Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients
Li Che, Yan-Hua Yuan, Jun Jia, Jun Ren
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Medical Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Introduction
Liver cancer, especially hepatocellular carcinoma (HCC), is a malignancy of worldwide significance (1,2).Although the increased global incidence of HCC is correlated with the increasing prevalence of chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) (2,3), some of the mechanisms associated with the initiation and progression of this disease remain elusive.
Dysregulation of the hedgehog (HH) pathway is implicated in the carcinogenesis of multiple tissue types (1,4).HH was first identified inDrosophiladuring screening of genes that are important in early embryonic development (5).This pathway is activated during binding of sonic HH (SHH) or Indian hedgehog (IHH) ligand to their receptors, Patched (PTCH).The unboundPTCHacts as a tumor suppressor that can bind to and repress smoothened (SMOH), thereby preventing the SMOH proto-oncoprotein from activating downstream of the transcription factors, such as glioma-associated oncogene-1(GLI1).By contrast, the ligand-boundPTCHfacilitates the release ofSMOHand activation ofGLI1resulting in the transcription of target genes includingPTCHandGLI1(1).
The HH activation has been observed in other types cancers such as basal cell carcinomas of the skin (6-9),prostate cancer (10,11), lung cancer (12,13), gastrointestinal cancers (14-19), breast cancer (20,21), and ovarian cancer (22).Although, it is required in liver embryogenesis (14,23), the HH signaling pathway is not well-sustained in the adult liver because of the insufficient HH pathway activity of mature hepatocytes (1,23).Recent studies showed that the HH pathway is frequently activated in HCC (1-3).The increased expression of GLI1 protein in breast tumor and hepatoblastoma is also reportedly correlated with significantly poor prognosis (24-26).The HH pathway mediates the progression of breast cancer from noninvasive to invasive and serves as a significant independent prognostic indicator in gastric and bladder cancers (27,28).
In our previous studies, we found an association with poor clinical prognosis of the HH pathway in human HCC (29) as well as the simultaneous expression ofGLI1in HCC and liver tissues adjacent to the tumor.These findings prompted us to expand our sample size to 46 HCC patients and extend their follow ups to confirm whether the expression of HH pathway components is associated with HCC progression and clinical outcome.
Materials and methods
Patients and tumor samples
This study included 46 HCC patients consisting of 40(86.96%) males and 6 (13.04%) females with ages ranging from 35 years to 79 years (median age =49 years).All patients underwent surgical treatment from April 2002 to July 2005 in Beijing Cancer Hospital.The clinical and pathologic data included patients’ demographics (age, gender), tumor size,degree of histological differentiation, and complete followup record.The exclusion criterion was preoperative therapy.The study was approved by the Ethics Committee of Human Experimentation of Beijing Cancer Hospital.
All samples of tumor and adjacent normal liver tissues were freshly obtained immediately after surgery.The samples of tumor tissue were collected from the luminal aspect of the malignancy, whereas those of paired adjacent normal tissue were from the luminal aspect of the liver tissues 2 cm away from the tumor margin.All tissue samples were snap-frozen in liquid nitrogen for 30 min after resection and stored at -80 °C.
RNA extraction and complementary DNA (cDNA)preparation
The total RNA was isolated from 100 mg of each tissue sample using TRIzol reagent (Invitrogen, Carlsbad, CA,USA) following the manufacturer’s instructions, and stored at -80 °C for further use.The reverse transcription (RT)of the total RNA (1 μL) was performed using SuperScript First-Strand Synthesis System (Invitrogen).The internal control was glyceraldehyde-3-phosphate dehydrogenase(GAPDH; forward primer: 5'-TCA ACG GAT TTG GTC GTA TT-3', reverse primer: 5'-AGT CTT CTG GGT GGG AGT GAT-3', 540 bp).
Polymerase chain reaction (PCR) amplification
To analyze the expression of individual HH gene, 1 μL of cDNA was amplified with 6.25 units of AmpliTaq Gold(Roche, Basel, Switzerland) in 25 μL reaction solution containing 0.5 mmol/L dNTPs and 1.5 mmol/L MgCl2.The primer sequences for PCR of each gene were designed according to a previous study (9) or using Genbank sequences(Table 1).All reactions were carried out in a PTC-100 Peltier Thermal Cycler (MJ Research, Waltham, MA, USA).Electrophoresis was performed by loading 8 μl of each sample on a 1% agarose gel.The reaction result was visualized by ethidium bromide staining using the Bio-imaging System(Ultra-Violet Products, UVP, Cambridge, UK).
DNA sequencing
The representative PCR products of each gene were measured by Beijing AuGCT Biotechnology Co.Ltd.and were screened using Chromas 2.3 shareware (Technelysium, Australia).
