Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer
Gil-Su Jang*, Min-Jeong Kim*, Hong-Il Ha, Jung Han Kim, Hyeong Su Kim, Sung Bae Ju, Dae Young Zang
1Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang 431-070, South Korea;2Department of Diagnostic Radiology, Hallym University Sacred Heart Hospital, Anyang 431-070, South Korea;3Department of Internal Medicine, Kangnam Sacred Heart Hospital, Seoul 136-705, South Korea
Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer
Gil-Su Jang1*, Min-Jeong Kim2*, Hong-Il Ha2, Jung Han Kim3, Hyeong Su Kim3, Sung Bae Ju3, Dae Young Zang1
1Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang 431-070, South Korea;2Department of Diagnostic Radiology, Hallym University Sacred Heart Hospital, Anyang 431-070, South Korea;3Department of Internal Medicine, Kangnam Sacred Heart Hospital, Seoul 136-705, South Korea
Corresponding to:Dae Young Zang, MD, PhD. Division of Hemato-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, 896 Pyeongchon-dong, Dongan-gu, Anyang 431-070, South Korea. Email: fhdzang@hallym.or.kr.
Objective:Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.0 (RECIST 1.0) was proposed as a new guideline for evaluating tumor response and has been widely accepted as a standardized measure. With a number of issues being raised on RECIST 1.0, however, a revised RECIST guideline version 1.1 (RECIST 1.1) was proposed by the RECIST Working Group in 2009. This study was conducted to compare CT tumor response based on RECIST 1.1vs.RECIST 1.0 in patients with advanced gastric cancer (AGC).
Methods:We reviewed 61 AGC patients with measurable diseases by RECIST 1.0 who were enrolled in other clinical trials between 2008 and 2010. These patients were retrospectively re-analyzed to determine the concordance between the two response criteria using the κ statistic.
Results:The number and sum of tumor diameters of the target lesions by RECIST 1.1 were significantly lower than those by RECIST 1.0 (P<0.0001). However, there was excellent agreement in tumor response between RECIST 1.1 and RECIST 1.0 (κ=0.844). The overall response rates (ORRs) according to RECIST 1.0 and RECIST 1.1 were 32.7% (20/61) and 34.5% (20/58), respectively. One patient with partial response (PR) based on RECIST 1.0 was reclassified as stable disease (SD) by RECIST 1.1. Of two patients with SD by RECIST 1.0, one was downgraded to progressive disease and the other was upgraded to PR by RECIST 1.1.
Conclusions:RECIST 1.1 provided almost perfect agreement with RECIST 1.0 in the CT assessment of tumor response of AGC.
Response Evaluation Criteria in Solid Tumors guideline version 1.0 (RECIST 1.0); Response Evaluation Criteria in Solid Tumors guideline version 1.1 (RECIST 1.1); gastric cancer; tumor response
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/3073/3975
Introduction
Objective assessment of the change in tumor burden is a critical component in the evaluation of cancer therapeutics (1). The Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.0 (RECIST 1.0) was proposed as a new guideline for evaluating tumor response. Key features of RECIST 1.0 include definitions of minimum size of measurable lesions, instructions on how many lesions to follow (up to 10, a maximum of five per organ), and the use of uni-dimensional rather than bi-dimensional measures for evaluation of tumor burden (2). RECIST 1.0 has been widely accepted as a standardized measure of tumor response, particularly in oncologic clinical trials with objective response or time to progression as primary endpoints (3). However, a number of issues wereraised on RECIST 1.0, which included the total number of lesions to be assessed, the assessment of lymph nodes (LNs), and the utility of newer imaging technologies such as multidetector computed tomography (MDCT) and positron emission tomography (PET) (4,5).

Table 1 Summary of major changes in RECIST 1.1 compared with RECIST 1.0
In 2009, a revised RECIST guideline version 1.1 (RECIST 1.1) was presented by the RECIST Working Group, based in part on investigations using a database consisting of more than 6,500 patients with about 18,000 target lesions (1,4,6,7). Major changes in RECIST 1.1 included LN measurement, the maximum number of target lesions, and the definition of disease progression (Table 1) (6-8).
