Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
Xian-Huan Yu, Lei-Bo Xu, Hong Zeng, Rui Zhang, Jie Wang and Chao Liu
Guangzhou, China
Original Article / Biliary
Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
Xian-Huan Yu, Lei-Bo Xu, Hong Zeng, Rui Zhang, Jie Wang and Chao Liu
Guangzhou, China
BACKGROUND:Combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) is an uncommon subtype of primary hepatic carcinoma, and its prognosis is poor. This study was undertaken to investigate the prognosis and the clinicopathological characteristics of cHCC-CC, including their possible cellular origin.
METHODS:Among 852 patients with a primary hepatic carcinoma who underwent hepatectomy from January 1998 to April 2008 at our hospital, cHCC-CC was identified in 14 patients. The clinicopathological characteristics of the 14 patients were analyzed retrospectively. The expression of the liver stem cell markers (c-kit, CD90, CD133 and CK19) in the tumor tissue was detected by immunohistochemistry, and the Kaplan-Meier method was used to evaluate survival.
RESULTS:Among the 14 patients, 9 presented with abdominal pain, 3 with anorexia and debilitation, and the remaining two patients were asymptomatic. The mean age was 53.6±3.0 (range 38-74) years. Among the included patients, 11 had an elevated serum alpha-fetoprotein level, 13 were infected with hepatitis B virus, 9 had vascular invasion and 1 had lymph node metastasis. The average diameter of the tumors was 9.9±1.1 (range 5.0-16.0) cm. The median overall survival time was 7.9±1.0 months. In addition, the presence of the liver stem cell markers, c-kit, CD90, CD133 and CK19 was 71.4%, 85.7%, 92.9% and 78.6%, respectively. All four markers were simultaneously expressed in eight cases.
CONCLUSIONS:cHCC-CC has aggressive characteristics and the prognosis is extremely dismal. The high expression of liver stem cell markers in the tumor tissue suggests that these tumors may derive from liver stem cells.
(Hepatobiliary Pancreat Dis Int 2011; 10: 620-625)
liver neoplasms; hepatocellular carcinoma; cholangiocarcinoma; prognosis; stem cells
Introduction
There are three subtypes of primary hepatic carcinomas (PHCs): hepatocellular carcinoma (HCC), combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC), and cholangiocellular carcinoma (CC). cHCC-CC is an uncommon subtype that has a mixture of both hepatocellular and cholangiocellular differentiation. In 1903, Wells et al reported the first case of cHCC-CC, a subtype which now accounts for 0.4%-14.2% of all PHC cases worldwide.[1,2]In 1949, Allen and Lisa[3]classified cHCC-CC into three types: 1) type A, in which separate neoplastic masses may comprise entirely cells of either the liver cell type or the bile duct type; 2) type B, in which contiguous masses with different characteristics mingle as they grow; and 3) type C, in which individual masses display features of two cell types so intimately that they can be interpreted only as arising from the same site. Though each of these three possibilities is illustrated by authentic cases in the literature, recent emerging evidence indicates that only tumors of Allen's type C exhibit a mixture of both hepatocellular and cholangiocellular differentiation and appear to be true combined tumors.[4,5]
Tumors characteristics of Allen's type C cHCC-CC are well-known histopathologically, but their clinical behavior is poorly understood. Also, the cellular origin of cHCC-CC, as well as other liver malignancies, remains undefined.[6]In the present study, the demo-graphic features and prognoses of 14 patients with histopathologically proven cHCC-CC who underwent partial hepatectomy from January 1998 to April 2008 were retrospectively analyzed. Moreover, the expression of liver stem cell markers (c-kit, CD90, CD133 and CK19) in the tumor tissues was investigated by immunohistochemistry.
Methods
Sample collection
From January 1998 to April 2008, 852 patients underwent surgical treatment for hepatic tumors at Sun Yat-Sen Memorial Hospital. cHCC-CC was identified histopathologically in 14 patients. The medical records of these 14 patients, including the characteristics of their underlying liver disease, the tumor characteristics, and their prognoses, were retrospectively reviewed. Furthermore, paraffin-embedded tumor tissues from each case were obtained and cut into 4 μm sections consecutively. Routine histological examination was performed with hematoxylin and eosin staining in the Department of Pathology.
The present study was approved by the Ethics Committee of the hospital and was in accordance with the Helsinki Declaration of 1975. Our institutional review board approved the current study and waived the requirement for patient consent for this retrospective review.
