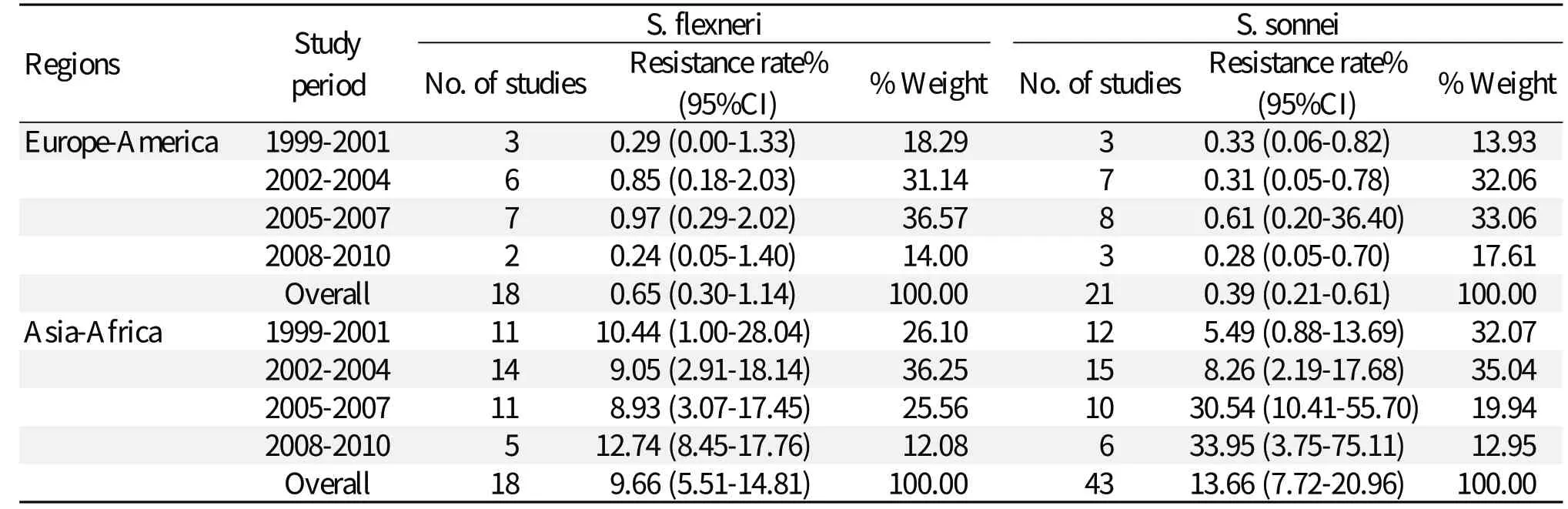
Table 3 Rates of resistance to gentamicin in S. flexneri and S. sonnei isolated from Europe-America and Asia-Africa during 1999-2010
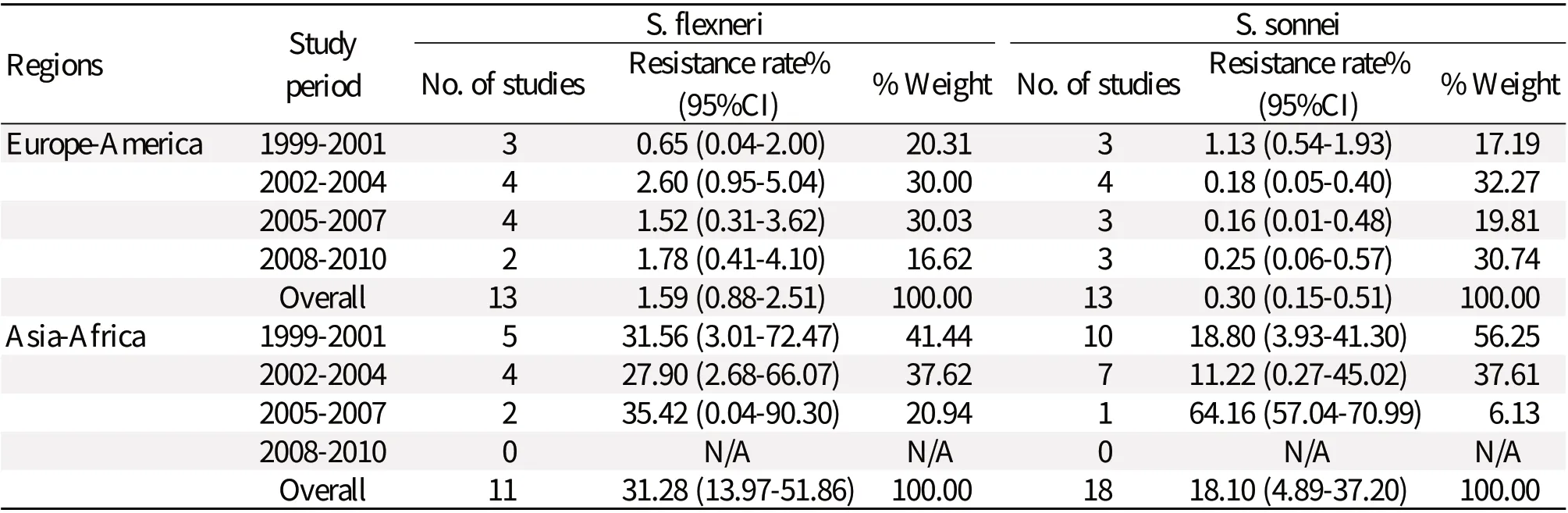
Table 4 Rates of resistance to kanamycin reported for S. flexneri and S. sonnei isolated from Europe-America and Asia-Africa during 1999-2010
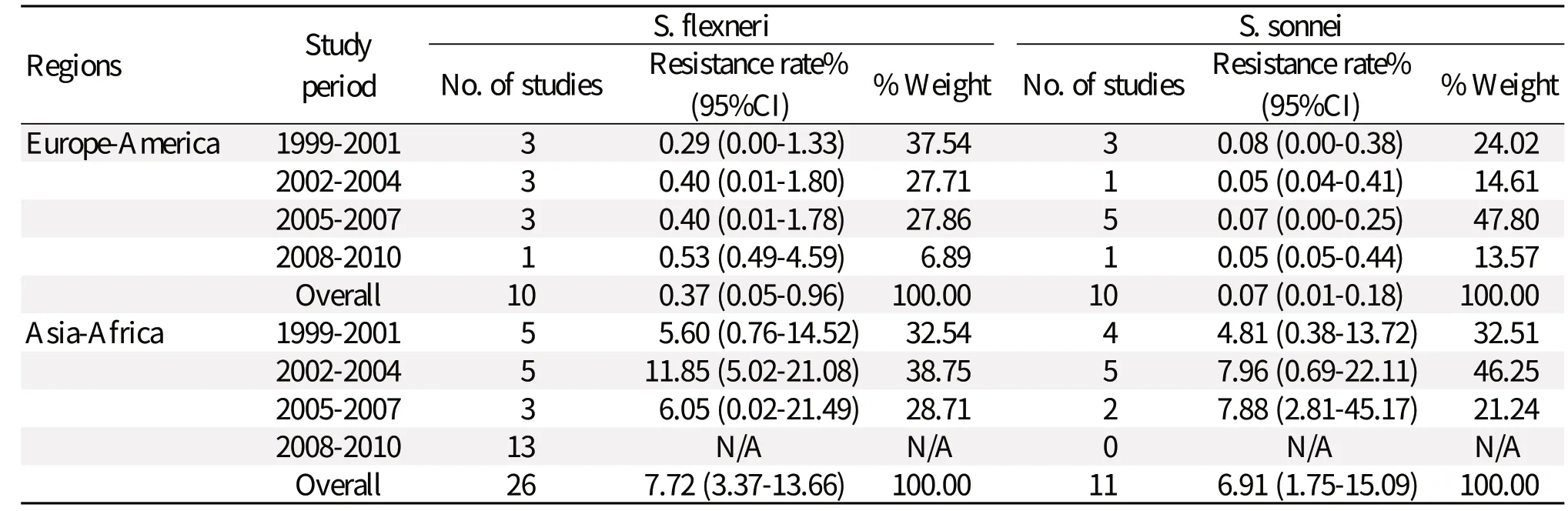
Table 5 Rates of resistance to amikacin reported for S. flexneri and S. sonnei isolated from Europe-America and Asia-Africa during 1999-2010
losis. Children are at a higher risk of being affected by the disease, which might be a reflection of secondary infection from the adults as well as poor personal hygiene[19]. Because of increasing antimicrobial resistance to Shigella, empiric treatment options are dwindling. In a recent study, aminoglycosides showed higher in vitro activity against members of the family Enterobacteriaceae. The function of aminoglycoside antibiotics for empirical treatment of patients with serious infections caused by Shigella merits our attention.
As with other classes of antibiotics, significant differences in the spectrum of antimicrobial activity exist among various aminoglycosides[20]. Aminoglycosides bind to the bacterial ribosome and inhibit protein synthesis. Generally, newer aminoglycosides, such as gentamicin, tobramycin, amikacin, netilmicin, isepamicin, dibekacin, and arbekacin, have broader spectra of activity than older compounds like streptomycin and kanamycin. Aminoglycosides are often administered in combination with other antibacterial agents. Despite their potential nephrotoxicity, ototoxicity and problems associated with aminoglycoside-resistant organisms, aminoglycoside antibiotics remain valuable and sometimes indispensable for the treatment of various infections and prophylaxis in special situations[21]. Several mechanisms have been proposed for bacterial resistance to aminoglycoside antibiotics, including decreased antibiotic uptake and accumulation, modification of the ribosomal target, efflux of antibiotic, and enzymatic modification of aminoglycosides.