Western blot analysis
The total proteins were extracted from the prepared samples of fresh HCC and corresponding adjacent normal liver tissues, and the concentration was measured by bicinchoninic acid (BCA) protein assay.The protein sample(70 μg) was separated on 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) gels, and then transferred onto a polyvinylidene difluoride (PVDF)membrane (Millipore, Bedford).The primary antibodies were rabbit anti-human SHH polyclonal antibody (Santa Cruz Biotechnology, sc-9024) and anti-GAPDH antibody(Zhongshan Goldenbridge Biotechnology, Ta-08).The secondary antibodies were anti-rabbit IgGs conjugated to horseradish peroxidase (HRP).The blots were developed with the Pico West illumination kit (Promega, Wisconsin,USA).The results were compared by ImageJ (NIH,Maryland, USA) and the ratio of protein gray levels of SHH/GAPDH was calculated.
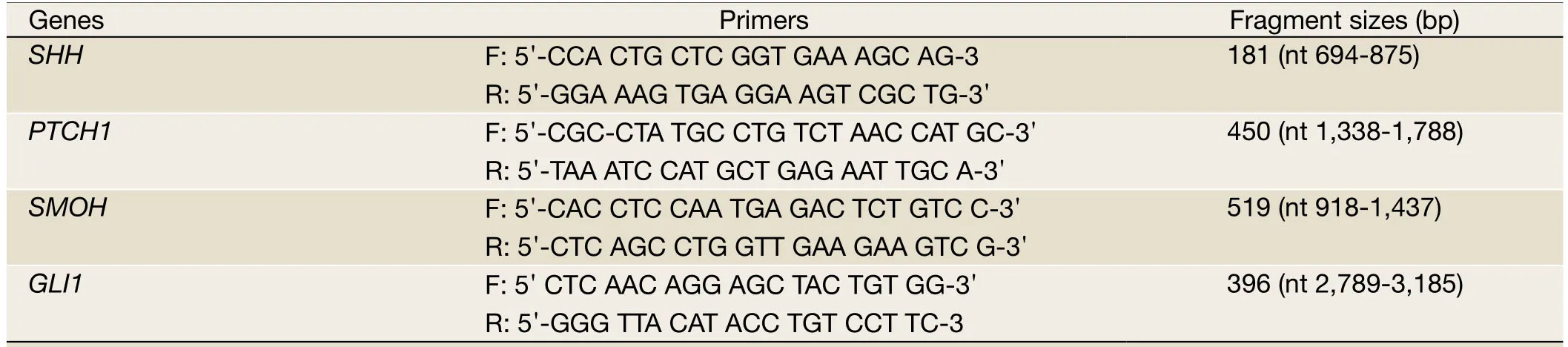
Table 1 Primers and fragment sizes of HH signaling genes
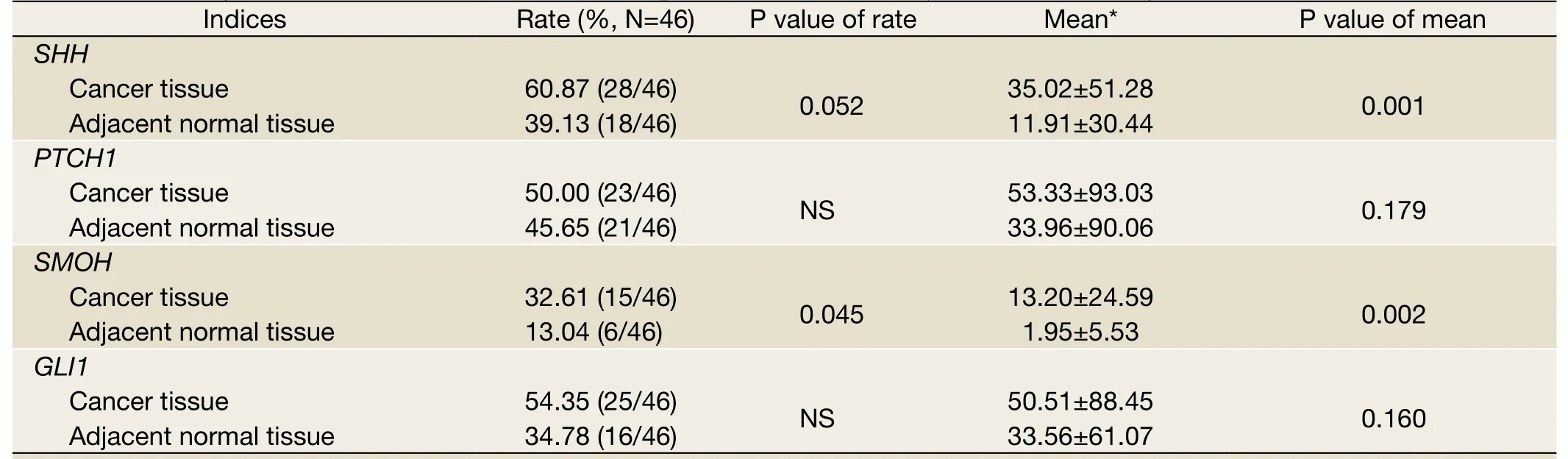
Table 2 Summary of SHH, PTCH1, SMOH and GLI1 expression in HCC and adjacent liver tissues by RT-PCR
Statistical analysis
The disease-free survival (DFS) is the time from initial diagnosis to relapse or metastasis.The overall survival (OS)is the time from initial diagnosis to death due to any cause or the date of last follow up.Statistical data were analyzed using the SPSS software (SPSS Inc., Chicago, IL, USA).The pairwise correlation between the continuous clinical outcomes and target gene expression levels were estimated using Spearman’s rank correlation (Ρ).P<0.05 (two tailed)was considered statistically significant.The survival time distribution was estimated using the Kaplan-Meier method.
