Prognostic significance and clinical relevance of Sprouty 2 protein expression in human hepatocellular carcinoma
Kang Song, Qiang Gao, Jian Zhou, Shuang-Jian Qiu, Xiao-Wu Huang, Xiao-Ying Wang and Jia Fan
Shanghai, China
Prognostic significance and clinical relevance of Sprouty 2 protein expression in human hepatocellular carcinoma
Kang Song, Qiang Gao, Jian Zhou, Shuang-Jian Qiu, Xiao-Wu Huang, Xiao-Ying Wang and Jia Fan
Shanghai, China
BACKGROUND:In vitroexperiments and mice models have confirmed the importance of Sprouty 2 (Spry2) in inhibiting tumorigenesis and the progression of human cancer. However, the prognostic value of Spry2 in cancer patients remains unknown. This study is aimed to investigate the clinical relevance and prognostic significance of Spry2 expression in patients with hepatocellular carcinoma (HCC).
METHODS:With samples from 240 randomly-selected HCC patients who underwent surgery, immunohistochemistry was used to investigate Spry2 expression on tissue microarrays. The correlation of Spry2 expression with survival was estimated by the Kaplan-Meier method and univariate/multivariate Cox proportional hazard regression analysis. Spry2, ERK and phospho-ERK expression in HCC cell lines was detected by Western blotting.
RESULTS:Among the patients, 86.3% (207 of 240) exhibited down-regulation of Spry2 expression. Patients negative for Spry2 showed poorer survival (P=0.002) and increased recurrence (P=0.003). Multivariate analysis further established Spry2 as an independent predictor of postoperative recurrence in HCC patients (HR=1.47; 95% CI, 1.02-2.08;P=0.037). Downregulation of Spry2 was associated with highly malignant phenotypes like vascular invasion and advanced tumor stages, and was positively correlated with the metastatic potential of HCC cell lines.
CONCLUSION:In the era of molecular targeted therapy, the expression of Spry2 in HCC may have relevant clinical significance and turn out to be a key factor in prognostic assessment and in treatment planning.
(Hepatobiliary Pancreat Dis Int 2012;11:177-184)
hepatocellular carcinoma; Sprouty 2; tumor suppressor; prognosis
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and its recurrence rate can be as high as 50% at 3 years, even for those who undergo resection.[1]Although the etiology of HCC is better-defined than other types of cancer, the molecular genetics and signaling pathways underlying hepatic carcinogenesis and disease progression are still poorly understood.
An unbiased and integrative analysis of multidimensional genomic datasets can effectively screen for potential driver genes and provides novel mechanistic and clinical insights into the pathobiology of HCC. By genomic screening, Sprouty 2 (Spry2) was identified as one of the prominent genes to be differentially expressed in HCC compared with non-tumor liver tissue[2,3]and was independently recaptured by correlating expression arrays and array-based comparative genomic hybridization data.[4]Typically, Spry2 antagonizes growth factor-mediated cell proliferation, migration, and differentiation by modulating receptor tyrosine kinase (RTK) signaling and suppressing the MAPK-ERK signaling pathway.[5]There is emerging evidence that this protein is an important modulator of vital pathways central to the development or progression of many types of cancer, such as angiogenesis, cell growth, invasion,migration, and cytokinesis. Since Spry2 is known to act downstream of many RTK pathways that have wellappreciated roles in HCC, including FGF, VEGF and HGF,[6-8]it may play an important part in suppressing the progression of HCC. Also, given the significance of the MAPK pathway in HCC, which can be activated by important etiologic factors (hepatitis virus B and C) and mitogenic growth factors, modulation of Spry2 could have profound effects on the development or progression of HCC. Although a paradoxical role of sprouty proteins in human malignant transformation has been reported,[9]the inhibitory role and the underlying molecular mechanisms of Spry2 in checking HCC development are beginning to come out.[10]
As for clinical utility, Spry2 is included in the gene signature that predicts the sensitivity to MEK-1/2 targeted therapy in melanoma.[11]Despite the established role of the MAPK-ERK signaling pathway in HCC, phospho-ERK (p-ERK) expression shows no correlation with postoperative recurrence in HCC patients,[12]suggesting that upstream molecules like Spry2 play a more important role. Recently, sorafenib, a multikinase inhibitor targeting Raf kinase and RTK, has been shown to be effective in prolonging the survival of patients with advanced HCC, heralding a new era of molecular targeting therapy and reinforcing the utility of blocking Ras/MAPK signals in the treatment of HCC.[13]Thus, considering the significant role of Spry2 in HCC, elucidating its clinical relevance, histopathological associations and prognostic implications seems mandatory and may provide novel insight into the role of Spry2 in promoting HCC development.