Immunohistochemistry
The immunohistochemical staining of tissue sections for c-kit (Neomarkers, USA), CD90 (BIOS, China), CD133 (BIOS), and CK19 (Neomarkers) was completed according to the protocol defined in the Power Vision Two-Step Kit (ZSG, Beijing, China) at the Medical Research Center of Sun Yat-Sen Memorial Hospital.
The evaluation of staining was performed independently by two investigators, both of whom were unaware of the clinical data. The immunostained sections were first scanned under light microscopy at low magnification of ×40, and five non-overlapping fields were then examined at a final magnification of ×400. The results were described by the rate of positive cells and the staining intensity. The rate of positive cells (A) was divided into five grades: 0 (no positive cells), 1 (positive cells ≤10%), 2 (positive cells 11%-50%), 3 (positive cells 51%-75%) and 4 (positive cells >75%). The score of staining intensity (B) was classified as 0 (no staining), 1 (mild staining), 2 (moderate staining) and 3 (strong staining). The final score for each section was given as A×B, which ranged from 0 to 12. A negative result was defined as a score of 0-3 and a positive result was a score of 4-12.
Follow-up
During the first six postoperative months, patients were re-examined every one to two months. After that period, patients were re-examined every three to six months. At each follow-up visit, clinical and laboratory data were collected. Chest X-ray and abdominal ultrasound (or computed tomography scan) were performed. The deadline of follow-up was April 2009.
Statistical analysis
Statistical analysis was made with the SPSS software package (version 13.0, SPSS, Chicago, IL., USA). Quantitative data were presented as mean±standard error (SE). The Kaplan-Meier method was used to evaluate survival, and the log-rank test was used to compare the differences. A bivariate correlation was used to evaluate the statistical strength of the independent associations among the clinicopathologic features and the expression of liver stem cell markers. For all tests, a P value less than 0.05 was considered to be statistically significant. All P values were the results of two-sided tests.
Results
Clinical data
All 14 cHCC-CC patients had an Allen's type C tumor and underwent partial hepatectomy. The incidence of cHCCCC is 1.6% (14/852). The general characteristics of these patients are summarized in Table 1. In the 14 patients, 12 were men and 2 women. The mean and median ages were 53.6±3.0 and 50.5 years (range 38-74) years, respectively. Nine patients presented with abdominal pain, three with anorexia and debilitation, and the remaining two were asymptomatic. Of the included patients, 13 had a hepatitis B virus (HBV) infection and 10 of these patients developed liver cirrhosis. Eleven patients had an elevated level of serum alpha-fetoprotein (AFP) and five had an elevated level of serum carbohydrate antigen 19-9 (CA19-9) preoperatively. The diameter of the primary tumor was more than 5 cm in all patients (range 5.0-16.0 cm) with a mean of 9.9±1.1 cm. Six patients had macroscopic vascular invasion, 3 had microscopic vascular invasion, and 1 had lymph node metastasis. Moreover, two (14.3%) patients were classified as stage I, three (21.4%) as stage II, five (35.7%) as stage IIIA, three (21.4%) as stage IIIB, and one (7.2%) as stage IVA according to the AJCC TNM Staging and Grouping System, 7th Edition (2010).[7]
Relapse and overall survival of the 14 cHCC-CC patients
The follow-up span was 1-12 (mean 7.9±3.3; median7.9±1.0) months. Two patients (14.3%) were lost to follow-up. By the end of the follow-up, four patients experienced tumor recurrence in the remnant liver, and three experienced extra-hepatic metastases. Of the four patients with recurrence after surgery, one received percutaneous ethanol intratumoral injection, and the other three just received support therapy because of poor liver function and physical status (Table 1). The survival time was 1 to 12 months postoperatively, with a median overall survival time of 7.9±1.0 months (Fig. 1).