Results
Expression of individual SHH gene
We detected the expression of HH signaling molecules in 46 paired normal liver and HCC samples by RT-PCR.In the HCC samples,SMOHwas detected in 15 (32.61%)samples,PTCH1in 23 samples (50.00%),SHHin 28 samples (60.87%), andGLI1in 25 (54.35%) samples.No significant difference was observed between the expression levels ofPTCH1andGLI1in the normal liver and tumor tissues, whereas the overexpression ofSHHwas observed in HCC samples (P=0.001).The expression of the signaling transmembrane protein geneSMOHwas significantly increased in HCC samples (P=0.002) (Figure 1, Table 2).
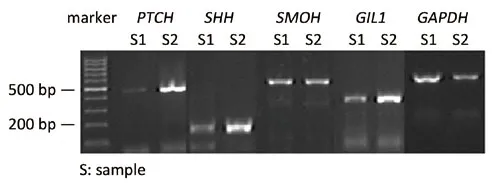
Figure 1 Expression of SHH, PTCH1, SMOH and GLI1 genes in HCC samples
Expression of individual SHH protein
The molecular weight of SHH protein was about 27 kD.The GAPDH protein expression levels were stable in all tissues samples.SHH protein expression was detected in 12 cases of HHC and corresponding adjacent normal liver tissues, whoseSHHmRNA was positively expressed in liver tumor tissues.We found that SHH protein was significantly positively expressed in human HCC tissues but negatively or weakly expressed in adjacent normal liver tissues.However, in 1 case of severe cirrhotic adjacent nontumor liver tissue, SHH protein was strongly positively expressed (Figure 2).This was consistent with the result of our previous immunohistochemical detection (29).
Correlation between expression of SHH signaling genes and clinical prognosis of HCC
All 46 enrolled patients had a complete follow-up record.The median follow-up time was 30 months (range: 1-83 months).
We found no significant relationship between the expression levels of SHH signaling genes (exceptGLI1) in tumor tissues and clinical prognosis in the 46 enrolled HCC patients.The expression of transcriptional factorGLI1in tumor tissues showed a significant relationship with DFS (P=0.042) and OS(P=0.030) (Figure 3A,B).The co-overexpression ofSHHandGLI1genes in tumor tissues showed a significant relationship with DFS (P=0.024) and a trend of influence on the OS of 46 HCC patients (P=0.083) (Figures 3C,D).
For adjacent non-tumor liver tissues, we found the expression levels ofSHH,SMOHandPTCH1did not show a significant relationship with the clinical prognosis, while the expression ofGLI1showed a significant relationship with DFS and OS (P<0.05).The 4-year DFS rates of patients whoseGLI1expressed positively and negatively in adjacent liver tissues were 8.9% and 50.4%, respectively (P=0.041).The 5-year OS rates were 21.4% and 34.7%, respectively (P=0.042) (Figure 3E,F).
The co-overexpression ofGLI1gene in tumor tissues and adjacent liver tissues was significantly associated with clinical prognosis (P<0.05).Compared with patients whoseGLI1gene was not simultaneously positively expressed in tumor tissues and adjacent liver tissues, those patients withGLI1co-overexpression had 2-year DFS rates of 21.2% and 57.3%, respectively (P=0.029), as well as 5-year OS rates of 18.5% and 42%, respectively (P=0.025) (Figure 3G,H).
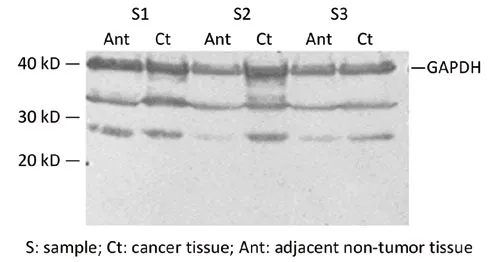
Figure 2 Western blot analysis for SHH.S1.SHH protein expression is positive in some cirrhotic adjacent non-tumor liver tissues; S2 and S3.SHH protein expression is significantly positive in human HCC tissues and negative or weakly observed in adjacent non-tumor liver tissues
Correlation between expression of SHH signaling genes and patients’ characteristics
The patients’ characteristics are listed inTable 3, and the relationship between gene expression patterns and patients’characteristics were analyzed.Overall, the 46 tumor samples increased inSMOHproto-oncogene expression(mean: 13.20±24.59).SMOHexpression was up-regulated in 15 HCC samples (32.61%).Furthermore,SMOHprotooncogene expression in tumor positively correlated with the HCC tumor size (ρ=0.306, P=0.041) (Figure 4A).We also found that theGLI1mRNA transcript levels had a trend of correlating with the HCC tumor size (ρ=0.277,P>0.065), whereas theSHHmRNA transcript levels had no correlation with the size of liver tumor (P>0.20).