However, most current studies are limited toin vitroor mouse model analysis of Spry2 functions. In this study, we aimed to elucidate the clinical roles, if any, of Spry2 in human HCC. We investigated Spry2 expression in tumor samples from a large HCC cohort. Here, for the first time, we demonstrated that downregulation of Spry2 in HCC significantly correlated with tumor aggressiveness, and was an independent predictor of increased recurrence.
Methods
Patient selection and follow-up
Tumor stage was determined according to the TNM staging system of the International Union Against Cancer (6th edition).[15]Tumor differentiation was graded by the Edmondson-Steiner system. The liver function of all patients was classified as Child-Pugh stage A. Informed consent was given by all patients, and this study was approved by the Research Ethics Committee of Zhongshan Hospital.
The patient follow-up and postoperative management were administrated abiding our established guidelines as described previously.[16-18]In brief, patients were followed up every 2-4 months as appropriate. Alphafetoprotein (AFP) test, liver ultrasonography, computed tomography, magnetic resonance imaging and bone scan were selected as needed. If HCC recurrence were confirmed, a second hepatectomy, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or external radiotherapy was given according to the number, size and sites of the recurrent tumor. Overall survival (OS) was defined as the interval between the date of surgery and death. Time to recurrence (TTR) was defined as the interval between the date of surgery and the first recurrence, or from the date of surgery to the date of last follow-up for the patients without recurrence. Data were censored at last follow-up for patients without relapse or death. The median follow-up period was 39.0 months (range, 1.5-95.0; SD, 22.7). At last follow-up (March 31, 2010), 127 (52.9%) patients were confirmed as having relapsed: 89 with intrahepatic recurrence, 17 with extrahepatic metastasis, and 21 with both. A total of 122 (50.8%) patients died: 52 because of liver failure or bleeding from the gastrointestinal tract, and 70 with tumor recurrence. Details of the clinicopathologic characteristics are summarized in Table 1.
Cell lines and Western blotting
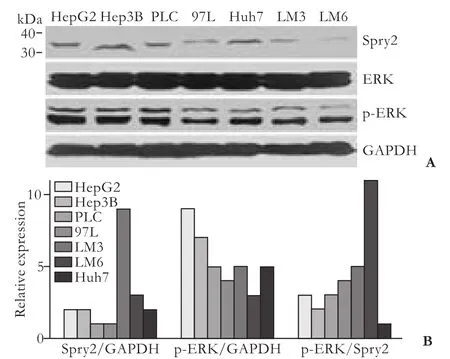
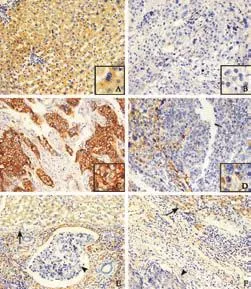
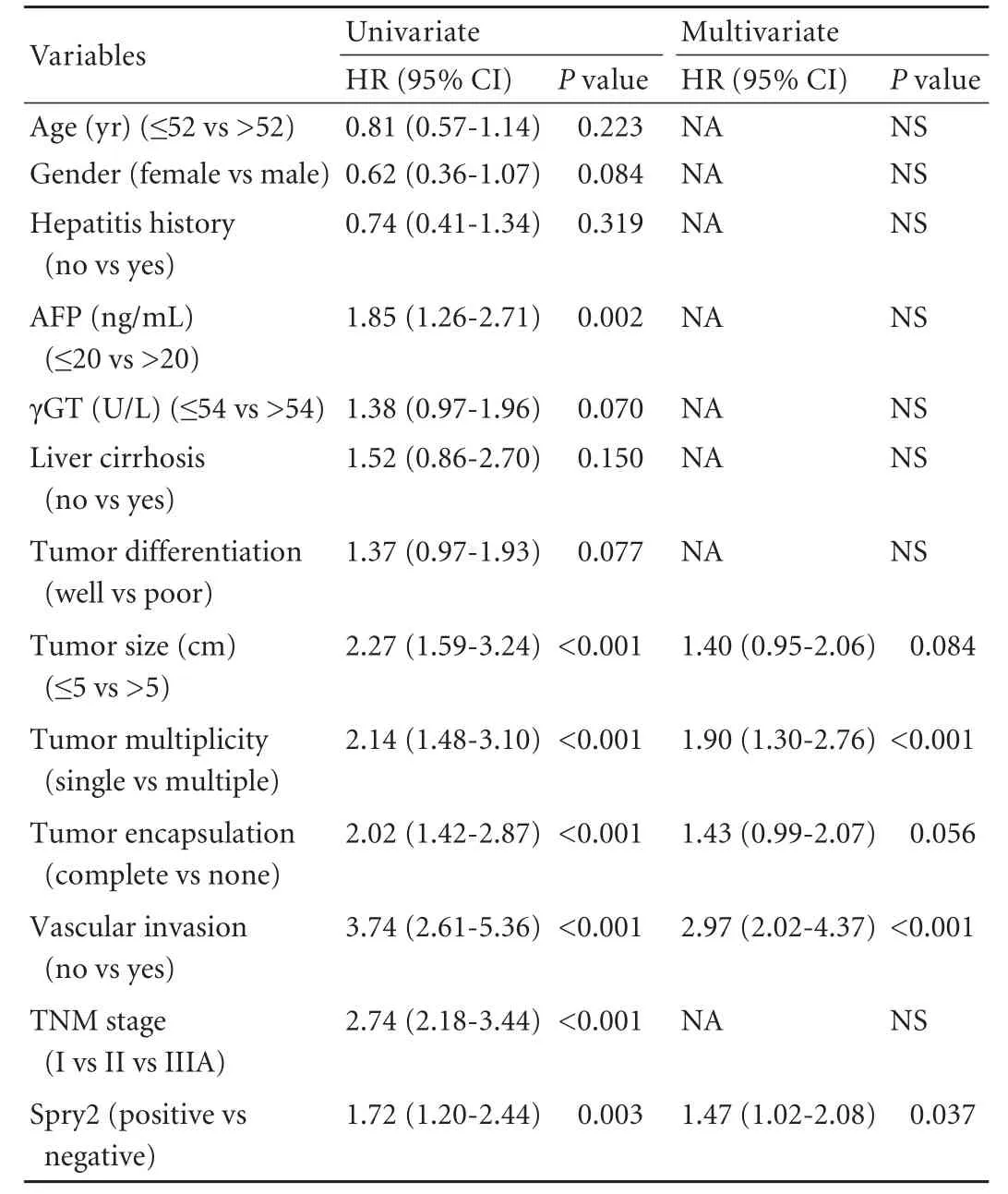
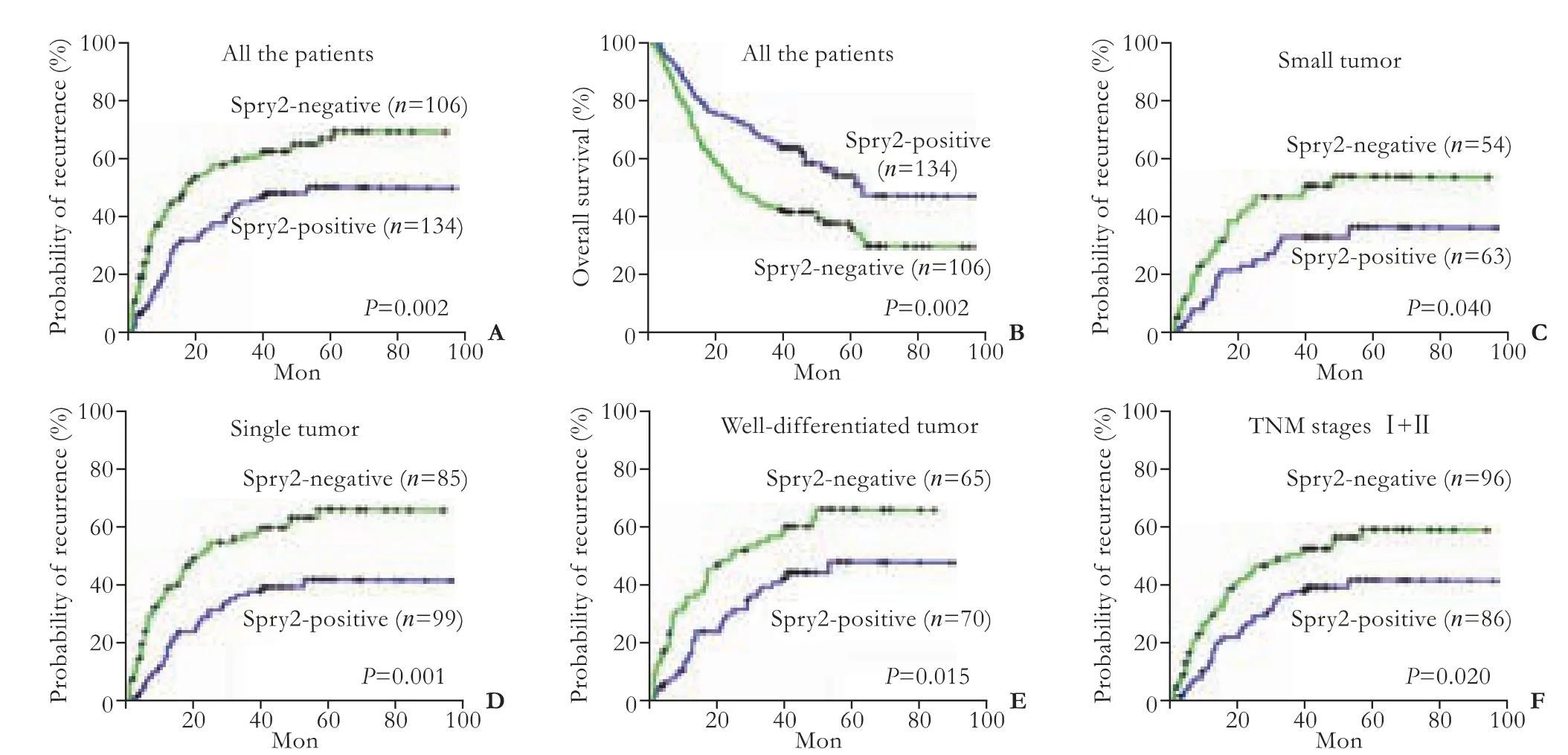
Human HCC cell lines, including three cell lines (MHCC97L, HCCLM3 and HCCLM6) with serially increased metastatic potential (MHCC97L Cell lysis, protein extraction, concentration measurement, and Western blotting were done as previously described[18,22]with the following primary antibodies: mouse anti-human monoclonal antibodiesto Spry2 (3 μg/mL, clone ab60719, Abcam, Cambridge, MA), ERK (1 μg/mL, clone ab54230, Abcam), p-ERK (1:2000, clone E10, Cell Signaling Technology, Danvers, MA), and GAPDH (1:5000, clone 6C5, Chemicon, Temecula, CA) as an internal loading control. The results presented are representative of three experiments. Table 1. Correlation between Spry2 expression and clinicopathological characteristics Tissue microarray construction and immunohistochemistry Tissue microarrays were produced as described previously.