The resistance of clinical isolates to aminoglycoside antibiotics varies with the specific drug, the microorganism, mechanism of resistance, geographic area, and many other factors. Of the 580 articles and abstracts fully evaluated for this meta-analysis, only 68 published reports met our strict inclusion criteria. Several studies which we reviewed were not included because they did not report on the outcomes of the study drugs in the study period or did not meet other inclusion criteria. However, use of a statistical tool to assess potential heterogeneity among included studies (STATA) gave us further confidence in the metaanalysis results. This meta-analysis demonstrated that the resistance to aminoglycoside is a significant issue in Asia and Africa, as well as in Europe and America. Reported aminoglycoside resistance in Shigella varies greatly from country to country and is likely to be an important problem in certain regions. The goals of our study were to analyze the distribution of antimicrobial resistance associated aminoglycoside-resistant Shigella based on articles reported between January 1999 and December 2010 and to examine issues related to reasonable treatment about shigellosis.

Table 6 Antimicrobial resistance in children and adults
The first noteworthy finding of this study was that all of these three drugs, in the European-American regions, had an obviously lower resistance rate during the 12 years than in the Asian-African regions. Regardless of its origins and mechanisms, the widespread resistance that we found among S. flexneri and S. sonnei suggests that infections due to drug-resistant Shigella are now endemic around the world. Although mild illness can resolve without antimicrobial treatment, current recommendations guide clinicians to treat Shigella infections with antimicrobial therapy to reduce the duration and severity of clinical symptoms and decrease the shedding period of Shigella. Because isolation and antimicrobial susceptibility testing for Shigella take only several days, antimicrobial agents were generally selected empirically. Combined with the characteristics of antibiotics use in different areas, we found that the use trends of aminoglycoside for shigellosis were not very serious. Selected literature reported from America[22,23]showed that trimethoprimsulfamethoxazole and ampicillin can no longer be considered appropriate empirical therapies. A Chinese article found that[18,24], for the treatment of shigellosis, 35 to 56 different antibiotics were used, with penicillin, cephalosporin, and macrolides accounting for the largest total volume. In some cases, aminoglycoside is effective and approved for use as treatment of Enterobacteriaceae. Stronger antibiotics like aminoglycoside may also present notable side effects. Rising resistance rates of these stronger antibiotics have a major impact on the ability of physicians to treat common infections. Patients, especially from Asian-African regions, faced more severe infections with increased duration as resistance increases. They may also experience heightened toxicity associated with the use of stronger antibiotics. Clinicians eventually encountered infections caused by highly resistant pathogens for which no effective antibiotics are available.
To compare the resistance difference among Shigella species, additional clinical data from large-scale observational studies were needed to evaluate the link between in vitro aminoglycoside resistance and different subtypes. We found that S. flexneri resistance to these antibiotics was greater than the resistance calculated for S. sonnei, and this effect was not influenced by district. In some hospital-based surveillance studies, S. flexneri infections have been more frequently detected[25]. One explanation for this could be that S. flexneri infections result more frequently in hospitalizations than S. sonnei infections. The notion that S. sonnei is less virulent than S. flexneri is supported by the possibly shorter duration of diarrhea occurring among patients infected with S. sonnei than among those with S. flexneri. Moreover, the molecular mechanisms of different antimicrobial resistance rate in Shigella may be associated with the function of integrons. In Shigella species, antimicrobial resistance is often associated with the presence of class 1 and class 2 integrons that contain resistance gene cassettes. Multiple and complex expression regulation mechanisms involving mobile genetic elements in integrons have been developed in the evolution of Shigella strains. S. sonnei and S. boydii strains often contain a single integron of class 2, whereas S. flexneri and S. dysenteriae strains carry a class 1 integron, either alone or associated with a class 2 integron[26,27]. Finally, S. flexneri could be linked with more complex drug resistant genes. This may be one of the explanations why S. flexneri resistance was greater than S. sonnei. Information about the rates of Shigella-resistance to kanamycin and amikacin in Asian-African regions was not reported, which stresses the need for continuous surveillance of resistance in those countries.
The third significant finding is that, in some countries, the prevalence of S. sonnei resistance was greater in 2008-2010. Obviously, the resistance rates among S. sonnei have an increasing trend. In some areas, S. sonnei has become the primary cause of shigellosis. Some of the S. sonnei isolates recovered showed resistance to several kinds of antimicrobial agents. There are distinct phenotypic and genotypic differences in terms of biotypes, antimicrobial susceptibilities and PFGE profiles, and antimicrobial susceptibilities.[70,72,73]. An increasing trend in the use of strong antibiotics, such as gentamicin and kanamycin, for shigellosis might be responsible for the acquisition of resistance to these antibiotics in S. sonnei isolates. The increased prevalence of S. sonnei resistance prompted us to suspect that S. sonnei may play a significant role in aminoglycosides-resistance in the future. It is essential to curb the spread of antibiotic resistance and diffusion of S. sonnei should be prevented. Overall, the surveillance of antimicrobial resistance of S. sonnei isolates should be continued, particularly to monitor the emergence of strains fully resistant to aminoglycosides. Thus, analyses of subtypes-based experiences in Shigella resistance can provide an important contribution to the understanding of real-world resistance issues from the perspective of day-to-day medical practice. This analysis also tells us that we should protect against S. flexneri, which caused more hospitalizations in the past, as well as S. sonnei, which caused the majority of shigellosis cases in this study.