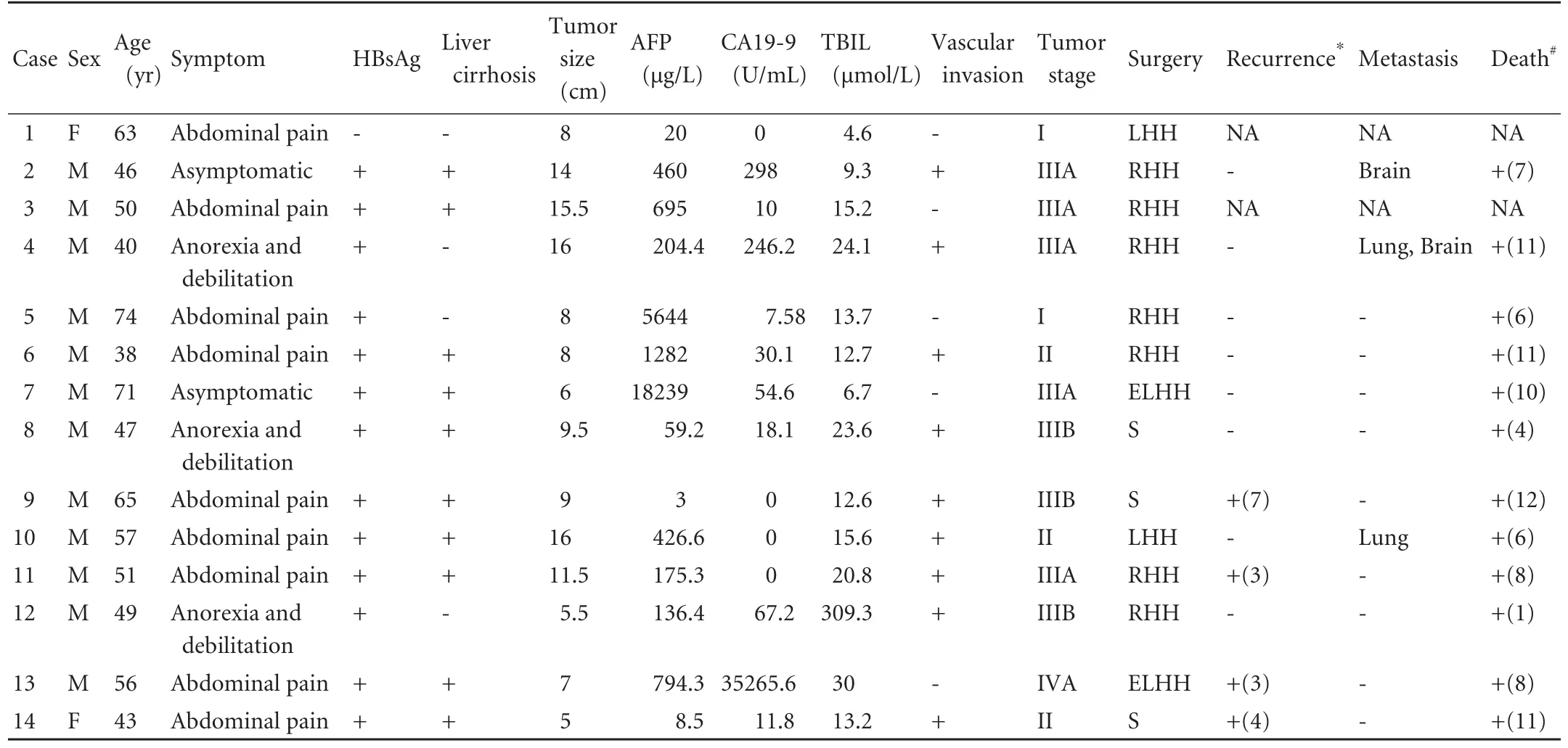
Table 1. Clinical features of patients with cHCC-CC
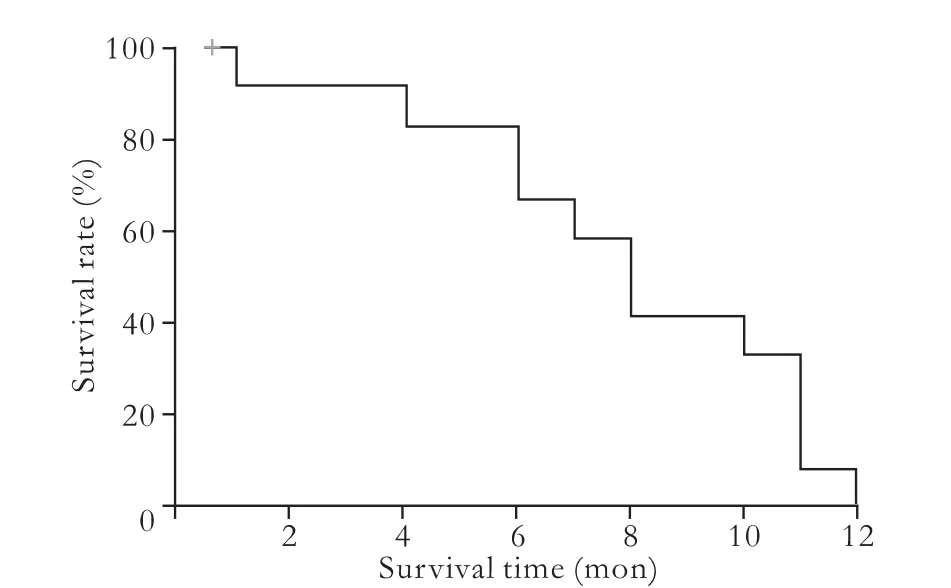
Fig. 1. Overall postoperative survival of the 14 patients with cHCC-CC.