[14]Briefly, after HCC cases were histologically reviewed by hematoxylin and eosin staining, three 1-mm diameter cores from representative areas were punched for each case. Immunohistochemistry was carried out on 4-μm sections as previously described.[14,17]In brief, neutralization of endogenous peroxidase was done with 0.3% H2O2and antigen retrieval was performed with microwave oven in pH 6.0 citrate buffer. After incubating with the primary antibody (mouse anti-human Spry2 monoclonal antibody, 3 μg/mL), immunostaining was carried out using a twostep protocol (Novocastra, Newcastle, UK). Tissue microarrary staining, including negative staining controls, was done in a single experiment. Evaluation of immunohistochemical findings The immunoreactivity of Spry2 was evaluated independently by 2 researchers who were blinded to patient outcome. Scoring accounted for both the representation of the area and intensity of the stain. Briefly, the score was the sum of the percentage of positive cells (1, <25% positive; 2, 25%-50% positive; and 3, >50% positive) and the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong). Sums between 0 and 3 were scored as negative, and sums of 4 to 6 were scored as positive. Both the tumor and paired peritumor liver tissue were evaluated. Statistical analysis Statistical analyses were done with SPSS 15.0 software. The association between Spry2 expression and clinicopathologic variables was analyzed using the chi-square test. The survival curves were estimated by the Kaplan-Meier method and compared by the logrank test. The Cox proportional hazards regression model was used to perform univariate and multivariate analyses, including all the clinicopathologic features as covariates with stepwise manner (backward, likelihood ratio). AP(two-tailed) <0.05 was considered statistically significant. Spry2 expression pattern in HCC cell lines and tissue samples A 33-kDa band corresponding to the Spry2 protein was detected in the human HCC cell lines (Fig. 1A). Strikingly, the ratio of p-ERK to Spry2 expression displayed a concordant elevation with their stepwise metastatic potential in cell lines MHCC97L, HCCLM3,and HCCLM6. Similarly, Spry2 expression alone excellently and inversely correlated with the metastatic potential, while ERK and p-ERK expression were not in parallel with the metastatic potential (Fig. 1B). Fig. 1. Spry2, ERK and p-ERK expression in HCC cell lines at different levels (A). Densitometric analysis of Western blotting (B). In HCC tumors, according to the immunostaining scores, the protein levels of Spry2 were significantly reduced (P<0.001, the Mann-Whitney test) in HCC tissues compared with those in peritumor noncancerous tissues; and the expression of Spry2 was down-regulated in 86.3% (207/240) of the HCC samples examined in this study. The Spry2 expression was seen in the cell membrane, cytoplasm, or both, in a focal or scattered pattern, while it was evenly scattered throughout the peritumor noncancerous tissue (Fig. 2). Among the 240 cases, 134 (55.8%) showed positive staining for Spry2, with the remaining 106 (44.2%) identified as negative. Spry2 expression and patient outcome The five-year recurrence-free survival and overall survival of the 240 patients with HCC were 40.1% and 46.4%, respectively. Of the clinicopathologic predictors investigated, univariate analysis revealed that serum AFP, tumor size, encapsulation, multiplicity, vascular invasion and tumor stage adversely affected recurrence as well as survival (Table 2). In addition, tumor differentiation and serum γ-glutamyl transpeptidase (γGT) adversely affected survival but not recurrence (Table 3). Fig. 2. Examples of immunohistochemical detection of Spry2. Positive Spry2 expression in peritumor noncancerous liver tissue (A). An example of a poorly-differentiated HCC with negative Spry2 expression (B). Examples of HCC tumors with positive Spry2 expression in a diffuse pattern (C) or in a focal pattern (D). Peritumor liver tissue (arrow) showing positive while tumor thrombosis (arrowhead) showed negative Spry2 expression (E). A case of positive Spry2 in peritumor liver tissue (arrow) and negative Spry2 in cancerous tissue (arrowhead) (F) (original magnification ×400). Spry2-negative patients had significantly increased recurrence and poorer survival than Spry2-positive patients. The median TTR and OS were 16 and 27 months for Spry2-negative patients compared with 52 and 63 months for Spry2-positive patients (Fig. 3A and 3B). Furthermore, multivariate Cox proportional hazards regression analysis was used to confirm the prognostic significance of Spry2 expression with regard to TTR and OS including all the variables to control for confounders. Multivariate analysis (Table 2) showed that Spry2 (P=0.037), tumor multiplicity (P<0.001) and vascular invasion (P<0.001) were independent prognostic factors for TTR. In particular, Spry2-negative patients were 1.5 times more likely to suffer from recurrence [hazard ratio (HR)=1.47; 95% confidence interval (CI), 1.02-2.08;P=0.037] than Spry2-positive patients. However, for OS, Spry2 was no longer significant on multivariate analysis (Table 3). This may be attributed to the fact that cirrhosis, a life-threatening condition, is present in more than 80% of patients with HCC, and significantly affects OS.[23] Furthermore, subgroup analysis revealed significant differences in recurrence between Spry2-positve and Spry2-negative patients with early-stage disease: small tumor (P=0.040), single tumor (P=0.001), welldifferentiated tumor (P=0.015), and TNM stages I+II (P=0.020) (the log-rank test; Fig. 3C-F), as well as potential significance in tumor with no vascular invasion (P=0.093, the log-rank test). In 184 patients with only one tumor nodule, the results were then validated by multivariate analysis: Spry2-negative patients (n=99) were nearly twice as likely to suffer from relapse than Spry2-positive patients (n=85) (HR=1.79; 95% CI, 1.16-2.77;P=0.009). Table 2. Univariate and multivariate analyses of factors associated with recurrence Table 3. Univariate and multivariate analyses of factors associated with survival Fig. 3. Kaplan-Meier curves demonstrating recurrence or survival differences between patients with Spry2-positive or -negative tumors among overall