Our fourth finding was that, in the pediatric group, the resistance to aminoglycosides was higher than in the adult group population. For children and adults with acute infectious gastroenteritis, the use of specific antimicrobial therapy should be limited to welldefi ned bacterial and protozoal agents. However, the use of antimicrobial agents in humans for many conditions, including therapy for children with diarrheal disease, is widespread. Although aminoglycosides showed higher in vitro activity against members of the family Enterobacteriaceae, antimicrobial therapy should be considered for specifi c clinical circumstances including the safety and tolerability of antimicrobial agents, particularly in young children. Considering potential side effects associated with aminoglycoside antibiotics to children, aminoglycoside would not be the best therapeutic choice for gastrointestinal diseases of children. For empiric treatment of diarrheal infections among children, in the report of Abu Elamreen et al.[28,29], ampicillin and trimethoprim-sulfamethoxazole are most often used. In the meantime, the greater aminoglycoside resistance rates to Shigella in the pediatric group cannot be ignored. This meta-analysis provides an important synthesis of the reported aminoglycoside resistance rates for S. flexneri and S. sonnei. Based on these findings, aminoglycoside resistance is consistently present in a variable proportion of multiple populations. We found that Shigella showed greater resistance to gentamicin, kanamycin or amikacin in the pediatric group than it did in the adult group. As interest evolves in the resistance patterns and rates of Shigella to aminoglycoside antibiotics, these results can be used to guide treatment decisions and to formulate consensual recommendations for appropriate treatment paradigms, especially for children. The meta-analysis technique used here can help to develop appropriate guidelines governing antibiotic use and to monitor drug resistance trends in different population groups.
Analysis of data on the use of various aminoglycoside antibiotics in different countries and regions of the world indicates that a correlation exists between the selective pressure of antibiotics and the patterns of combinations of aminoglycoside resistance mechanisms. For example, gentamicin has been most frequently used in the USA, while amikacin was used more extensively in Japan. In that time, the significant mechanisms of aminoglycoside resistance in the USA were production of ANT(2")-I (resistance to gentamicin, tobramycin, dibekacin, and kanamycin), and AAC(3)-I (resistance to gentamicin), whereas in Japan, Europe and Latin America, in addition to ANT(2")-I, AAC(6')-I (resistance to amikacin, netilmicin, tobramycin, dibekacin, and kanamycin but not to gentamicin) was identified[20,30]. The epidemiology of aminoglycoside resistance is becoming more complex, in part because of the multitude of aminoglycosidemodifying enzymes that exist for these antibiotics and also from the presence of disparate additional mechanisms for antibiotic resistance other than enzymatic resistance determinants. Because the genes for the aminoglycoside-modifying enzymes are often located on plasmids or transposons, together with the genes encoding resistance to other classes of antibacterials, the total consumption of non-aminoglycosides can also significantly influence the epidemiological features of aminoglycoside resistance[20].
In summary, this meta-analysis has provided important information on resistance by S. flexneri and S. sonnei to aminoglycosides in European-American and Asian-African countries. The use of the metaanalysis technique has allowed us to summarize data from individual studies and to determine robust values for both overall resistance and resistance among subgroups. Because identifying resistance patterns can be informative for empiric treatment recommendations, these results will be helpful in developing future guidelines and treatment paradigms for S. flexneri and S. sonnei, as well as in helping to direct future research on the impact of bacterial resistance and appropriate antimicrobial use.
Acknowledgements
We would like to thank all participants for their assistance with data collection and valuable discussion.
[1] Mota MI, Gadea MP, González S, González G, Pardo L, Sirok A, et al. Bacterial pathogens associated with bloody diarrhea in Uruguayan children. Rev Argent Microbiol 2010; 42: 114-7.
[2] Djie-Maletz A, Reither K, Danour S, Anyidoho L, Saad E, Danikuu F, et al. High rate of resistance to locally used antibiotics among enteric bacteria from children in Northern Ghana. J Antimicrob Chemother 2008; 61: 1315-8.
[3] Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis 1999; 5: 607-25.
[4] Abu Elamreen FH, Abed AA, Sharif FA. Detection and identification of bacterial enteropathogens by polymerase chain reaction and conventional techniques in childhood acute gastroenteritis in Gaza, Palestine. Int J Infect Dis 2007; 11: 501-7.
[5] American Medical Association, Centers for Disease Control and Prevention, Center for Food Safety and Applied Nutrition, Food and Drug Administration, Food Safety and Inspection Service, US Department of Agriculture: Diagnosis and management of foodborne illnesses: a primer for physicians. MMWR Recomm Rep 2001; 50: 1-69.
[6] Jamal W, Rotimi VO, Pal T, Sonnevend A, Dimitrov TS. Comparative in vitro activity of tigecycline and other antimicrobial agents against Shigella species from Kuwait and the United Arab of Emirates. J Infect Public Health 2010; 3: 35-42.