C-kit, CD90, CD133 and CK19 expression in cHCC-CC tissues
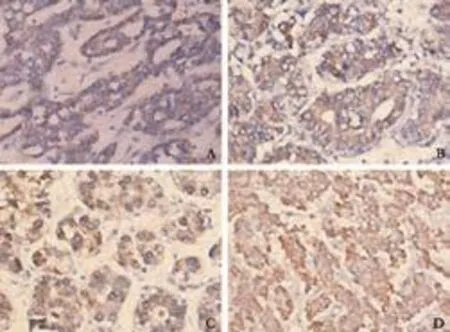
Fig. 2. Positive staining of liver stem cell markers in the membrane and cytoplasm of cHCC-CC (A: c-kit; B: CD90; C: CD133; D: CK19) (power vision two-step method, original magnification ×400).
All four liver stem cell markers were primarily stained in the tumor tissues. Positive staining of the four markers was seen in the membrane and cytoplasm of tumor cells (Fig. 2). The results evaluated by the two investigators were identical for all slides. Ten patients (10/14, 71.4%) were positive for c-kit, 12 (12/14, 85.7%) were positive for CD90, 13 (13/14, 92.9%) were positivefor CD133, and 11 (11/14, 78.6%) were positive for CK19. The simultaneous expression of all four liver stem cell markers was found in eight patients (8/14, 57.1%). There was no significant association between c-kit, CD90, CD133, or CK19 expression and the clinicopathologic data on gender, age, HBV infection, liver cirrhosis, tumor size, AFP levels, total bilirubin levels, and vascular invasion (Table 2). In addition, log-rank testsshowed that there was no significant association between the expression of the four liver stem cell markers and prognosis (Fig. 3).
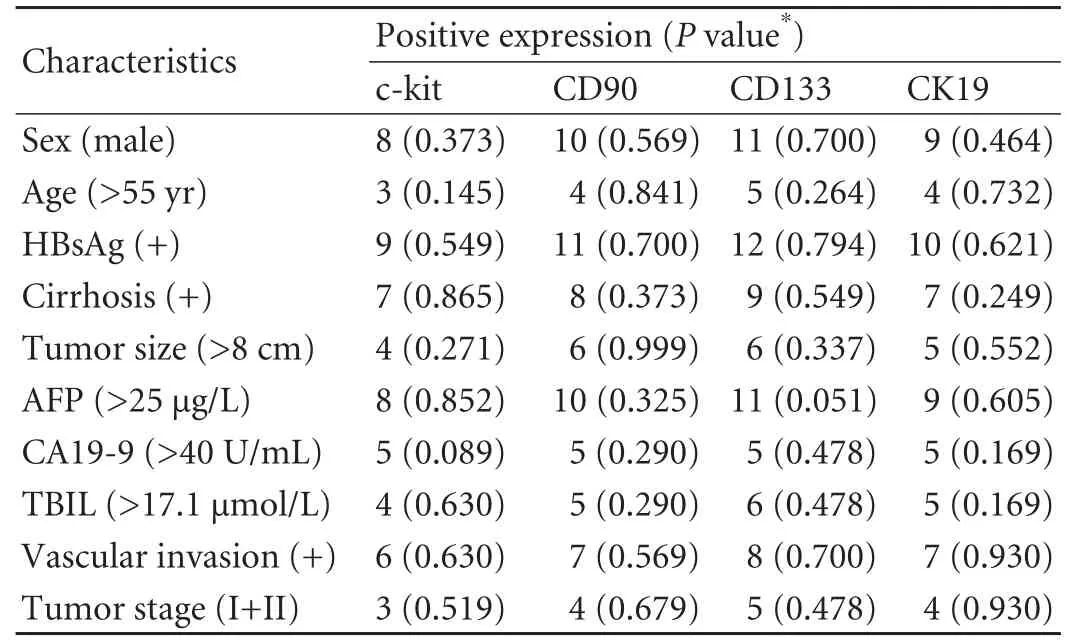
Table 2. Correlations between clinicopathological data and c-kit, CD90, CD133 and CK19 expression
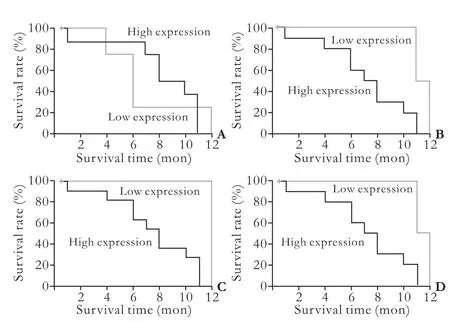
Fig. 3. Survival curves of patients with high or low expression of liver stem cell markers. A: The mean survival time was 8.3 months for the c-kit high expression group and 7.0 months for the c-kit low expression group; log-rank tests showed no statistically significant difference (P=0.779). B: The mean survival time was 7.2 months for the CD90 high expression group and 11.5 months for the CD90 low expression group; log-rank tests showed no statistically significant difference (P=0.050). C: The mean survival time was 7.5 months for the CD133 high expression group and 12.0 months for the CD133 low expression group; log-rank tests showed no statistically significant difference (P=0.080). D: The mean survival time was 7.2 months for the CK19 high expression group and 11.5 months for the CK19 low expression group; logrank tests showed no statistically significant difference (P=0.050).
Discussion
The incidence of cHCC-CC reported in previous studies is considerably diverse, ranging from 0.4% to 14.2% of PHCs worldwide.[1,2]Also, the local incidence of cHCCCC varies considerably between different regions of China. In the mainland, Zhang et al[8]reported that the incidence of cHCC-CC is 1.05% among resected PHCs. In Hong Kong, Liu et al[9]reported an incidence of 2.3%, whereas Ng et al[10]reported an incidence of 5.3%. In Taiwan, Lin et al[11]reported that cHCC-CC accounts for 5.0% of 599 PHC patients. We found the incidence in our study to be 1.6%.