patients (A and B), as well as patients with less aggressive clinicopathologic features (C-F). Correlations between Spry2 expression and clinicopathologic parameters To evaluate the association of Spry2 with tumor biology, comparisons of the clinicopathologic features with Spry2 expression were made. Patients with low Spry2 expression were more likely to exhibit aggressive clinicopathologic features, i.e. Spry2-negative patients harbored more tumors with vascular invasion, high serum AFP (P=0.016), poor differentiation (P=0.007) and advanced TNM stages (P=0.047) (Table 1). This is consistent with the results from the HCC cell lines, in which highly metastatic cell lines showed high p-ERK/ Spry2 ratios as well as low Spry2 expression, and the well-differentiated HCC cell line Huh7 showed the lowest p-ERK/Spry2 ratio. Previous high-throughput surveys of genes involved in hepatic carcinogenesis and malignant progression suggested that Spry2, a negative feedback inhibitor of the MAPK-ERK pathway, is a candidate tumor suppressor in HCC.[3,4]Initially, Spry2 was identified as one of the most significantly and differentially expressed genes comparing HCC tissue with paired noncancerous peritumor liver tissue (P<0.01 by Student'sttest).[2]Further study suggested that Spry2 but not Spry1 transcript is underexpressed in HCC, and genes involved in the angiogenic process and tumor invasiveness show strong correlations with Spry2 expression.[3]However, no prognostic information was provided and its association with clinical and pathologic features remained unknown. We here present the first large-scale study using tissue microarray analyses to determine the clinical significance of tumor Spry2 expression in surgically resected HCC patients. We found that Spry2 was an independent and powerful prognostic predictor for the progression of surgically resected primary HCC. The HCC patients whose primary tumors exhibited negative expression of Spry2 evidently had a much higher probability of recurrence. Why Spry2 expression is a potential prognostic factor may be due to an evolutionarily conserved inhibitor of RTKs and the MAPK-ERK cascade that heavily contribute to the molecular pathogenesis of HCC. The down-regulation of Spry2 in HCC cells results in unchecked activation of the MAPK-ERK pathway and in turn provides a proliferative advantage for cancer cells, while in normal hepatocytes the high expression of Spry2 significantly repressed the proliferative signaling.[4]In mouse HCC models, blocking Spry2 activity via a dominant negative form of Spry2 cooperates with c-met to promote hepatic carcinogenesis and recapitulates the subgroup of human HCC with a clinically aggressive phenotype.[10] Besides its prognostic value, our data further suggest that Spry2 is associated with aggressive phenotypes like advanced TNM stages, vascular invasion and poor differentiation in HCC patients. In support of this result, the ratio of p-ERK to Spry2 may display a concordant elevation with the stepwise metastatic potential of the HCC cell lines. Thus, down-regulation of Spry2 in HCC may indicate aggressive tumor behavior. In contrast, being consistent with the conclusion of no significant correlation of pERK1/2 expression with recurrence-free survival in HCC patients,[12]ERK and p-ERK expression are not in parallel with the metastatic potential in HCC cell lines. In line with these results, several reports also demonstrate that the expression of p-ERK is not associated with the dependence of the MAPK pathway and the output of the ERK pathway.