The serum levels of tumor markerα-fetoprotein (AFP)are clinically used in the diagnosis and follow-up of patients with malignant liver tumors.Therefore, we examined the relationships among preoperative serum AFP levels, tumor size, as well as the expression ofPTCHtumor-suppressor gene andSMOHproto-oncogene.In our cohort study,the serum AFP levels were elevated in 35 cases (76.09%).However, no empirical evidence suggested that the tumor size correlated with the preoperative serum AFP level(ρ=0.193, P=0.325).The serum AFP levels also had no correlation with the expression levels ofSHH,PTCH,SMOHorGLI1gene in the tumor samples (P>0.30).But interestingly, the serum AFP level inversely correlated with DFS (ρ=-0.483, P=0.009) and OS (ρ=-0.390, P=0.040).Moreover the tumor size also inversely correlated with DFS(ρ=-0.131, P=0.389 and OS (ρ=-0.189, P=0.214) although the relationship was not statistically significant.
We found that HCC withPTCHoverexpression had significantly higherSHH(ρ=0.381, P=0.009) (Figure 4B)andSMOHexpressions (ρ=0.558, P<0.001) (Figure 4C).Moreover, HCC withPTCHoverexpression in adjacent non-tumor liver tissues also tended to have higherSMOH(ρ=0.485, P=0.001) andSHHexpressions (ρ=0.359,P=0.015) in tumor tissues.This result suggested thatSHHoverexpression in some of the tumors was associated with increased HH activity.
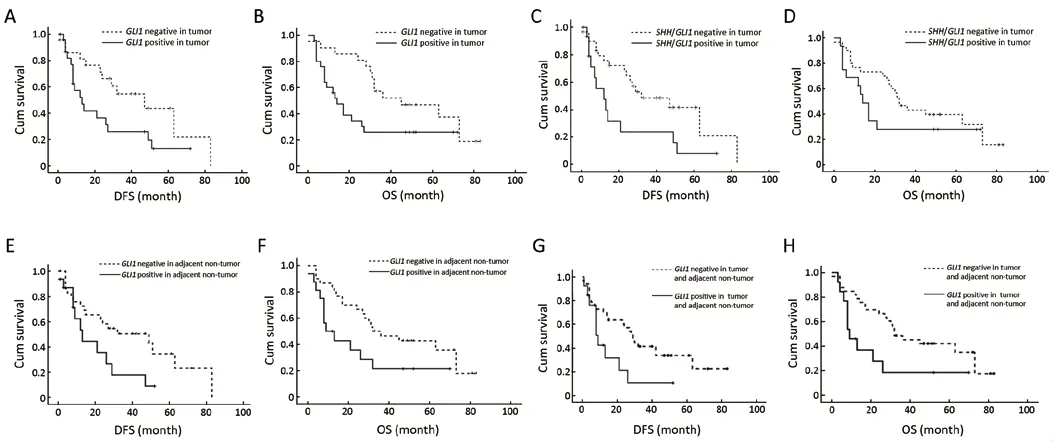
Figure 3 The expression of GLI1 and SHH in tumor tissues and adjacent non-tumor liver tissues was correlated with DFS and OS.The expression of GLI1 in tumor tissues was correlated with DFS(A, P=0.042) and OS (B, P=0.030) of 46 HCC patients.The expression of GLI1 and SHH in tumor tissues was correlated with DFS (C, P=0.024) and OS (D, P=0.083) of the patients.GLI1 expression in adjacent nontumor liver tissues was correlated with DFS (E, P=0.041) and OS (F, P=0.042) of the patients.GLI1 expression in tumor tissues and adjacent non-tumor liver tissues was correlated with DFS (G, P<0.029) and OS (H, P<0.025) of the patients
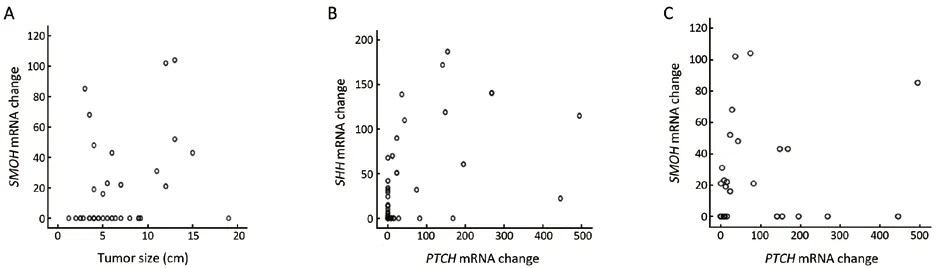
Figure 4 Correlation among SHH component gene expressions in HCC and matched non-tumor liver tissues.A.SMOH mRNA expression was correlated with the tumor size (ρ=0.306, P=0.041); B.SHH expression was correlated with PTCH in tumor tissues (ρ= 0.381, P=0.009); C.SMOH expression was correlated with PTCH in tumor tissues.(ρ=0.558, P<0.001)
Discussion
TheHHgene family, which codes a much more sophisticated set of secreted proteins, was first identified inDrosophilain 1980 (5) and essential for early embryo patterning.Previous studies have reviewed that the HH signaling pathway plays key roles in various processes, such as embryogenesis, maintenance of adult tissue homeostasis,tissue repair during chronic persistent inflammation, and carcinogenesis (3,30,31).SHH is active only in stem cells and/or endodermal progenitor in adults (16,23).Recent studies showed that aberrant signaling of this pathway is involved in a variety of human cancers (6-20,21-32).