The new criteria recommend measurement of LNs on their short axis and propose measurement rules for categorizing an LN that is at least 15 mm on its short axis to be considered a target lesion. An LN with at least 10 mm but less than 15 mm on its short axis, although it may be pathologic, is considered a non-target lesion. An LN with less than 10 mm on its short axis is regarded as normal. The maximum number of target lesions has been reduced from ten to five in total, and from five to two per organ. Disease progression has been clarified: an absolute increase of at least 5 mm as well as a 20% increase in sum is now required to be defined as progressive disease (PD).
RECIST 1.1 showed almost perfect agreement with REICST 1.0 in tumor response assessment of patients with non-small cell lung cancer (NSCLC) (9-11). However, it still remains to be revealed how RECIST 1.1 affects the selection and CT measurement of target lesions, assessment of tumor response, and time to progression in malignancies of other primary sites.
This study was conducted to compare the CT measurement and tumor response based on RECIST 1.1vs.RECIST 1.0 in patients with advanced gastric cancer (AGC).
Patients and methods
Patients
This study was performed under an Institutional Review Board’s waiver according to the Korean ethical guidelines for epidemiological research. We evaluated 61 AGC patients with at least one measurable disease by RECIST 1.0 who were enrolled in other clinical trials between 2008 and 2010 at Hallym University Sacred Heart Hospital, Anyang, Korea and Asan Medical Center, Seoul, Korea. The patient was eligible for this study if he or she met the following criteria: histologically confirmed adenocarcinoma or signet-ring cell carcinoma of the stomach, radiologically or histologically confirmed metastatic disease, having at least one measurable lesion by RECIST version 1.0, having no other cancers, no history of chemotherapy and/or radiotherapy, and tumor assessment by MDCT at baseline and post-chemotherapy. These patients had received the first-line chemotherapy with cisplatin plus capecitabine or oxaliplatin plus 5-FU/leucovorin.
CT tumor measurement
All CT scans were performed on a 64-MDCT scanner with a slice thickness of 5 mm, and the images were transferred to the Picture Archiving Communication System (PACS). The post-chemotherapy CT scans were performed every two cycles of cisplatin plus capecitabine and four cycles of oxaliplatin plus 5-FU/leucovorin.