[24,25] The advent of sorafenib as an effective molecular targeting therapy has recently changed the scope of clinical investigations in HCC. In a phase II clinical trail, patients whose tumors expressed higher pretreatment pERK levels had a longer time to tumor progression following treatment with sorafenib.[26]Anin vitrostudy from our group further demonstrated that reducing the baseline pERK level made HCC cells significantly less sensitive to sorafenib-mediated growth inhibition,[27]suggesting pERK as a potential biomarker of treatment sensitivity. More importantly, mechanistic studies have indicated that relief of upstream feedback within the MAPK pathway can attenuate the response to selective inhibitors of RAF and MEK and contribute to drug resistance.[28]In this context, we suggest that a combination of p-ERK and Spry2, for example the ratio of p-ERK to Spry2, may help to better define which subsets of patients will benefit from sorafenib. As such, a further understanding of the role of feedback elements in promoting transformation and attenuating the drug response may thus inform the development of combination strategies that maximize tumor response. Still, we found the prognostic value of Spry2 inHCC in early stage patients operated on. The HCC patients with a single nodule, if associated with negative Spry2, failed to have a favorable outcome. Spry2-negative patients were about twice as likely to develop postoperative tumor recurrence. Consequently, the prognostic uncertainties of HCC patients in the early stages and in the absence of highly aggressive phenotypes may partly be resolved by a combination with Spry2 expression. However, in this study, Spry2 was not down-regulated in all patients and its prognostic value remained inferior to classical pathologic factors like tumor vascular invasion. Several lines of evidence may be attributed to these results. First, in addition to Spry2, the MAP kinase phosphatases (MKPs or DUSPs) are equally important physiologic feedback inhibitors of the MAPK-ERK pathway.[29]Second, in the scenario of BRAF mutant tumors, the upstream feedback at the level of the RAF is disrupted by the inability of Spry2 to bind and inhibit mutant BRAF instead of Spry2 down-regulation.[30]Finally, this study is limited by its retrospective nature and the inclusion only of patients with resectable tumors. Larger population prospective studies of Spry2 are needed to further validate the usefulness of this system. In summery, considering previous mechanistic studies showing that inactivation of Spry2 promotes hepatic carcinogenesis as well as the invasion and migration of cancer cells, this study further recognizes Spry2 as a new signal-transducing target for prognostic prediction and prevention and as a biomarker for the treatment sensitivity of HCC. The restoration