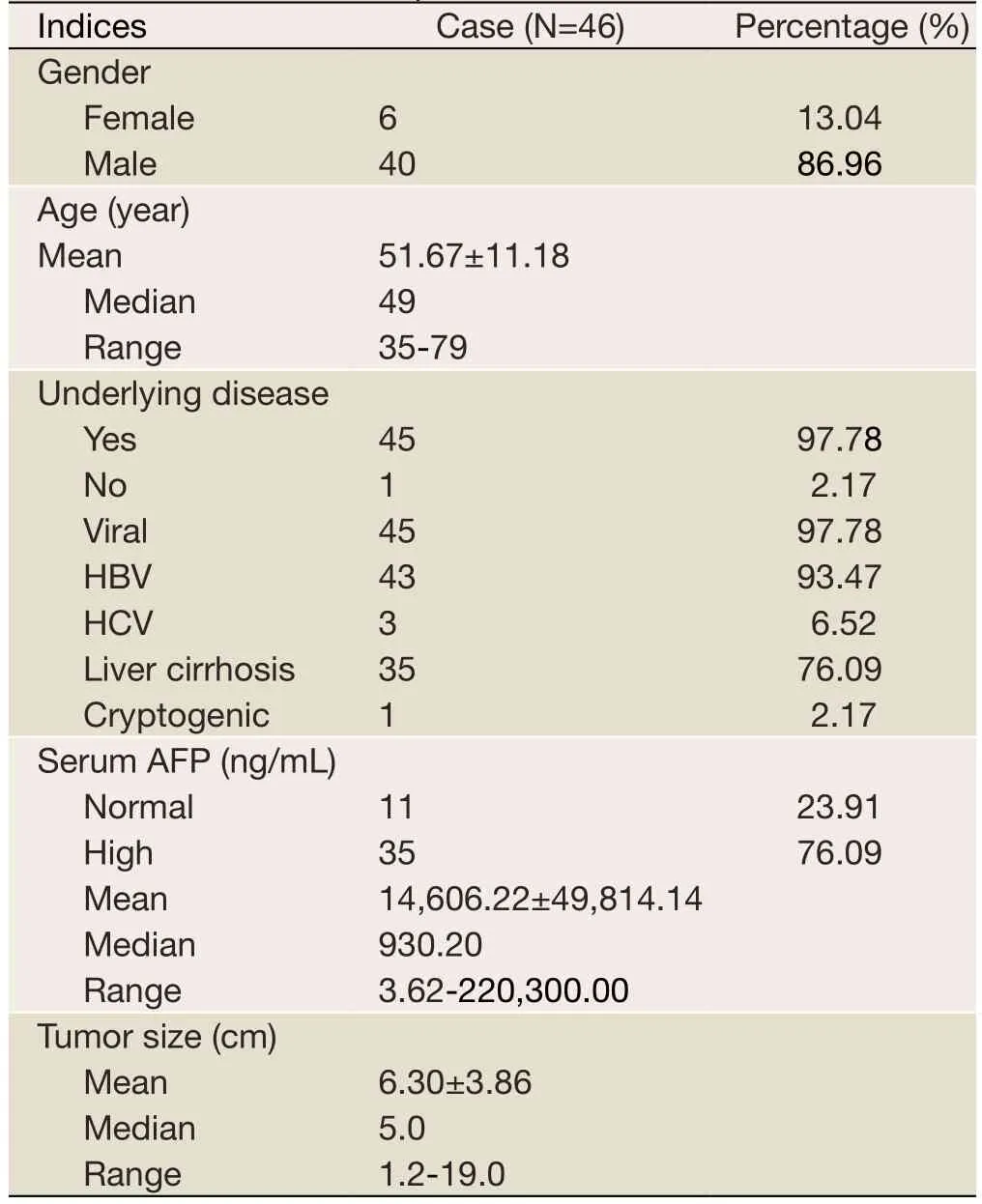
Table 3 Demographics, underlying diseases and tumor related factors for the cohort study
The HH pathway essentially consists of PTCH, the motive protein, SMOH, and the transcriptional factors GLI2 and GLI3.GLI1 is induced only upon activation of the HH pathway (24,26).Therefore, as a transcription factor, GLI1 expression is a good indicator of HH pathway activation.Recent studies showed that Gli1 controls several biological characteristics, such as proliferation and invasion,in several types of cancers (17,19,26).