The longest diameter of each target lesion was manually measured on an axial CT image using calipers as a measuring tool on PACS. The target lesion description and CT size measurement, the sum of the longest tumor diameters of target lesions for each imaging study, andtumor response for each patient were recorded by a board-certified abdomen radiologist using RECIST 1.0 and RECIST 1.1. Briefly, the target lesions recorded in the original measurements were reassessed if they met the criteria of RECIST 1.1: LNs less than 15 mm on the short axis were excluded from target lesions; when the number of target lesions exceeded the limits according to RECIST 1.1 (up to five in total and up to two per organ), smaller lesions were eliminated from target lesions; shortaxis measurements were used for LNs instead of long-axis measurements.
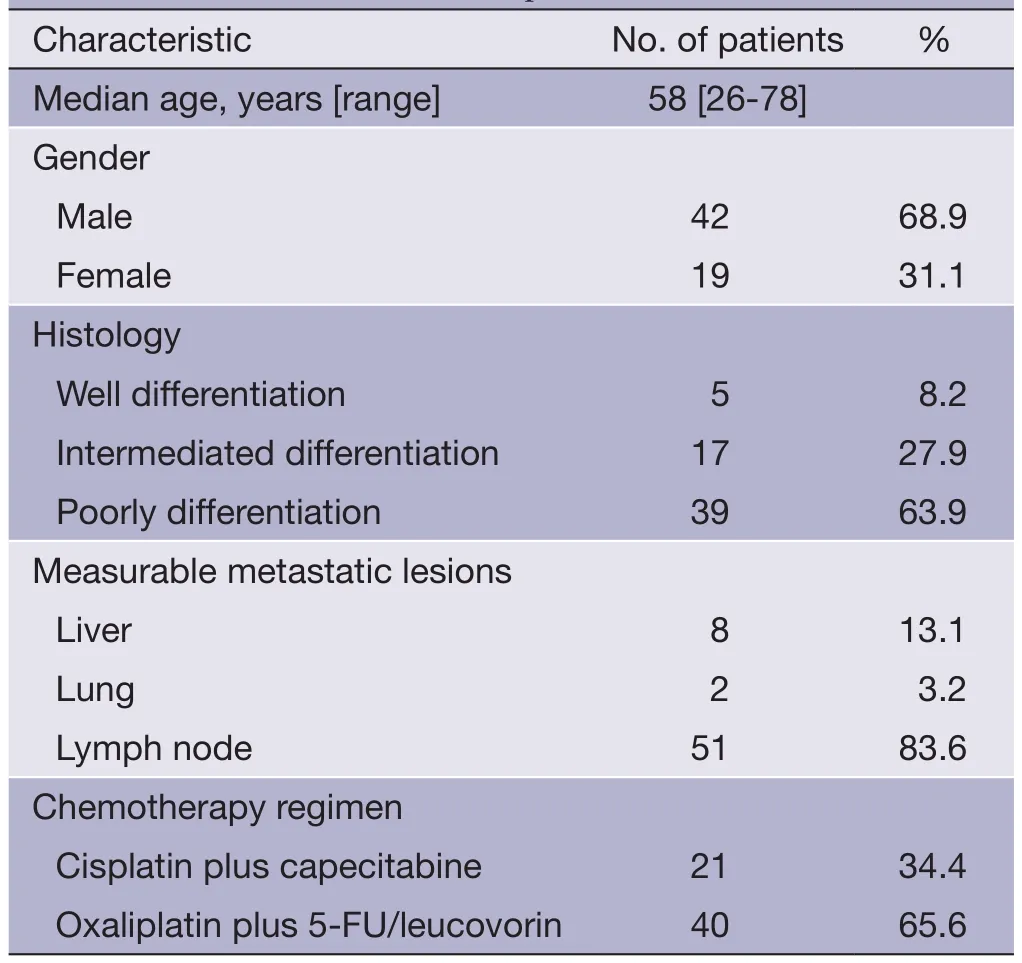
Table 2 Characteristic of the 61 patients
The number of RECIST 1.0 and RECIST 1.1 target lesions and the sum of tumor diameters at baseline and first follow-up were each calculated and recorded. Tumor responses were assessed separately using RECIST 1.0 and RECIST 1.1, respectively.
Statistical analysis
A paired Student’st-test was used to assess the statistical significance of changes in the number of target lesions and the sum of lesion diameters at baseline between RECIST 1.0 and RECIST 1.1. A P value of less than 0.05 was considered statistically significant. Concordance between the tumor responses by RECIST 1.0vs.RECIST 1.1 was assessed using the κ statistic. A kappa value of more than 0.75 was interpreted as showing excellent agreement.
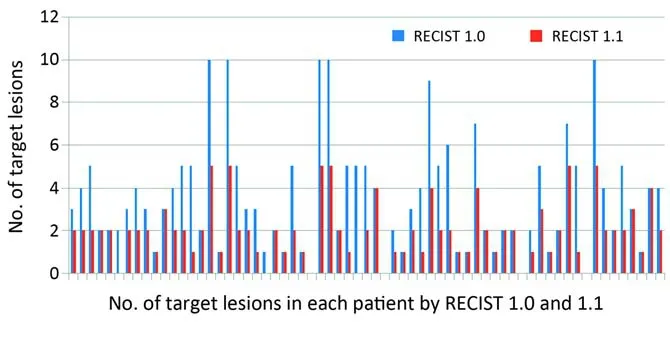
Figure 1 Number of target lesions according to Response Evaluation Criteria in Solid Tumors guideline version 1.0 (RECIST 1.0)vs.Response Evaluation Criteria in Solid Tumors guideline version 1.1 (RECIST 1.1). Number of target lesions by RECIST 1.1 was significantly lower than that by RECIST 1.0 (P<0.0001, paired Student’st-test).
Results
Patient characteristics
Patients’ baseline characteristics are summarized inTable 2.The patients consisted of 42 male (68.9%) and 19 female patients with a median age of 58 years (range, 26-78 years). Thirty-nine patients (63.9%) had poorly differentiated adenocarcinoma, and five (8.2%) well differentiated adenocarcinoma. The most common metastatic site with measurable lesions was the LN (83.6%), followed by the liver (13.1%) and lung (3.2%). Forty patients (65.6%) received cisplatin plus capecitabine as a first-line chemotherapy, and the remaining 21 (34.4%) were treated with oxaliplatin plus 5-FU/leucovorin.