The histopathological tumor characteristics of cHCCCC are well-known, but their clinical behavior is poorly understood. Though cHCC-CC is defined as the intimate intermingling of both an HCC component and a CC component, there is increasing evidence that cHCCCC differs markedly in the etiologic, epidemiologic, and clinical features from HCC and CC.[12]The exact cause of cHCC-CC is unknown, but multiple factors have been implicated in its pathogenesis, such as HBV and HCV infection, aflatoxin B1 intake, alcohol abuse, dioxin exposure and liver flukes.[13]It has been reported that the likelihood of having concurrent viral hepatitis or liver cirrhosis is lower in patients with cHCC-CC than in patients with HCC but higher than in patients with CC.[1,9]In the present study, the prevalence of HBV (13/14, 92.9%) and of liver cirrhosis (10/14, 71.4%) in the cHCCCC group far exceeded other reports (17%-58% and 39%-50%, respectively).
cHCC-CC is usually clinically silent until it presents with advanced disease, and successful preoperative diagnosis is elusive. Symptoms include painless jaundice, fatigue, abdominal discomfort, weight loss, and anorexia. Although a simultaneous increase in serum CA19-9 and AFP plus a liver mass on imaging studies may strongly indicate cHCC-CC, neither CA19-9 nor AFP is specific for cHCC-CC.[13]In addition, Choi et al[14]reported that certain features are helpful in differentiating this special tumor from other liver tumors, including 1) a hypoechoic mass with a central hyperechoic area, or a target appearance, on sonography; 2) a hypovascular mass with central hypervascular portions on angiography; or 3) partial retention of iodized oil in non-necrotic tumors on iodized-oil CT. Nagaoka et al[15]also reported that a fusion of positron emission tomography plus computed tomography (CT) is useful in detecting cHCC-CC, with adetection rate as high as 98%. A recent study of 12 cHCCCC patients showed that though MRI and triple-phase CT demonstrated a high sensitivity for lesion detection (100% and 78%, respectively), no imaging feature, whether shape, size, margins or location in the liver helped to suggest the presence of a cHCC-CC tumor.[16]However, the results are variable in different centers because the results depend on the histological proportions of the HCC and CC components and the volume of fibrous stroma. In the present study, 9 (64.3%) patients presented with abdominal discomfort, 11 (78.6%) had an elevated level of serum AFP and 5 (35.7%) had an elevated level of serum CA19-9 preoperatively. Although the diagnosis of PHC was definite on account of clinical, laboratory and radiological data for all patients, cHCC-CC was identified histopathologically after surgery.