Although the activation of the HH pathway is involved in several types of gastrointestinal cancers and other cancers,its role in HCC pathogenesis is not well understood.The normal hepatocytes lack the HH signaling pathway (1,23).However, the activation of the HH pathway in endodermal progenitors is essential for liver development.Thus, we hypothesized that regulation of the HH signaling may be involved in hepatocarcinogenesis.
Our data indicated that HH signaling is frequently activated in HCC.SHHand its target genes,PTCH1,SMOHandGLI1, were frequently expressed in the tumor tissues than in the adjacent liver tissues.These data support our hypothesis that activation of the HH pathway is essential in the development of HCC.Since the HH signaling pathway is frequently activated in HCC, the markers for the activation, includingSHH,SMOH,PTCH1andGLI1, may be useful for diagnosis of liver cancers.
Sicklick (1) reported that the expression ofSMOHprotooncogene is positively correlated with HCC tumor size.Our results also showed that overexpression ofSMOHmRNA in HCC was positively correlated with HCC tumor size.Thus, it can be a prognostic indicator in HCC biology.Although the serum AFP level was inversely correlated with DFS and OS, it was not related to the expression of SHH pathway genes.Moreover, the tumor size was also inversely correlated with DFS and OS, but with no statistically significant relationship.This data showed thatSMOHactivation is a potential prognostic indicator of human HCC.Ten Haaf,et al.(24) found that the increased expression of GLI1 protein in breast cancer is significantly correlated with unfavorable OS.Souzaki,et al.(26) reported that the%GLI1 nuclear translocation in lymph nodes with micrometastasis was higher than that in ductal carcinoma in situ(DCIS) with microinvasion and DCIS.The progression from DCIS to invasive ductal carcinoma (IDC) requires a certain level of %GLI1 nuclear translocation and the HH pathway contributes to the progression from DCIS.He,et al.(28)also reported that patients with positive expression of SHH,PTCH1 and GLI1 proteins showed poorer DFS and OS than those with negative expression, and these proteins were independent, unfavorable prognostic factors.
In this study, we found thatGLI1expression in HCC tissues showed a significant relationship with DFS and OS.The simultaneous positive expression ofGLI1gene in tumor and adjacent non-tumor liver tissues was significantly related with clinical prognosis.These data suggest that the activation of SHH signaling pathway is potential prognostic indicator in human HCC.The markers for HH signaling activation, especiallyGLI1, may be useful for the judgment of clinical prognosis.
In general, the enhanced HH pathway activation leads to downstream expression of target genes, includingPTCHandGLI1.Thus, the levels of these transcripts are often used as surrogate markers of HH pathway activity (33).However, recent studies suggested that other less-understood mechanisms also influence the levels ofPTCHandGLI1transcripts.HCC often develops in cirrhotic livers (34,35)and others have demonstratedPTCHtranscripts in some cirrhotic patients (36,37).IncreasedSMOHmRNA levels are also observed in some cirrhotic patients (36).Up to threeforth (35/46) of the HCC patients in our study had underlying cirrhosis.Therefore, the interindividual differences inPTCHexpression in non-neoplastic liver tissues also influence our results.
In our study,SHHoverexpression resulted in higherPTCHexpression.Thus, the HCC with higherPTCHlevels tends to have higherSMOHexpression.This suggests that dysregulation of HH signaling occurs during hepatocarcinogenesis and likely resulted from increasedSMOHthat occurs without the accompanying increase inPTCHexpression, which is typically observed in other gastrointestinal tumors (14,15).This observation was consistent with other previous studies (1,3,4).
Several studies have demonstrated that SHH signaling pathway not only plays a critical role in the development of human gastrointestinal tract, but also has a close relationship with gastrointestinal adenocarcinoma and precancerosis (32,38), while others have found thatSHHgene expression was higher during the early disease stage during which more undifferentiated cells were found, than in advanced disease stage (3,15,20,38).HH signaling pathway is also reportedly activated during the fibroproliferative response to chronic cholestatic biliary injury in primary biliary cirrhosis (39).These suggested that HH signaling pathway has an early and critical role in carcinogenesis (20).