Number of target lesions and sum of tumor diameters
The number of target lesions on RECIST 1.1 was significantly lower than that on RECIST 1.0 (P<0.0001), with a decrease of target lesions in 38 patients (62.3%) (Figure 1). The median number of target lesions was 3 (range, 1-10) by RECIST 1.0 and 2 (range, 0-5) by RECIST 1.1, respectively. Three patients had no longer target lesion by RECIST 1.1 because their LN target lesions by RECIST 1.0 were excluded by the new LN size criteria of REICST 1.1 (at least 15 mm on its short axis). The numbers of target lesions decreased by RECIST 1.1 were one in eight patients, two in ten, three in nine, four in four, and five in seven, respectively.
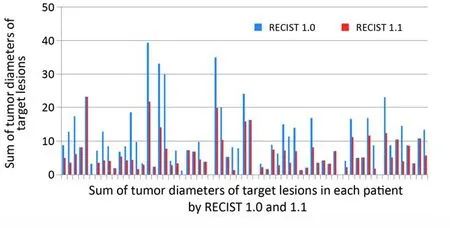
Figure 2 The sum of tumor diameters of target lesions according to Response Evaluation Criteria in Solid Tumors guideline version 1.0 (RECIST 1.0)vs.Response Evaluation Criteria in Solid Tumors guideline version 1.1 (RECIST 1.1). The sum of tumor diameters of target lesions by RECIST 1.1 was significantly lower than that by RECIST 1.0 (P<0.0001, paired Student’st-test).
The sum of diameters of the target lesions using RECIST 1.1 was also significantly lower than that using RECIST 1.0 (10.9±8.52 cmvs.6.33±5.12 cm, P<0.0001) as a result of significant decreases in the target lesions (Figure 2).
Best tumor response
The comparison of tumor responses between RECIST 1.0 and RECIST 1.1 is shown inTable 3. There was an excellent agreement in the CT assessment of tumor response between RECIST 1.1 and RECIST 1.0, with a kappa value of 0.844. While tumor responses by RECIST 1.0 were 32.7% (20/61) of partial response (PR), 62.2% (38/61) of stable disease (SD), and 4.9% (3/61) of PD, respectively, and tumor responses by RECIST 1.1 were 34.5% (20/58) PR, 58.6% (34/58) SD, and 6.9% (4/58) PD. One patient with PR based on RECIST 1.0 was reclassified as SD by RECIST 1.1 because 4 LN target lesions with a short axis of less than 15 mm were excluded by RECIST 1.1. Of two patients with SD by RECIST 1.0, one was downgraded to PD and the other was upgraded to PR by the new criteria of up to two lesions per organ in RECIST 1.1.
Discussion
In this study, we compared CT tumor measurement and tumor response based on RECIST version 1.0vs.1.1 in patients with AGC who had received first-line chemotherapy with cisplatin plus capecitabine or oxaliplatin plus 5-FU/leucovorin. Our results showed that RECIST 1.1 significantly decreased the number of target lesions as well as the sum of tumor diameters of the target lesions in AGCpatients at baseline of the first-line chemotherapy. However, the best tumor response assessment showed almost perfect agreement between two RECIST versions.