[7] Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the National Antimicrobial Resistance Monitoring System from 1999 to 2002. Antimicrob Agents Chemother 2006; 50: 49-54.
[8] Salam MA, Bennish ML. Antimicrobial therapy for shigellosis. Rev Infect Dis 1991; 13: 332-41.
[9] Chang CY, Lu PL, Lin CC, Lee TM, Tsai MY, Chang LL. Integron types, gene cassettes, antimicrobial resistance genes and plasmids of Shigella sonnei isolates from outbreaks and sporadic cases in Taiwan. J Med Microbiol 2011; 60: 197-204.
[10] Panbangred W, Jayanetra P, Pilantanapak A. Epidemiological study of sulfonamide and trimethoprim resistance genes in Enterobacteriaceae. Southeast Asian J Trop Med Public Health 1990; 21: 175-84.
[11] Murray BE. Problems and mechanisms of antimicrobial resistance. Infect Dis Clin North Am 1989; 3: 423-39.
[12] Replogle ML, Fleming DW, Cieslak PR. Emergence of antimicrobial-resistant shigellosis in Oregon. Clin Infect Dis 2000; 30: 515-9.
[13] Mache A. Antibiotic resistance and sero-groups of Shigella among paediatric out-patients in south west Ethiopia. East Afr Med J 2001; 78: 296-9.
[14] Yu HL, Chang ZR, Zhang LS, Zhang J, Li ZJ, Xu JG, et al. Analysis on the status of Shigella spp antimicrobial resistance through data from the National Shigellosis Surveillance System in China, in 2005. Zhonghua liuxingbingxue zazhi 2007; 28: 370-3.
[15] Gu B, Cao Y, Pan S,Yu R, Peng Z, Qian H, et al. Int J Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Antimicrob Agents 2012; 40: 9-17.
[16] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing by disk diffusion, 15th informational supplement. 2005, 100-S15. Wayne, Pa.
[17] Freeman MF, Tukey JW. Transformations related to the angular and square root. Ann Math Stat. 1950; 21: 607-11.
[18] Xia S, Xu B, Huang L, Zhao JY, Ran L, Zhang J, et al. Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006. J Clin Microbiol 2011; 49: 232-42.
[19] Wilson G, Easow JM, Mukhopadhyay C, Shivananda PG. Isolation & antimicrobial susceptibility of Shigella from patients with acute gastroenteritis in western Nepal. Indian J Med Res 2006; 123: 145-50.
[20] Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 2003; 16: 430-50.
[21] Antimicrobial Therapy, Inc. The sanford guide to antimicrobial therapy 2011.
[22] Wong MR, Reddy V, Hanson H, Johnson KM, Tsoi B, Cokes C, et al. Antimicrobial resistance trends of Shigella serotypes in New York City, 2006-2009. Microb Drug Resist 2010; 16: 155-61.
[23] Replogle ML, Fleming DW, Cieslak PR. Emergence of antimicrobial-resistant shigellosis in Oregon. Clin Infect Dis 2000; 30: 515-9.
[24] Zhang WX, Shen Y, Wang Y, Chen Y, Huang M, Zeng Q, et al. Antibiotic use in five children's hospitals during 2002-2006: the impact of antibiotic guidelines issued by the Chinese Ministry of Health Pharmacoepidemiol. Drug Safety 2008; 17: 306-11.
[25] Chompook P, Samosornsuk S, Von Seidlein L, Jitsanguansuk S, Sirima N, Sudjai S, et al. Estimating the burden of shigellosis in Thailand: 36-Month populationbased surveillance study. Bull World Health Organ 2005; 83: 739-46.
[26] Ke X, Gu B, Pan S, Tong M. Epidemiology and molecular mechanism of integron-mediated antibiotic resistance in Shigella. Arch Microbiol 2011; 193: 767-74.
[27] Gu B, Pan S, Wang T, Zhao W, Mei Y, Huang P, et al. Novel cassette arrays of integrons in clinical strains of Enterobacteriaceae in China. Int J Antimicrob Agents 2008; 32: 529-33.
[28] Abu Elamreen FH, Sharif FA, Deeb JE. Isolation and antibiotic susceptibility of Salmonella and Shigella strains isolated from children in Gaza, Palestine from 1999 to 2006. J Gastroenterol Hepatol 2008; 23: 330-3.
[29] American Medical Association, American Nurses Association-American Nurses Foundation, Centers for Disease Control and Prevention, Center for Food Safety and Applied Nutrition, Food and Drug Administration, Food Safety and Inspection Service, US Department of Agriculture: Diagnosis and management of foodborne illnesses: a primer for physicians and other health care professionals. MMWR Recomm Rep 2004; 53: 1-33.
[30] Miller GH, Sabatelli FJ, Hare RS, Glupczynski Y, Mackey P, Shlaes D, et al. The most frequent aminoglycoside resistance mechanisms-changes with time and geographic area: a reflection of aminoglycoside usage patterns? Aminoglycoside Resistance Study Groups. Clin Infect Dis 1997; 24: 46-62.