Huang,et al.(3) indicated that tissue abnormalities were present in these adjacent liver tissues with expression ofGLI1andPTCH1, ranging from small cell dysplasia,dysplastic nodules to microscopic HCC, whereas a noncancerous liver tissue did not have any detectable expression ofSHH,PTCH1andGLI1.This indicated that HH signaling activation occurs in early lesions of HCC.Our data showed active SHH signaling genes in adjacent liver tissues of HCC, especially in some cirrhotic patients.The overexpression ofGLI1gene in adjacent liver tissues suggests a worse prognosis.This provided evidence that HH signaling may play a previously unsuspected role in the progression from cirrhosis to liver cancer.Although the pathological examination found no cancer cells existed in the tissue samples expressingGLi1gene, the possibility of the tumor-induced abnormal cell differentiation in these tissue cannot be ruled out.The SHH pathway genes were not only overexpressed in malignant tumors, but also play a multifaceted role.The HH pathway activation may occur as an early event during the evolution of hepatic neoplasm.Overall, these data support that tumorigenesis occurs is an injury/repair-related process, and suggest that dysregulation of the HH pathway contributes to liver regeneration,namely liver cancer.
Sicklick (1) reported the missense mutation of oncogenicSMOHgene in HCC.This does not rule out the possibility that other unidentifiedSMOHmutations may exist in HCC and that HH signaling may play a previously unsuspected role in the progression from cirrhosis to liver cancer.Thus,the mutation statuses ofPTCH,SMOH,GLI1and other signaling molecules in HCC requires further analysis to determine whether the mutations of these genes may be present during HCC development.
Several studies have indicated that the HH pathway may be a potent therapeutic target for tumors including HCC.A series of studies with a HH pathway inhibitor, cyclopamine,has brought about this expectation.Cyclopamine was discovered through epidemiological investigations of malformed sheep (40).Cyclopamine can reportedly inhibit HH ligand-dependent and independent HH pathway activation through direct interaction withSMOH(41-44).The HH pathway may be a potential therapeutic target in HCC.
Acknowledgements
This work was supported by the Project 2009CB521700 of the National Basic Research Program of China (“973”Program) and the Capital Development Grant (2007-2053).The funder had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
Disclosure:The authors declare no conflict of interest.
1.Sicklick JK, Li YX, Jayaraman A, et al.Dysregulation of the hedgehog pathway in human hepatocarcinogenesis.Carcinogenesis 2006;27:748-57.
2.Patil MA, Zhang J, Ho C, et al.Hedgehog signaling in human hepatocellular carcinoma.Cancer Biol Ther 2006;5:111-7.
3.Huang S, He J, Zhang X, et al.Activation of the hedgehog pathway in human hepatocellular carcinomas.Carcinogenesis 2006;27:1334-40.
4.Beachy PA, Karhadkar SS, Berman DM.Tissue repair and stem cell renewal in carcinogenesis.Nature 2004;432:324-31.
5.Nüsslein-Volhard C, Wieschaus E.Mutations affecting segment number and polarity in Drosophila.Nature 1980;287:795-801.
6.Johnson RL, Rothman AL, Xie J, et al.Human homolog of patched, a candidate gene for the basal cell nevus syndrome.Science 1996;272:1668-71.
7.Hahn H, Wicking C, Zaphiropoulous PG, et al.Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome.Cell 1996;85:841-51.
8.Reifenberger J, Wolter M, Weber RG, et al.Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive euroectodermal tumors of the central nervous system.Cancer Res 1998;58:1798-803.
9.Xie J, Murone M, Luoh SM, et al.Activating smoothened mutations in sporadic basal-cell carcinoma.Nature 1998;391:90-2.
10.Karhadkar SS, Bova GS, Abdallah N, et al.Hedgehog signalling in prostate regeneration, neoplasia and metastasis.Nature 2004;431:707-12.
11.Sanchez P, Clement V, Ruiz i Altaba A.Therapeutic targeting of the hedgehog- GLI pathway in prostate cancer.Cancer Res 2005;65:2990-2.
12.Watkins DN, Berman DM, Burkholder SG, et al.Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer.Nature 2003;422:313-7.
13.Velcheti V, Govindan R.Hedgehog signaling pathway and lung cancer.J Thorac Oncol 2007;2:7-10.
14.Berman DM, Karhadkar SS, Maitra A, et al.Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumors.Nature 2003;425:846-51.
15.Monzo M, Moreno I, Artells R, et al.Sonic hedgehog mRNA expression by real-time quantitative PCR in normal and tumor tissues from colorectal cancer patients.Cancer Lett 2006;233:117-23.
16.Dimmler A, Brabletz T, Hlubek F, et al.Transcription of sonic hedgehog, a potential factor for gastric morphogenesis and gastric mucosa maintenance, is upregulated in acidic conditions.Lab Invest 2003;83:1829-37.