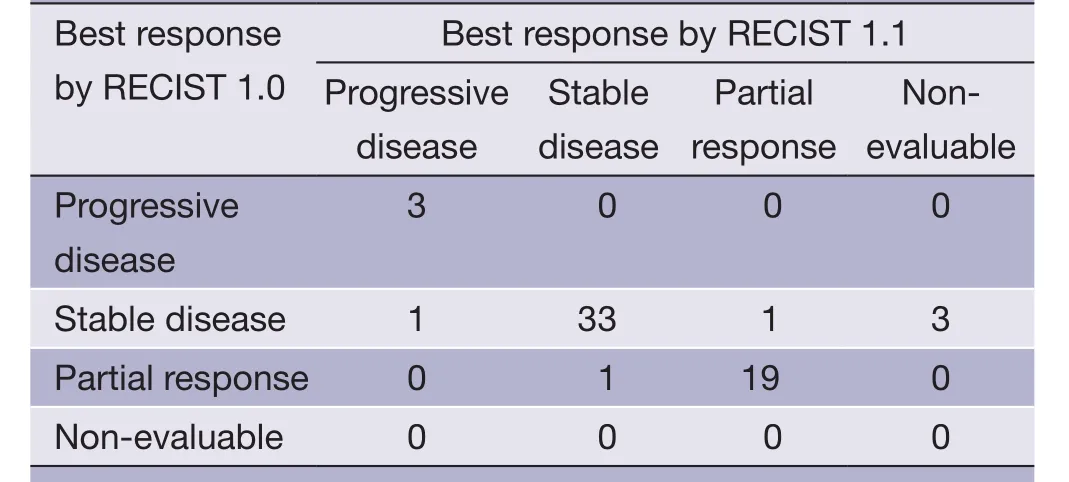
Table 3 Best response assessment by RECIST 1.0vs.RECIST 1.1
The decreases on the number of target lesions and the sum of tumor diameters of the target lesions were mainly resulted from the following two reasons. The first is a decrease in the number of LN target lesions due to the new size criteria of malignant LN by RECIST 1.1 (LNs only ≥15 mm in the short axis are considered measurable and assessable as target lesions). This new LN criteria of RECIST 1.1 affected 27 patients (44.3%) in our study. Of 61 patients who had at least one target lesion according to RECIST 1.0 at baseline of the first-line chemotherapy, interestingly, 3 (4.9%) had no longer target lesions by RECIST 1.1 with the new LN criteria. If a study using RECIST 1.1 had been planned, these patients would have been excluded from clinical trials. In another study of patients with AGC, Fuseet al. reported that the proportion of patients with target lesions was significantly decreased from 67% to 53% by the new LN criteria (12). These results indicate that RECIST 1.1 may alter the eligibility of patients for clinical trials in which overall response rate (ORR) is a primary end point. In this study, since patients had been enrolled in other clinical trials of which primary end point was overall responses, they all had at least one measurable disease at baseline according to the eligibility criteria by RECIST 1.0. Unlike in the study by Fuseet al., therefore, we had no opportunity to compare the proportions of patients with at least one target lesion between RECIST 1.0 and 1.1 in all consecutive patients with AGC.
Another cause of the decrease in the number of target lesions and the sum of tumor diameters was the change inthe maximum number of target lesions per organ (from a maximum of ten to a maximum of five and from five per organ to two per organ). In this study, 8 patients had more than 3 target lesions in an organ according to RECIST 1.0. Using RECIST 1.1, however, the sum of diameters of target lesions by RECIST 1.0 was decreased in 35 patients (57.4%). This decrease in the sum of tumor diameters was mostly due to the decrease in the number of target lesions.
In our study, only three patients showed disagreement of the best response between two RECIST versions. One patient with PR based on RECIST 1.0 was reclassified as SD by RECIST 1.1 because of 4 LN target lesions excluded by the new LN criteria of RECIST 1.1. Of two patients with SD by RECIST 1.0, one was downgraded to PD and the other was upgraded to PR by RECIST 1.1 with the new criteria of up to two lesions per organ. As patients showing PR or SD practically stay on the same therapy, patients with disagreement between PR and SD have no significant clinical impact of RECIST 1.1. In our study, only one patient showed disagreement of PDvs.SD, which would have impacted clinical decisions. Therefore, the clinical impact of RECIST 1.1 on changing therapeutic decisions seemed to be minimal.
In the present study, we did not incorporate PET scan in the evaluation of tumor response. PET scan has an important role in the assessment of tumor response using RECIST 1.1. The recent development of new target agents that induce necrosis in tumors but do not necessarily reduce the tumor size highlighted the limitations of using exclusively anatomic criteria. The RECIST Working Group acknowledged this limitation of RECIST 1.0 and included PET in RECIST 1.1. RECIST 1.1 specified that a positive finding at follow-up test after a negative finding at baseline PET should be considered as a new lesion and evidence of PD. Furthermore a positive finding at follow-up PET in patients who did not undergo PET at baseline should also be considered as a new lesion, indicating evidence of PD if the lesion was not seen at baseline CT but confirmed at follow-up CT (13). New lesions detected on PET may change the best tumor response from SD according to RECIST 1.0 to PD according to RECIST 1.1, which may lead to a lower concordance rate for tumor responses between two REICST versions. In the current study, however, no patients underwent PET scan because this test was not required in protocols at the time of clinical trials. Prospective studies with periodic PET scans are needed to evaluate its impact on the RECIST revision.