[31] Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the national antimicrobial resistance monitoring system from 1999 to 2002. Antimicrob Agents Chemother 2006; 50: 49-54.
[32] Al-Nimri S, Miller WA, Byrne BA, Guibert G, Chen L. A unified approach to molecular epidemiology investigations: tools and patterns in California as a case study for endemic shigellosis. BMC Infect Dis 2009; 9: 184.
[33] Drews SJ, Lau C, Andersen M, Guibert G, Chen L. Laboratory based surveillance of travel-related Shigella sonnei and Shigella flexneri in Alberta from 2002 to 2007. Global Health 2010; 6: 20.
[34] Merino LA, Hre?uk GE, Ronconi MC, Alonso JM. Antibiotic resistance and molecular epidemiology of Shigella spp. in northeastern Argentina. Rev Panam Salud Publica. 2004; 15: 219-24.
[35] Peirano G, Souza FS, Rodrigues DP, Shigella Study Group. Frequency of serovars and antimicrobial resistance in Shigella spp. from Brazil. Mem Inst Oswaldo Cruz. 2006; 101: 245-50.
[36] Mota MI, Gadea MP, González S, González G, Pardo L, Sirok A, et al. Bacterial pathogens associated with bloody diarrhea in Uruguayan children. Rev Argent Microbiol 2010; 42: 114-7.
[37] Haukka K, Siitonen A. Emerging resistance to newer antimicrobial agents among Shigella isolated from Finnish foreign travellers. Epidemiol Infect 2008; 136: 476-82.
[38] Nógrády N, Tóth á, Borbás K, Pászti J, Tóth I. Antimicrobial susceptibility, integron and virulencerelated gene carriage and genetic relationship of Shigellae isolated in Hungary from 1998 to 2008. Acta Microbiol Imm H 2009; 56: 216.
[39] Sire JM, Macondo EA, Perrier-Gros-Claude JD, Siby T, Bahsoun I, Seck A, et al. Antimicrobial resistance in Shigella species isolated in Dakar, Senegal (2004-2006). Jpn J Infect Dis 2008; 61: 307-9.
[40] Putnam SD, Riddle MS, Wierzba TF, Pittner BT, Elyazeed RA, El-Gendy A, et al. Antimicrobial susceptibility trends among Escherichia coli and Shigella spp. isolated from rural Egyptian paediatric populations with diarrhoea between 1995 and 2000. Clin Microbiol Infect 2004; 10: 804-10.
[41] Yismaw G, Negeri C, Kassu A. A five-year antimicrobial resistance pattern of Shigella isolated from stools in the Gondar University hospital, northwest Ethiopia. Trop Doct 2008; 38: 43-5.
[42] Tiruneh M. Serodiversity and antimicrobial resistance pattern of Shigella isolates at Gondar University Teaching Hospital, northwest Ethiopia. Jpn J Infect Dis 2009; 62: 93-7.
[43] Opintan J, Newman MJ. Distribution of serogroups and serotypes of multiple drug resistant Shigella isolates. Ghana Med J 2007; 41: 8-29.
[44] Djie-Maletz A, Reither K, Danour S, Anyidoho L, Saad E, Danikuu F, et al. High rate of resistance to locally used antibiotics among enteric bacteria from children in Northern Ghana. J Antimicrob Chemother. 2008; 61: 1315-8.
[45] Urio EM, Collison EK, Gashe BA, Sebunya TK, Mpuchane S. Shigella and Salmonella strains isolated from children under 5 years in Gaborone, Botswana, and their antibiotic susceptibility patterns. Trop Med Int Health 2001; 6: 55-9.
[46] Iwalokun BA, Gbenle GO, Smith SI, Ogunledun A, Akinsinde KA, Omonigbehin EA. Epidemiology of shigellosis in Lagos, Nigeria: Trends in antimicrobial resistance. J Health Popul Nutr 2001; 19: 183-90.
[47] Udo SM, Eja ME. Prevalence and antibiotic resistant Shigellae among primary school children in urban Calabar, Nigeria. Asia Pac J Public Health. 2004; 16:41-4.
[48] Davies NECG, Karstaedt AS. Shigella bacteraemia over a decade in Soweto, South Africa. Trans R Soc Trop Med Hyg 2008; 102: 1269-73.
[49] Temu MM, Kaatano GM, Miyaye ND, Buhalata SN, Shushu ML, Kishamawe C, et al. Antimicrobial susceptibility of Shigella flexneri and S. dysenteriae isolated from stool specimens of patients with bloody diarrhoea in Mwanza, Tanzania. Tanzan Health Res Bull 2007; 9: 186-9.
[50] Moyo SJ, Gro N, Matee MI,Kitundu J, Myrmel H, Mylvaganam H, et al. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dar es Salaam, Tanzania. BMC pediatr 2011 ; 11: 19.
[51] Samie A, Guerrant RL, Barrett L, Bessong PO, Igumbor EO, Obi CL. Prevalence of intestinal parasitic and bacterial pathogens in diarrhoeal and non-diarroeal human stools from Vhembe district, South Africa. J Health Popul Nutr 2009; 27: 739-45.