17.Isohata N, Aoyagi K, Mabuchi T, et al.Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus.Int J Cancer 2009;125:1212-21.
18.Ma X, Chen K, Huang S, et al.Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas.Carcinogenesis 2005;26:1698-705.
19.Feldmann G, Dhara S, Fendrich V, et al.Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers.Cancer Res 2007;67:2187-96.
20.Kubo M, Nakamura M, Tasaki A, et al.Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer.Cancer Res 2004;64:6071-4.
21.Mukherjee S, Frolova N, Sadlonova A, et al.Hedgehog signaling and response to Cyclopamine differ in epithelialand stromal cells in benign breast and breast cancer.Cancer Biol Ther 2006;5:674-83.
22.Chen X, Horiuchi A, Kikuchi N, et al.Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: It’s inhibition leads to growth suppression and apoptosis.Cancer Science 2007;98:68-76.
23.Deutsch G, Jung J, Zhang M, et al.A bipotential precursor population for pancreas and liver within the embryonic endoderm.Development 2001;128:871-81.
24.ten Haaf A, Bektas N, von Serenyi S, et al.Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival.BMC Cancer 2009;9:298.
25.Li YC, Deng YH, Guo ZH, et al.Prognostic value of hedgehog signal component expressions in hepatoblastoma patients.Eur J Med Res 2010;15:468-74.
26.Souzaki M, Kubo M, Kai M, et al.Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer.Cancer Sci 2011;102:373-81.
27.Saze Z, Terashima M, Kogure M, et al.Activation of the sonic hedgehog pathway and its prognostic impact in patients with gastric cancer.Dig Surg 2012;29:115-23.
28.He HC, Chen JH, Chen XB, et al.Expression of hedgehog pathway components is associated with bladder cancer progression and clinical outcome.Pathol Oncol Res 2012;18:349-55.
29.Che L, Ren J, Yuan YH, et al.Expression of genes related to Sonic Hedgehog signaling in human hepatocellular carcinomas.Beijing Da Xue Xue Bao (in Chinese)2008;40:616-23.
30.Katoh Y, Katoh M.Hedgehog signaling pathway and gastrointestinalstem cell signaling network (Review).Int J Mol Med 2006;18:1019-23.
31.Yang L, Xie G, Fan Q, et al.Activation of the hedgehogsignaling pathway in human cancer and the clinical implications.Oncogene 2010;29:469-81.
32.Hebrok M.Hedgehog signaling in pancreas development.Mech Dev 2003;120:45-57.
33.Watkins DN, Peacock CD.Hedgehog signalling in foregut malignancy.Biochem Pharmacol 2004;68:1055-60.
34.Fattovich G, Giustina G, Degos F, et al.Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients.Gastroenterology 1997;112:463-72.
35.Llovet JM, Burroughs A, Bruix J.Hepatocellular carcinoma.Lancet 2003;362:1907-17.
36.Shackel NA, McGuinness PH, Abbott CA, et al.Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression.Gut 2001;49:565-76.
37.Shackel NA, McGuinness PH, Abbott CA, et al.Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression.Am J Pathol 2002;160:641-54.
38.Ramalho-Santos M, Melton DA, McMahon AP.Hedgehog signals regulate multiple aspects of gastrointestinal development.Development 2000;127:2763-72.
39.Jung Y, McCall SJ, Li YX, et al.Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis.Hepatology 2007;45:1091-6.
40.Keeler RF, Binns W.Teratogeneic compaunds of Veratrum californicum (Durand).V.Comparison of cyclopian effects of steroidal alkaloids from the plant and structurally related compounds from other sources.Tetratology 1968;1:5-10.
41.Incardona JP, Gaffield W, Kapur RP, et al.The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction.Development 1998;125:3553-62.
42.Berman DM, Karhadkar SS, Hallahan AR, et al.Medulloblastoma growth inhibition by Hedgehog pathway blockade.Science 2002;297:1559-61.
43.Chen JK, Taipale J, Cooper MK, et al.Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened.Genes Dev 2002;16:2743-8.
44.Katano M.Hedgehog signaling pathway as a therapeutic target in breast cancer.Cancer Lett 2005;227:99-104.
 Chinese Journal of Cancer Research2012年4期
Chinese Journal of Cancer Research2012年4期
- Chinese Journal of Cancer Research的其它文章
- Combination chemotherapy with paclitaxel, cisplatin and fluorouracil for patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study
- NRS2002 assesses nutritional status of leukemia patients undergoing hematopoietic stem cell transplantation
- High-risk endometrial cancer may be benefit from adjuvant radiotherapy plus chemotherapy
- Circulating tumor cells (CTCs) in breast cancer: a diagnostic tool for prognosis and molecular analysis
- Evaluation of treatment response for breast cancer: are we entering the era of “biological complete remission”?
- Perivascular epithelioid cell tumor of male pelvic cavity: a case report and literature review