of inactivated Spry2 may prevent invasive progression and metastatic relapse to improve the prognosis for HCC patients. Contributors:SK and GQ contributed equally to this work. SK and FJ proposed the study. SK and GQ wrote the first draft, and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. FJ is the guarantor. Funding:This study was supported by grants from the National Key Sci-Tech Special Project of China (2008ZX10002-018/019), the Shanghai Rising-Star Program (10QA1401300), the National Natural Science Foundation of China (30901432) and the Research Fund for the Doctoral Program of Higher Education of China (20090071120026). Ethical approval:The study was approved by the Zhongshan Hospital Research Ethics Committee. Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. 1 El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-2576. 2 Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002;13:1929-1939. 3 Fong CW, Chua MS, McKie AB, Ling SH, Mason V, Li R, et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res 2006;66:2048-2058. 4 Lee SA, Ho C, Roy R, Kosinski C, Patil MA, Tward AD, et al. Integration of genomic analysis andin vivotransfection to identify sprouty 2 as a candidate tumor suppressor in liver cancer. Hepatology 2008;47:1200-1210. 5 Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 2002;4:850-858. 6 Lo TL, Fong CW, Yusoff P, McKie AB, Chua MS, Leung HY, et al. Sprouty and cancer: the first terms report. Cancer Lett 2006;242:141-150. 7 Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell 2005;8:689-701. 8 Cabrita MA, Christofori G. Sprouty proteins: antagonists of endothelial cell signaling and more. Thromb Haemost 2003; 90:586-590. 9 Lito P, Mets BD, Kleff S, O'Reilly S, Maher VM, McCormick JJ. Evidence that sprouty 2 is necessary for sarcoma formation by H-Ras oncogene-transformed human fibroblasts. J Biol Chem 2008;283:2002-2009. 10 Lee SA, Ladu S, Evert M, Dombrowski F, De Murtas V, Chen X, et al. Synergistic role of Sprouty2 inactivation and c-Met up-regulation in mouse and human hepatocarcinogenesis. Hepatology 2010;52:506-517. 11 Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D, et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244). Cancer Res 2010;70:2264-2273. 12 Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, et al. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol 2008;48:83-90. 13 Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390. 14 Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-979. 15 International Union Against Cancer (UICC). Sobin LH WC, eds. TNM classification of malignant tumors. 6th ed. New York: Wiley-Liss; 2002:81-83. 16 Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol 2007;47:684-690. 17 Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586-2593. 18 Yang XR, Xu Y, Shi GM, Fan J, Zhou J, Ji Y, et al. Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res 2008;14:3850-3859. 19 Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, et al. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer 1999;81:814-821. 20 Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J, et al. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics throughin vivoselection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol 2003;129:43-51. 21 Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL, et al. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutivein vivoselection and studies on metastatic characteristics. J Cancer Res Clin Oncol 2004;130: 460-468. 22 Weng YQ, Qiu SJ, Liu YK, Fan J, Gao Q, Tang ZY. Downregulation of beta-centractin might be involved in dendritic cells dysfunction and subsequent hepatocellular carcinoma immune escape: a proteomic study. J Cancer Res Clin Oncol 2008;134:179-186. 23 Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist 2010;15:23-33. 24 Pratilas CA, Hanrahan AJ, Halilovic E, Persaud Y, Soh J, Chitale D, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res 2008;68:9375-9383. 25 Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006;439:358-362. 26 Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24: 4293-4300. 27 Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from anin vitrostudy. BMC Med 2009;7:41. 28 Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A 2009;106:4519-4524. 29 Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 2008;27:253-261. 30 Brady SC, Coleman ML, Munro J, Feller SM, Morrice NA, Olson MF. Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Res 2009;69:6773-6781. Received November 3, 2010 Accepted after revision September 19, 2011 of 240 HCC patients who primary and curative resection of HCC between 2002 and 2006 at Liver Cancer Institute (Zhongshan Hospital, Fudan University), as described in our previous study,[14]was used as the study population. No patients received any anticancer treatments and no signs of distant metastases were detected before surgery. Clinicopathologic profile and follow-up data were reviewed and obtained from our prospectively established database. Author Affiliations: Liver Cancer Institute, Zhongshan Hospital (Song K, Gao Q, Zhou J, Qiu SJ, Huang XW, Wang XY and Fan J) and Institute of Biomedical Sciences (Zhou J and Fan J), Fudan University, Shanghai 200032, China Jia Fan, MD, PhD, Liver Cancer Institute, Zhongshan Hospital and Institute of Biomedical Sciences, Fudan University, 136 Yi Xue Yuan Road, Shanghai 200032, China (Tel/Fax: 86-21-64037181; Email: jiafan99@yahoo.com) ? 2012, Hepatobiliary Pancreat Dis Int. All rights reserved. 10.1016/S1499-3872(12)60145-3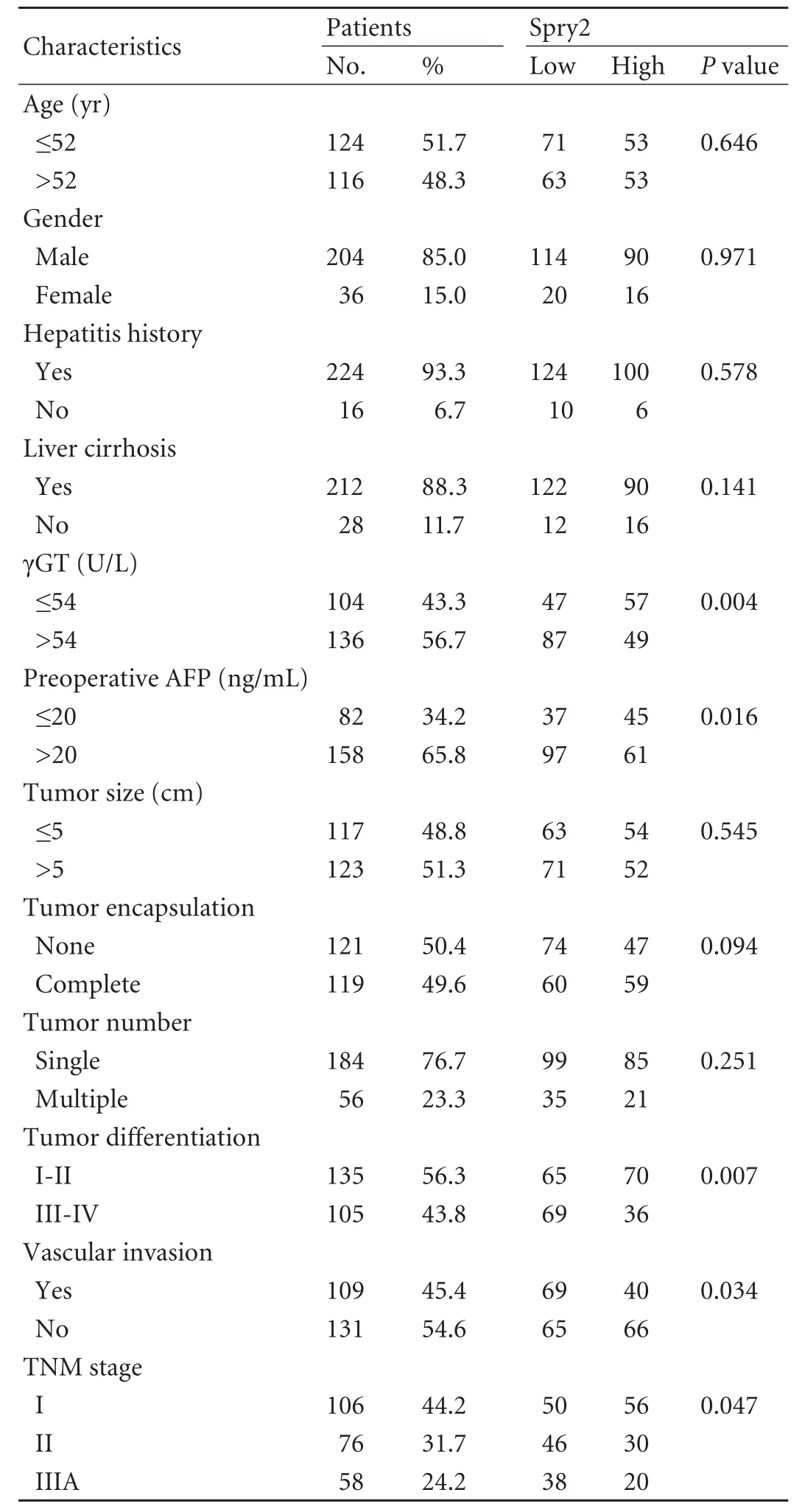
Results

Discussion
 Hepatobiliary & Pancreatic Diseases International2012年2期
Hepatobiliary & Pancreatic Diseases International2012年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreaticopleural fistula: etiology, treatment and long-term follow-up
- Rapamycin combined with allogenic immature dendritic cells selectively expands CD4+CD25+Foxp3+regulatory T cells in rats
- Inhibition of 12-lipoxygenase reduces proliferation and induces apoptosis of hepatocellular carcinoma cells in vitro and in vivo
- Management of hypersplenism in non-cirrhotic portal hypertension: a surgical series
- The impact of family history of hepatocellular carcinoma on its patients' survival
- Percutaneous transhepatic portal catheterization guided by ultrasound technology for islet transplantation in rhesus monkey