[52] Zhu JY, Duan GC, Yang HY, Fan QT, Xi YL. Atypical class 1 integron coexists with class 1 and class 2 integrons in multi-drug resistant Shigella flexneri isolates from China. Curr Microbiol 2011; 62: 802-6.
[53] Ding JX, Guo ZZ, Chen L. Six-year bacterial species distribution and drug sensitivity in childhood acute bacillary dysentery: An investigation of 290 cases. Chinese Journal of Contemporary Pediatrics 2005; 7: 54-6.
[54] Zhu DA, Sun JY, Fan HQ. Antimicrobial resistance and extended-spectrum β-lactamases genotypes of Shigella isolates in Shanghai. Chinese Journal of Infection and Chemotherapy 2009; 9: 2126-8.
[55] Wang XY, Du L, Von Seidlein L, Xu ZY, Zhang YL, Hao ZY, et al. Occurrence of shigellosis in the young and elderly in rural China: Results of a 12-month population-based surveillance study. Am J Trop Med Hyg 2005; 73: 416-22.
[56] Jones FR, Sanchez JL, Meza R, Batsel TM, Burga R, Canal E, et al. Short report: High incidence of Shigellosis among Peruvian soldiers deployed in the Amazon river basin. Am J Trop Med Hyg 2004; 70: 663-5.
[57] Navaneeth BV, Suganthi N, Belwadi MR. Antibiotic resistance among common bacterial enteric pathogens isolated from stool. Indian J Pediatr 2001; 68: 687-8.
[58] Rahman M, Shoma S, Rashid H, El Arifeen S, Baqui AH, Siddique AK, et al. Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: Resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. J Health Popul Nutr 2007; 25: 158-67.
[59] Bercion R, Njuimo SP, Boudjeka PM, Manirakiza A. Distribution and antibiotic susceptibility of Shigella isolates in Bangui, Central African Republic. Trop Med Int Health 2008; 13: 468-71.
[60] Ashkenazi S, Levy I, Kazaronovski V, Samra Z. Growing antimicrobial resistance of Shigella isolates. J Antimicrob Chemother 2003; 51: 427-9.
[61] Niyogi SK, Mitra U, Dutta P. Changing patterns of serotypes and antimicrobial susceptibilities of Shigella species isolated from children in Calcutta, India. Jpn J Infect Dis 2001; 54: 121-2.
[62] Dutta S, Rajendran K, Roy S, Chatterjee A, Dutta P, Nair GB, et al. Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of Shigellae strains isolated from Kolkata, India during 1995-2000. Epidemiol Infect 2002; 129: 235-43.
[63] Taneja N, Mohan B, Khurana S, Sharma M. Antimicrobial resistance in selected bacterial enteropathogens in north India. Indian J Med Res 2004; 120: 39-43.
[64] Uppal B, Arora VM. Changing resistance pattern of Shigella isolates in a Delhi hospital: An alarming trend. Indian J Med Microbiol 2004; 22: 199-200.
[65] Mamatha B, Pusapati BR, Rituparna C. Changing patterns of antimicrobial susceptibility of Shigella serotypes isolated from children with acute diarrhea in Manipal, South India, a 5 year study. Southeast Asian J Trop Med Public Health 2007; 38: 863-6.
[66] Nandy S, Mitra U, Rajendran K, Dutta P, Dutta S. Subtype prevalence, plasmid profiles and growing fluoroquinolone resistance in Shigella from Kolkata, India (2001-2007): a hospital-based study. Trop Med Int Health 2010; 15: 1499-507.
[67] MoezArdalan K, Zali MR, Dallal MM, Hemami MR, Salmanzadeh-Ahrabi S. Prevalence and pattern of antimicrobial resistance of Shigella species among patients with acute diarrhoea in Karaj, Tehran, Iran. J Health Popul Nutr 2003; 21: 96-102.
[68] Nowroozi J, Hakemi Vala M. Plasmid profile, antibiotic resistance, and phenotypic virulent strains of S. flexneri. Iran J Public Health 2006; 35: 43-8.
[69] Farshad S, Sheikhi R, Japoni A, Basiri E, Alborzi A. Characterization of Shigella strains in Iran by plasmid profile analysis and PCR amplification of ipa genes. J Clin Microbiol 2006; 44: 2879-83.
[70] Ranjbar R, Soltan-Dallal MM, Pourshafie MR, Mammina C. Antibiotic resistance among Shigella serogroups isolated in Tehran, Iran (2002-2004). J Infect Dev Ctries 2009; 3: 647-8.
[71] Hamedi A. Antibiotic resistance in children with bloody diarrhea. Acta Medica Iranica 2009; 47: 121-4.
[72] Ashtiani M, Monajemzadeh M, Kashi L. Trends in antimicrobial resistance of fecal Shigella and Salmonella isolates in Tehran, Iran. Indian J Pathol Microbiol 2009; 52: 52-5.