In conclusion, RECIST 1.1, despite the decreased number of target lesions and sum of tumor diameters, provided almost perfect agreement with RECIST 1.0 in the CT evaluation of tumor response of patients with AGC.
Acknowledgements
Disclosure:The authors declare no conflict of interest.
1. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
2. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
3. Nishino M, Jagannathan JP, Ramaiya NH, et al. Revised RECIST guideline version 1.1: what oncologists want to know and what raidologists need to know. AJR Am J Roentgenol 2010;195:281-9.
4. Moskowitz CS, Jia X, Schwartz LH, et al. A simulation study to evaluate the impact of the number of lesionsmeasured on response assessment. Eur J Cancer 2009;45:300-10.
5. Sargent DJ, Rubinstein L, Schwartz L, et al. Validation of novel imaging methodologies for use as cancer clinical trial endpoints. Eur J Cancer 2009;45:290-9.
6. Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer 2009;45:248-60.
7. Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer 2009;45:261-7.
8. Dancey JE, Dodd LE, Ford R, et al. Recommendations for the assessment of progression in randomized cancer treatment trials. Eur J Cancer 2009;45:281-9.
9. Sun JM, Ahn MJ, Park MJ, et al. Accuracy of RECIST 1.1 for non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors. Lung Cancer 2010;69:105-9.
10. Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) Guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to target therapy. AJR Am J Roentgenol 2010;195:W221-8.
11. Nishino M, Cardarella S, Jackman DM, et al. RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: comparison with RECIST 1.0. AJR Am J Roentgenol 2013;201:W64-71.
12. Fuse N, Nagahisa-Oku E, Doi T, et al. Effect of RECIST revision on classification of target lesions and overall response in advanced gastric cancer patients. Gastric Cancer 2013;16:324-8.
13. Chalian H, T?re HG, Horowitz JM, et al. Radiologic assessment of response to therapy: comparison of RECIST versions 1.1 and 1.0. Radiographics 2011;31:2093-105.
Cite this article as:Jang GS, Kim MJ, Ha HI, Kim JH, Kim HS, Ju SB, Zang DY. Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer. Chin J Cancer Res 2013;25(6):689-694. doi: 10.3978/j.issn.1000-9604.2013.11.09

10.3978/j.issn.1000-9604.2013.11.09
tudy with AGC patients who
the firstline chemotherapy with cisplatin plus capecitabine or oxaliplatin plus 5-FU/leucovorin, two RECIST versions showed almost perfect agreement in the evaluation of the best tumor response by CT (κ=0.844). The ORRs according to RECIST 1.0 and RECIST 1.1 were 32.7% (20/61) and 34.5% (20/58), respectively. This result is consistent with prior reports that were conducted in patients with NSCLC (9-11) or AGC (12). Nishinoet al. reviewed patients who had received erlotinib in a phase II clinical trial. Although the number of target lesions according to RECIST 1.1 decreased in 51.2% of patients (22/43), 93% of patients (40/43) showed the same results in the best tumor responses between two RECIST versions (κ=0.905). In other study of Korean NSCLC patients who received epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, the ORRs according to RECIST 1.0 and RECIST 1.1 were 35.6% and 38.5%, respectively, and only 6% of patients (6/104) showed disagreement in the tumor response assessment between two RECIST versions (9). Especially in the study of AGC by Fuseet al., the ORRs were 52% according to RECIST 1.0 and 55% according to RECIST 1.1, respectively.
Submitted Oct 18, 2013. Accepted for publication Nov 28, 2013.
*Gil-Su Jang and Min-Jeong Kim contributed equally to this study.
 Chinese Journal of Cancer Research2013年6期
Chinese Journal of Cancer Research2013年6期
- Chinese Journal of Cancer Research的其它文章
- Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis
- A “Stem Cells in Cancer” special issue in Translational Cancer Research
- The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells
- Spectral CT imaging as a new quantitative tool? Assessment of perfusion defects of pulmonary parenchyma in patients with lung cancer
- Locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of colon
- Clinical antiangiogenic effect of recombinant adenovirus-p53 combined with hyperthermia for advanced cancer