Numerous studies have demonstrated that the progression of cHCC-CC is more rapid than that of HCC or CC. In general, the survival rate of cHCC-CC is either worse than that reported for HCC or CC, or intermediate between that of HCC and CC. The survival of cHCC-CC reported is also diverse. Chantajitr et al[17]reported that the overall median survival time of cHCC-CC patients was nine months. Yano et al[5]reported that the median survival time was 22 months. Jarnagin et al[4]reported a median survival time of 32 months. Lee et al[18]reported the median time-to-recurrence and overall survival time of cHCC-CC patients after curative resection were 5.4 and 18.0 months, and cHCC-CC patients had a shorter time-to-recurrence than HCC and CC patients, and a shorter overall survival than HCC patients. Panjala et al[16]reported 12 cHCC-CC patients who underwent liver transplantation. In those patients, the median survival time was 3.6 years and the 1-, 3- and 5-year cumulative survival probabilities were 79%, 66% and 16%, respectively, with a 5-year survival comparable to or better than liver transplantation for intrahepatic CC but poorer than liver transplantation for HCC following the Milan criteria. In our study, each of the 12 patients who completed follow-up died within one year after surgery, and the overall median survival time was 7.9±1.0 months.
Several studies[19,20]have indicated that the prognostic factors are associated with survival of PHC patients. Wang et al[19]studied 438 HCC patients who underwent partial hepatectomy and revealed that the postoperative overall survival rates at 1, 3 and 5 years were 72.2%, 53.5% and 43.3%, respectively, and that Child-Pugh class, tumor size, capsular invasion, tumor stage, vascular invasion, and resection margin were independent prognostic factors for overall survival. Kim et al[20]studied 50 unresectable cHCC-CC patients who received transcatheter arterial chemoembolization. They found the median patient survival period was 12.3 months and suggested that tumor size, tumor vascularity, Child-Pugh class, and portal vein invasion were independent factors associated with patient survival. Poor prognostic factors for patient survival were big tumor size, hypovascular tumor, Child-Pugh class B, and presence of portal vein invasion. However, in the present study, we failed to find any significant prognostic factors associated with survival, probably because of the small number of patients.
It has long been a controversial issue whether PHC arises from the maturational arrest of liver stem cells or from the de-differentiation of mature hepatocytes/ cholangiocytes. It has been reported that PHC can derive from different levels of cells in the hepatic lineage, such as mature hepatocytes/cholangiocytes, ductular "bipolar" progenitor cells, and putative periductular stem cells. Different carcinogens may affect the three different levels of cells and induce PHC via different mechanisms.[6]As a subtype of PHC, numerous studies have demonstrated that cHCC-CC may originate from transformed hepatic progenitor cells or liver stem cells. A study[8]suggested that cHCC-CC is of hepatic progenitor cell origin, supporting the concept that human hepatocarcinogenesis may originate from the transformation of hepatic progenitor cells. Yano et al[21]established a human cHCCCC cell line (KMCH-2) that can be differentiated into both intrahepatic cholangiocarcinoma and HCC under different growth conditions, suggesting that cHCCCC may originate from stem cells. Other researchers[22]suggested that the transplantation of Bmi1, or β-catenintransduced cells clonally expanded from single hepatic stem/progenitor cells produce tumors that exhibit the histological features of cHCC-CC. The present study showed that the liver stem cell markers (c-kit, CD90, CD133 and CK19) were highly expressed in cHCC-CC, indicating that cHCC-CC may derive from liver stem cells.