[73] Pourakbari B, Mamishi S, Mashoori N, Mahboobi N, Ashtiani MH, Afsharpaiman S, et al. Frequency and antimicrobial susceptibility of Shigella species isolated in Children Medical Center Hospital, Tehran, Iran, 2001-2006. Braz J Infect Dis 2010; 14: 153-7.
[74] Soltan Dallal MM, Ranjbar R, Pourshafie MR. The study of antimicrobial resistance among Shigella flexneri strains isolated in Tehran, Iran. J Pediatr Infect Dis 2011; 6: 125-9.
[75] Izumiya H, Tada Y, Ito K, Morita-Ishihara T, Ohnishi M, Terajima J, Characterization of Shigella sonnei isolates from travel-associated cases in Japan. J Med Microbiol 2009; 58: 1486-91.
[76] Hirose K, Terajima J, Izumiya H, Tamura K, Arakawa E, Takai N, et al. Antimicrobial susceptibility of Shigella sonnei isolates in Japan and molecular analysis of S. sonnei isolates with reduced susceptibility to fluoroquinolones. Antimicrob Agents Chemother 2005; 49: 1203-5.
[77] Ahmed AM, Furuta K, Shimomura K, Kasama Y, Shimamoto T. Genetic characterization of multidrug resistance in Shigella spp. from Japan. J Med Microbiol 2006; 55: 1685-91.
[78] Oh JY, Yu HS, Kim SK, Seol SY, Cho DT, Lee JC. Changes in patterns of antimicrobial susceptibility and integron carriage among Shigella sonnei isolates from southwestern Korea during epidemic periods. J Clin Microbiol 2003; 41: 421-3.
[79] Seol SY, Kim YT, Jeong YS, Oh JY, Kang HY, Moon DC, et al. Molecular characterization of antimicrobial resistance in Shigella sonnei isolates in Korea. J Med Microbiol 2006; 55: 871-7.
[80] Jin YH, Oh YH, Jung JH, Kim SJ, Kim JA, Han KY, Sonnevend A, Dimitrov TS. Antimicrobial resistance patterns and characterization of integrons of Shigella sonnei isolates in Seoul, 1999-2008. J Microbiol 2010; 48: 236-42.
[81] Jamal W, Rotimi VO, Pal T, Sonnevend A, Dimitrov TS. Comparative in vitro activity of tigecycline and other antimicrobial agents against Shigella species from Kuwait and the United Arab of Emirates. J Infect Public Health 2010; 3: 35-42.
[82] Abu Elamreen FH, Abed AA, Sharif FA. Detection and identification of bacterial enteropathogens by polymerase chain reaction and conventional techniques in childhood acute gastroenteritis in Gaza, Palestine. Int J Infect Dis. 2007; 11: 501-7.
[83] Banajeh SM, Ba-Oum NH, Al-Sanabani RM. Bacterial aetiology and anti-microbial resistance of childhood diarrhoea in Yemen. J Trop Pediatr 2001; 47: 301-3.
[84] Al-Moyed KA, Harmal NS, Al-Harasy AH, Al-Shamahy HA. Increasing single and multi-antibiotic resistance in Shigella species isolated from Shigellosis patients in Sana'a, Yemen. Saudi Med J 2006; 27: 1157-60
[85] Meng CY, Smith BL, Bodhidatta L, Richard SA, Vansith K, Thy B, et al. Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr Infect Dis J 2011; 30: 331-5.
[86] Bhattacharya D, Sugunan AP, Bhattacharjee H, Thamizhmani R, Sayi DS, Thanasekaran K, et al. Antimicrobial resistance in Shigella - rapid increase & widening of spectrum in Andaman Islands, India. Indian J Med Res 2012; 135: 365-70.
[87] Zhang W, Luo Y, Li J, Lin L, Ma Y, Hu C, et al. Wide dissemination of multidrug resistant shigella isolates in china. J Antimicrob Chemother 2011; 66: 2527-35.
[88] Shiferaw B, Solghan S, Palmer A, Joyce K, Barzilay EJ, Krueger A, et al. Antimicrobial Susceptibility Patterns of Shigella Isolates in Foodborne Diseases Active Surveillance Network (FoodNet) Sites, 2000-2010. Clin Infect Dis 2012; 5(S)458-63.
[89] Shamsizadeh A, Nikfar R, Bavarsadian E. Neurological manifestations of shigellosis in children in southwestern Iran. Pediatr Int 2012; 54: 127-130.
[90] Tajbakhsh M, García Migura L, Rahbar M, Svendsen CA, Mohammadzadeh M, Zali MR, et al. Antimicrobialresistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother 2012; 67: 1128-33.
 THE JOURNAL OF BIOMEDICAL RESEARCH2013年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2013年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Immediate and delayed micro-tensile bond strength of different luting resin cements to different regional dentin
- Etiological risk factors for subfertility among Palestinian women in Gaza
- Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats
- Late anastomotic perforation following surgery for gastric neuroendocrine tumor complicated by perforated duodenal ulcer:a case report
- Integrated DNA-based/biochemical screening for early diagnosis of multiple endocrine neoplasia type 2A (MEN2A)
- Medical simulation-based education improves medicos' clinical skills