Tumors derived from liver stem cells tend to be more aggressive than those of mature hepatocyte/cholangiocyte origin. Lee et al[23]reported that patients with HCC who share a gene expression pattern with fetal hepatoblasts have a poorer prognosis than those who share gene expression with hepatocytes. Similarly, Durnez et al[24]reported that patients with CK19+HCC had a higher recurrence rate after transplantation, which suggests a worse prognosis for these HCCs in comparison to CK19-HCC. In the present study, our results showed that hepatic stem cell markers c-Kit, CD90, CD133 and CK19 were highly expressed in cHCC-CC tissue, which suggests that cHCC-CC may derive from liver stem cells. And the overall survival time was shorter in patients with a high expression of hepatic stem cell markers (CD90, CD133 and CK19) than in patients with low expression, thoughthe difference was not statistically significant.
In conclusion, cHCC-CC has aggressive characteristics and its prognosis is extremely dismal. The high expression of liver stem cell markers by tumor tissue suggests that they may derive from liver stem cells. We retrospectively analyzed 14 patients with cHCC-CC, so more clinical and experimental studies are needed to define the clinical and pathological characteristics of cHCC-CC in comparison to HCC and CC.
Funding:This study was supported by a grant from the Special Research Foundation of the National Natural Science Foundation of China (30872487).
Ethical approval:Not needed.
Contributors:LC and WJ designed the research. YXH, XLB and ZR performed the research. YXH, XLB and ZH analyzed the data. YXH, XLB and LC wrote the paper. LC is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg 2005;189:120-125.
2 Willekens I, Hoorens A, Geers C, Op de Beeck B, Vandenbroucke F, de Mey J. Combined hepatocellular and cholangiocellular carcinoma presenting with radiological characteristics of focal nodular hyperplasia. World J Gastroenterol 2009;15:3940-3943.
3 Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol 1949;25:647-655.
4 Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002; 94:2040-2046.
5 Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol 2003;33:283-287.
6 Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 2001;33:738-750.
7 Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010:237-246.
8 Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology 2008;52:224-232.
9 Liu CL, Fan ST, Lo CM, Ng IO, Lam CM, Poon RT, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg 2003;138:86-90.
10 Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellularcholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol 1998;13:34-40.
11 Lin G, Toh CH, Wu RC, Ko SF, Ng SH, Chou WC, et al. Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract 2008;62:1199-1205.
12 Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985;55:124-135.
13 Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract 2008;62:1271-1278.
14 Choi BI, Han JK, Kim YI, Kim HC, Park JH, Kim CW, et al. Combined hepatocellular and cholangiocarcinoma of the liver: sonography, CT, angiography, and iodized-oil CT with pathologic correlation. Abdom Imaging 1994;19:43-46.
15 Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int 2006;26:781-788.
16 Panjala C, Senecal DL, Bridges MD, Kim GP, Nakhleh RE, Nguyen JH, et al. The diagnostic conundrum and liver transplantation outcome for combined hepatocellularcholangiocarcinoma. Am J Transplant 2010;10:1263-1267.
17 Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg 2006;13:537-542.
18 Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol 2011;45:69-75.
19 Wang J, Xu LB, Liu C, Pang HW, Chen YJ, Ou QJ. Prognostic factors and outcome of 438 Chinese patients with hepatocellular carcinoma underwent partial hepatectomy in a single center. World J Surg 2010;34:2434-2441.
20 Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, et al. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology 2010;255:270-277.
21 Yano H, Iemura A, Haramaki M, Momosaki S, Ogasawara S, Higaki K, et al. A human combined hepatocellular and cholangiocarcinoma cell line (KMCH-2) that shows the features of hepatocellular carcinoma or cholangiocarcinoma under different growth conditions. J Hepatol 1996;24:413-422.
22 Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, et al. Enhanced self-renewal capability in hepatic stem/ progenitor cells drives cancer initiation. Gastroenterology 2007;133:937-950.
23 Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 2006;12:410-416.
24 Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138-151.
Received December 11, 2010
Accepted after revision May 13, 2011
Author Affiliations: Department of Hepato-Pancreato-Biliary Surgery (Yu XH, Xu LB, Zhang R, Wang J and Liu C) and Department of Pathology (Zeng H), Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China
Chao Liu, PhD, Department of Hepato-Pancreato-Biliary Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China (Tel: 86-20-34071172; Fax: 86-20-83755229; Email: mdliuchao@hotmail.com)
? 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60105-7
 Hepatobiliary & Pancreatic Diseases International2011年6期
Hepatobiliary & Pancreatic Diseases International2011年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: a meta-analysis
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
